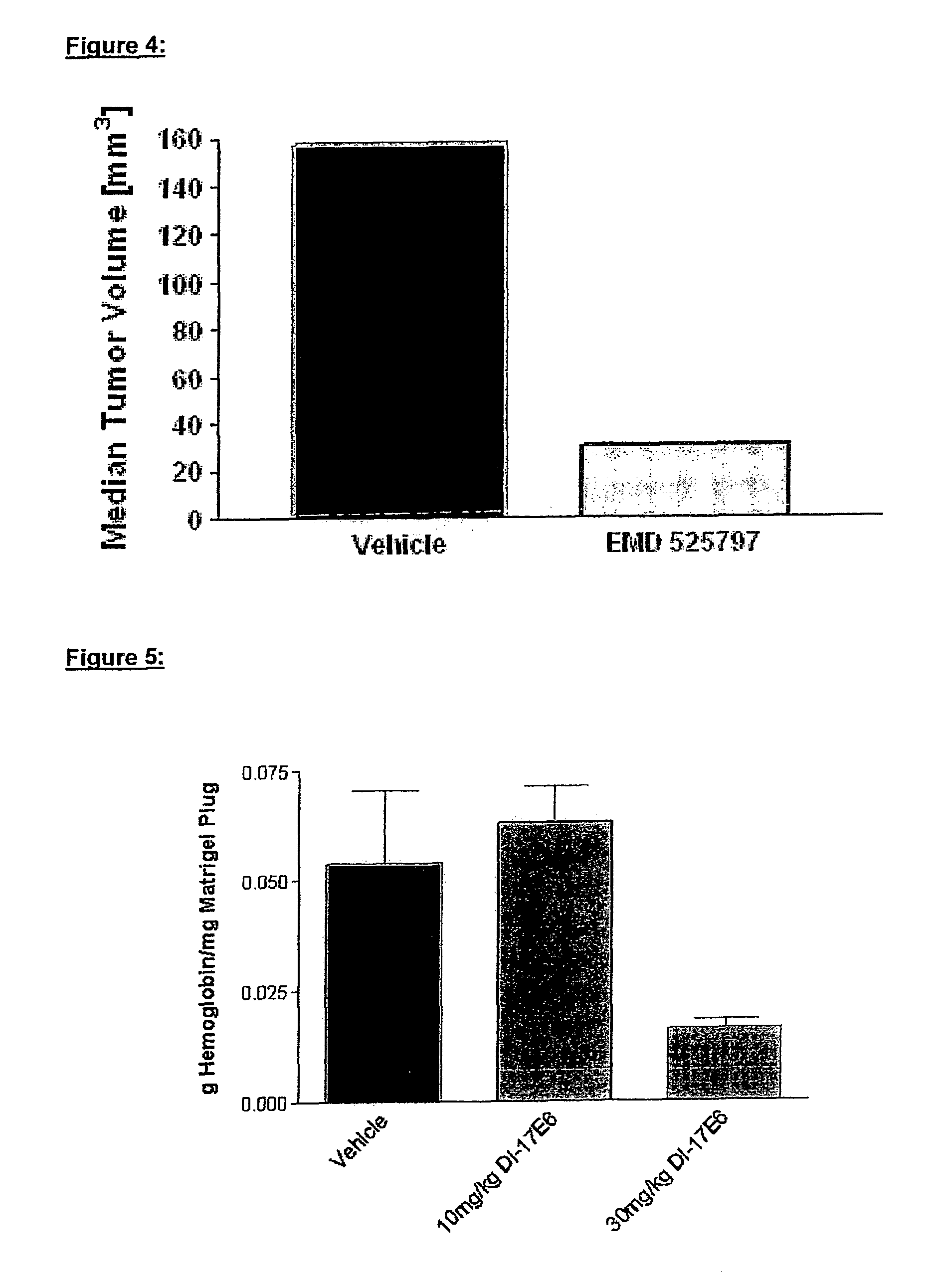
