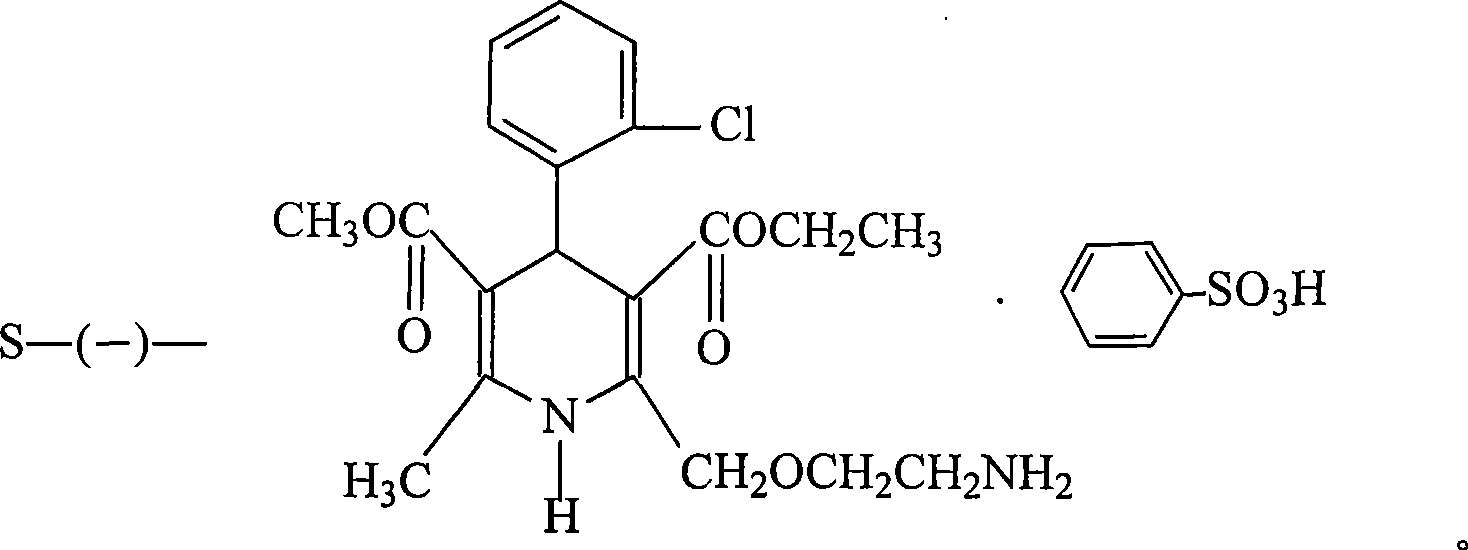
Production method of levamlodipine besylate
A kind of technology of levamlodipine besylate and its production method, which is applied in the field of production of levamlodipine besylate, can solve the problem that there is no system for temperature adjustment and prevention of feed liquid oxidation, it is not suitable for refined filtration treatment, and the filter cannot avoid light and other problems, to achieve the effect of convenient filtration operation and assembly and cleaning, stable filtrate quality, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] 1. Preparation of intermediate I crude product
[0016] Add 1kg (2.44mol) (±)-amlodipine and 84g (0.55mol) L-(+)-tartaric acid in a 10-liter reactor, then add 8 liters of DMF, stir at room temperature, react for 24 hours, suction filter, filter The cake was washed twice with DMF, 500ml each time, and the filter cake was vacuum-dried to prepare 440g of intermediate I crude product, with a yield of 36.9%.
[0017] 2. Refining of Intermediate I
[0018] Add 430g (0.89mol) of intermediate I crude product and DMF 1800ml into a 3-liter reactor, stir and react at 50°C for 5 hours, stop heating, cool, filter with suction, wash the filter cake with 500ml of DMF, and dry in vacuum to obtain Intermediate I. Sub-refined product 362g, yield 84.2%.
[0019] Add 360g (0.75mol) of the first refined product of intermediate I and 1500ml of DMF in a 3-liter reactor, stir and react at 50°C for 5 hours, stop heating, cool, filter with suction, wash the filter cake with 500ml of DMF, and d...
specific Embodiment approach
[0025] combined with figure 2Describe the specific implementation of the present invention using a special filter: a filter, including a filter top cover 1, ring groove filter plates 2 and 3, a filter barrel 4 and a filter bottom cover 5, a feed pipe 6, and an inert gas inlet pipe 7 , an inert gas outlet pipe 8, a cooling liquid outlet 9, a cooling liquid return pipe 10, and a temperature gauge 11 are respectively installed on the filter top cover 1, the filter top cover 1 is provided with an insulating layer 12 and an interlayer 13, and the filter barrel 4 is a cylindrical barrel Shaped, located at the bottom of the filter top cover 1, equipped with an insulating layer 27, an interlayer 28, an outer metal plate 15, a middle metal plate 16 and an inner metal plate 17, the filter bottom cover 5 is located at the bottom of the filter barrel 4, and is provided with an outlet Material pipe 18, coolant inlet 19, insulation layer 20 and interlayer 21, filter top cover 1 is hemisphe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com