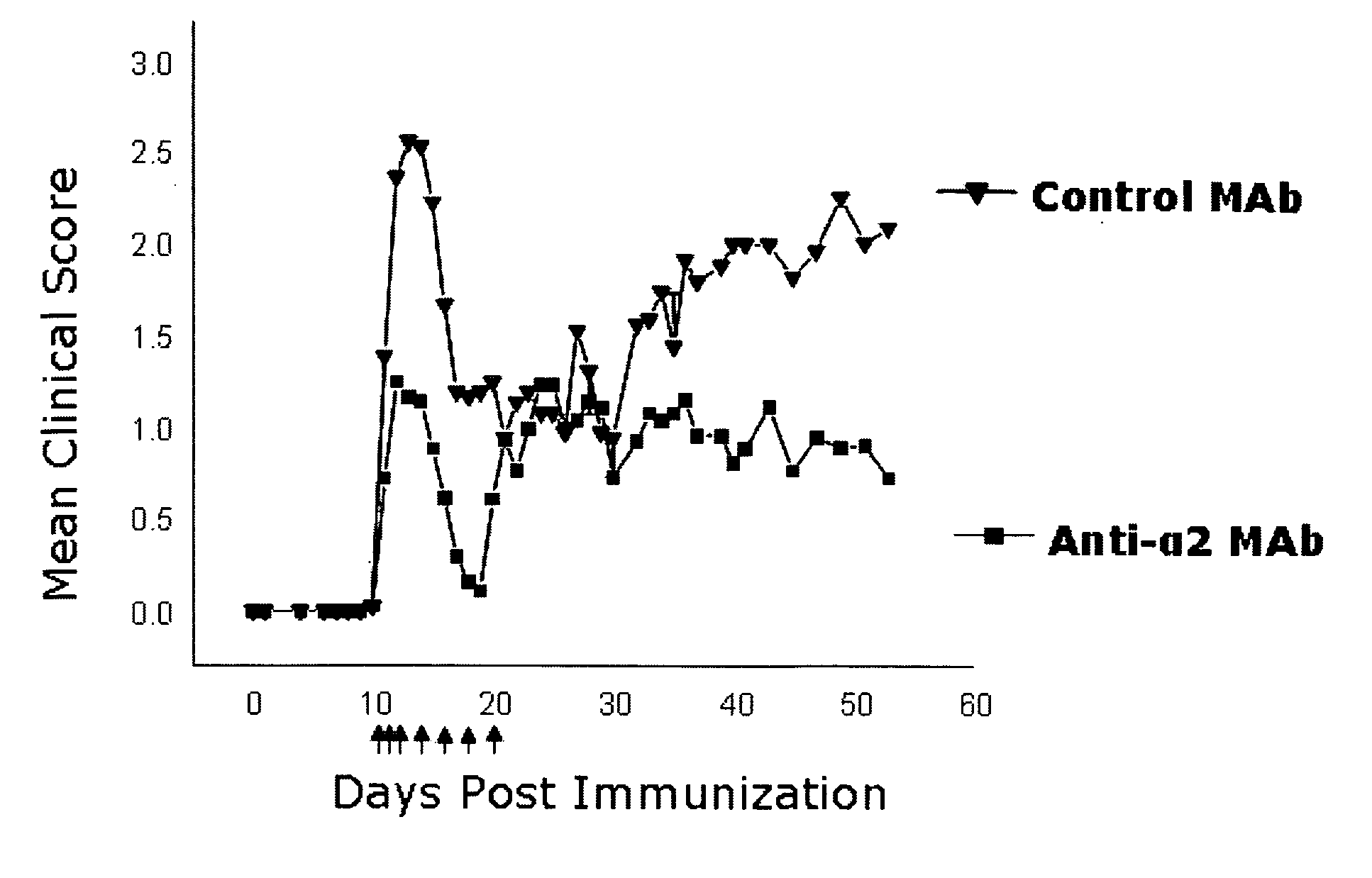
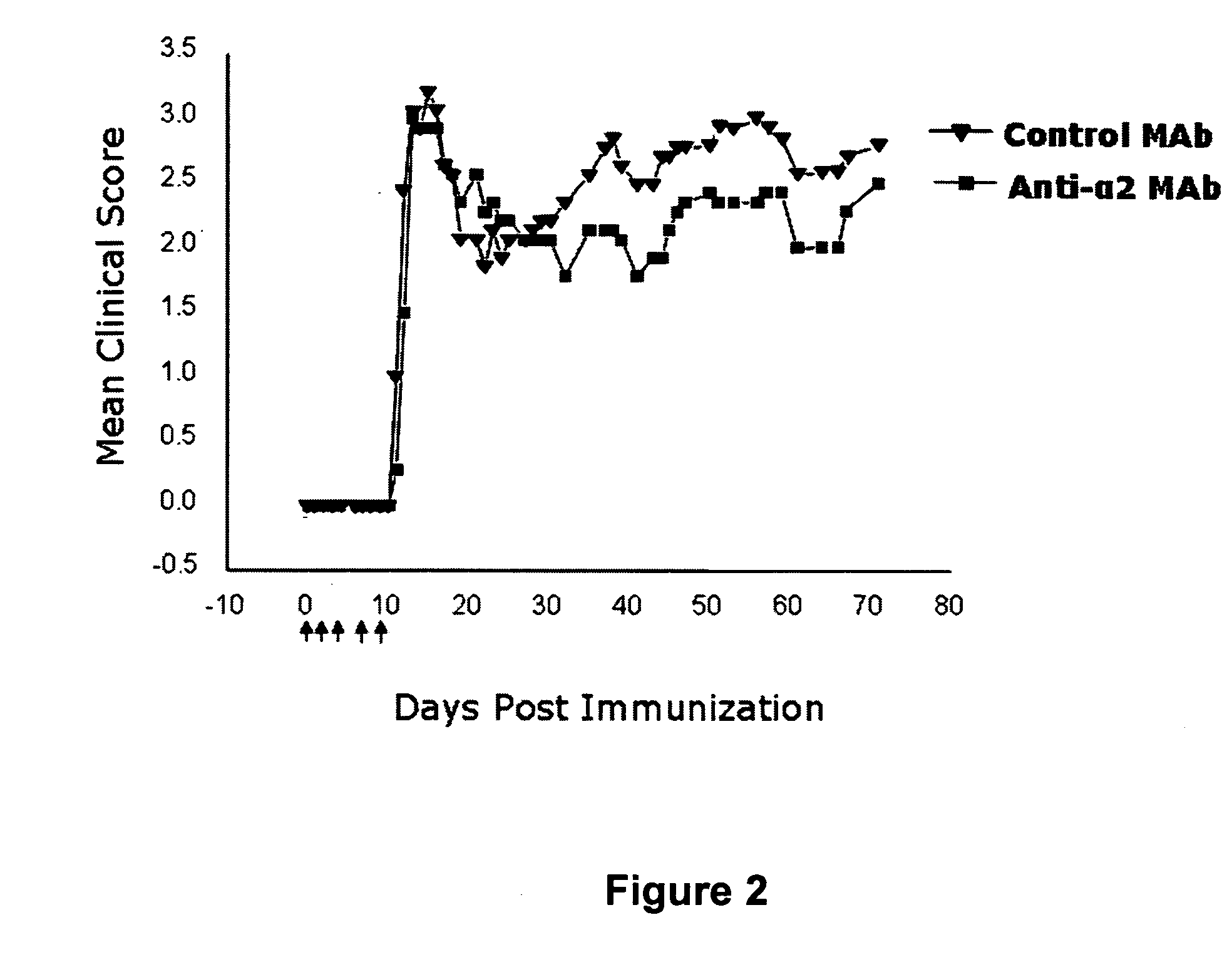
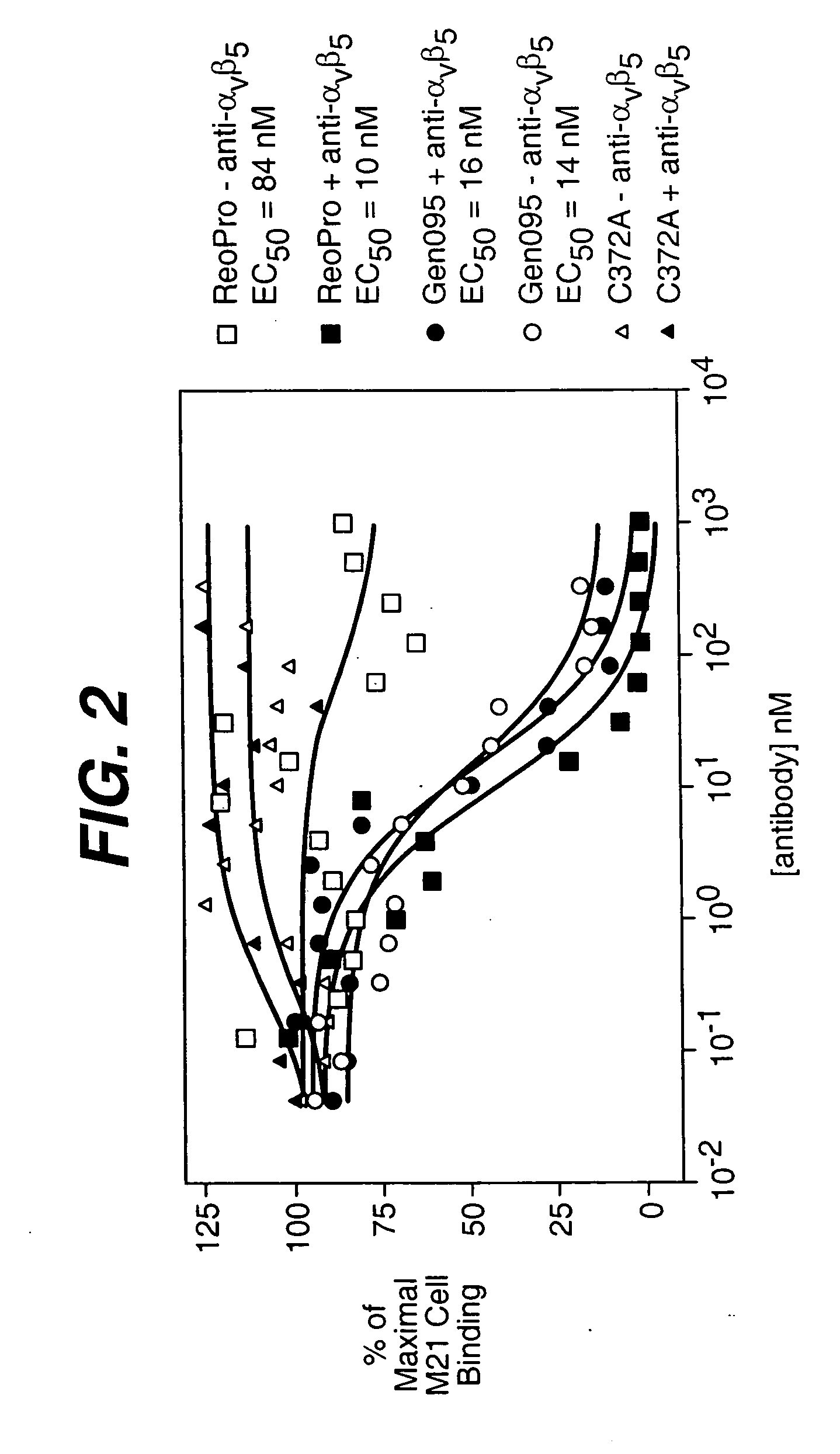
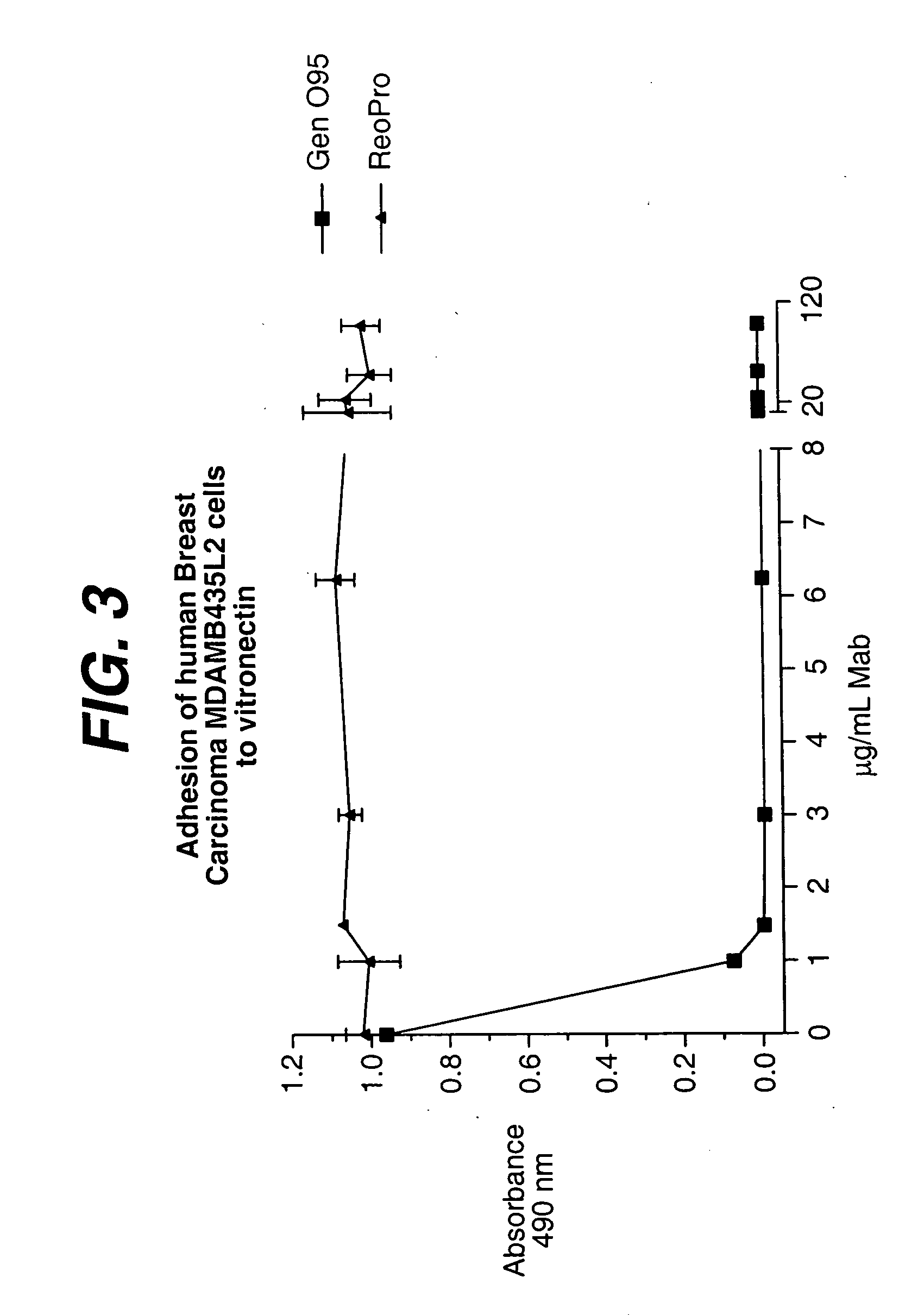
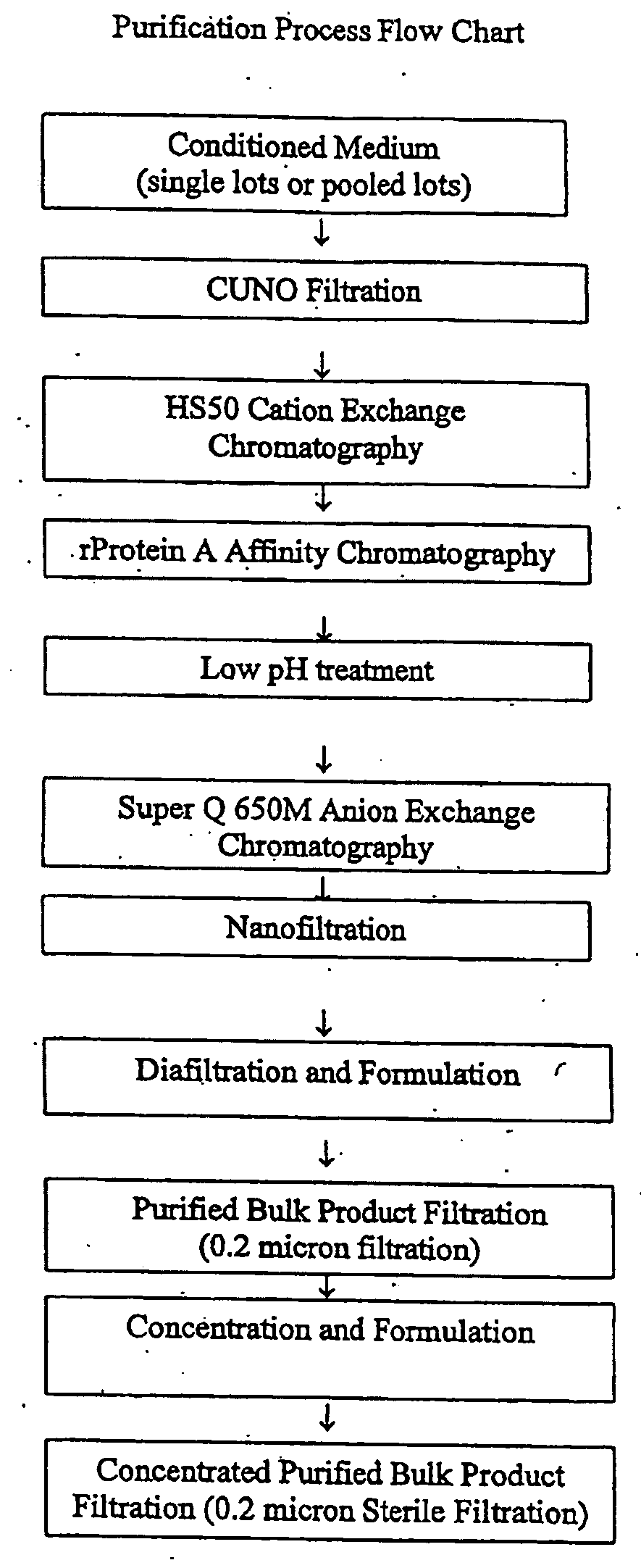
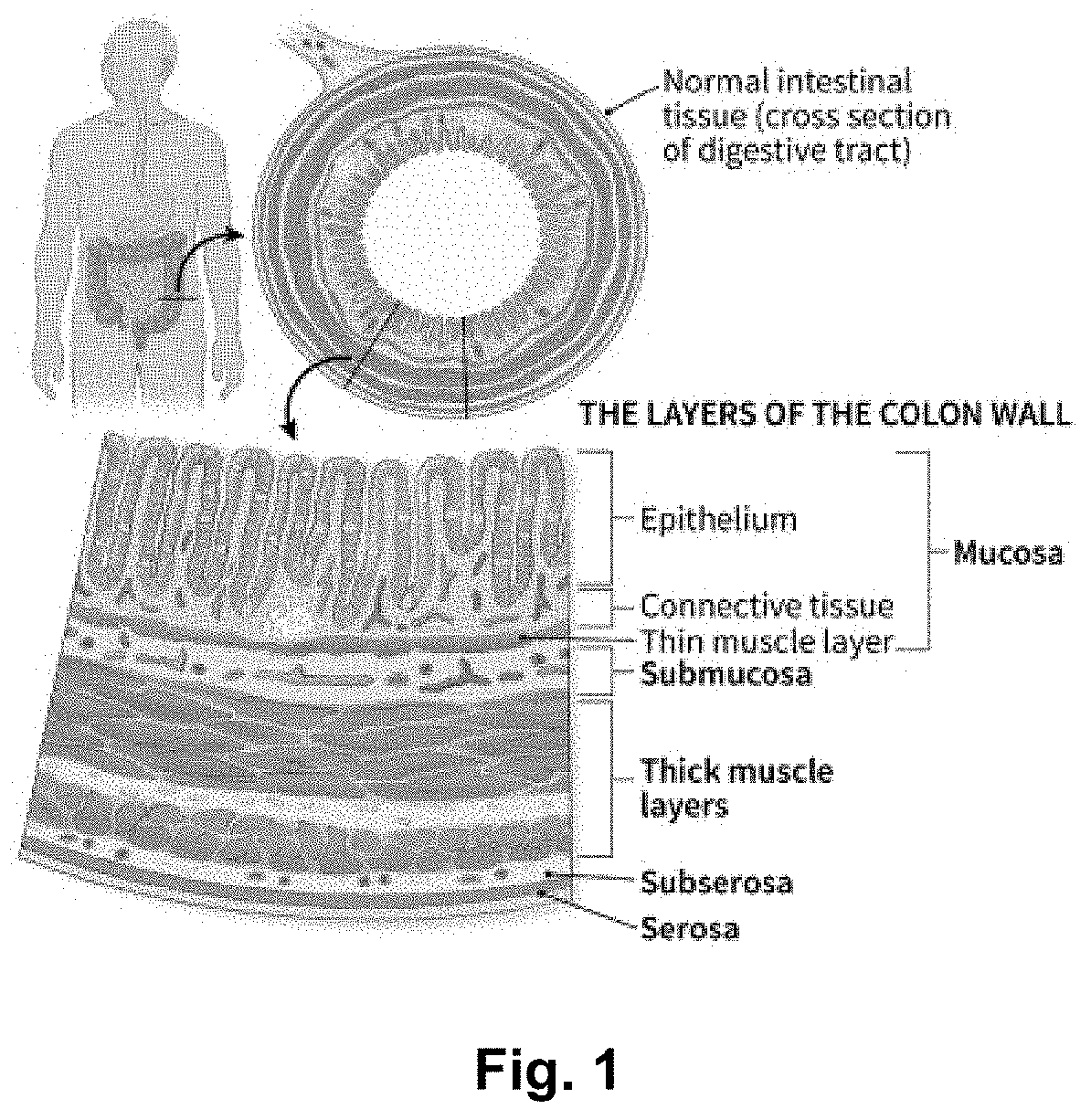
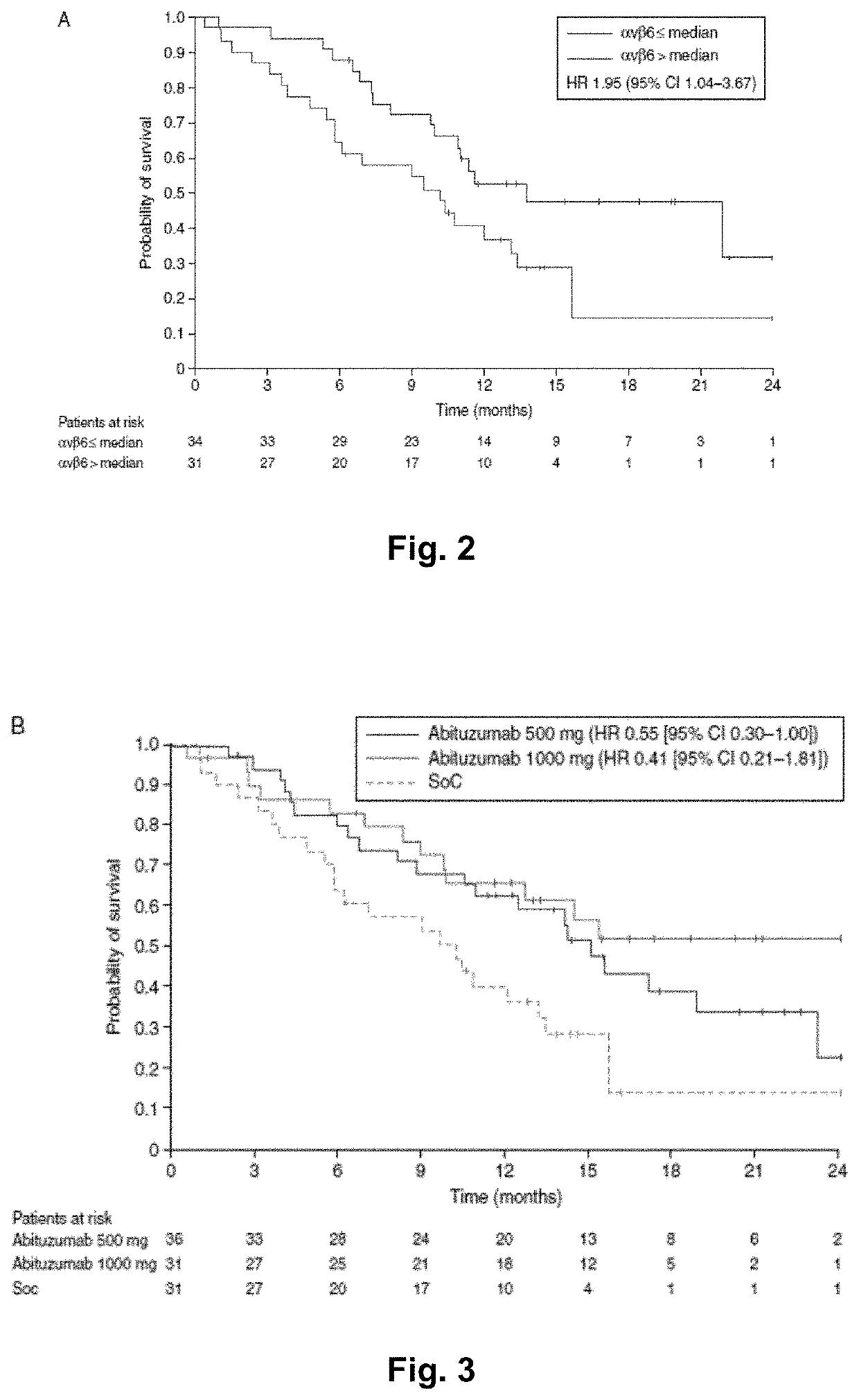
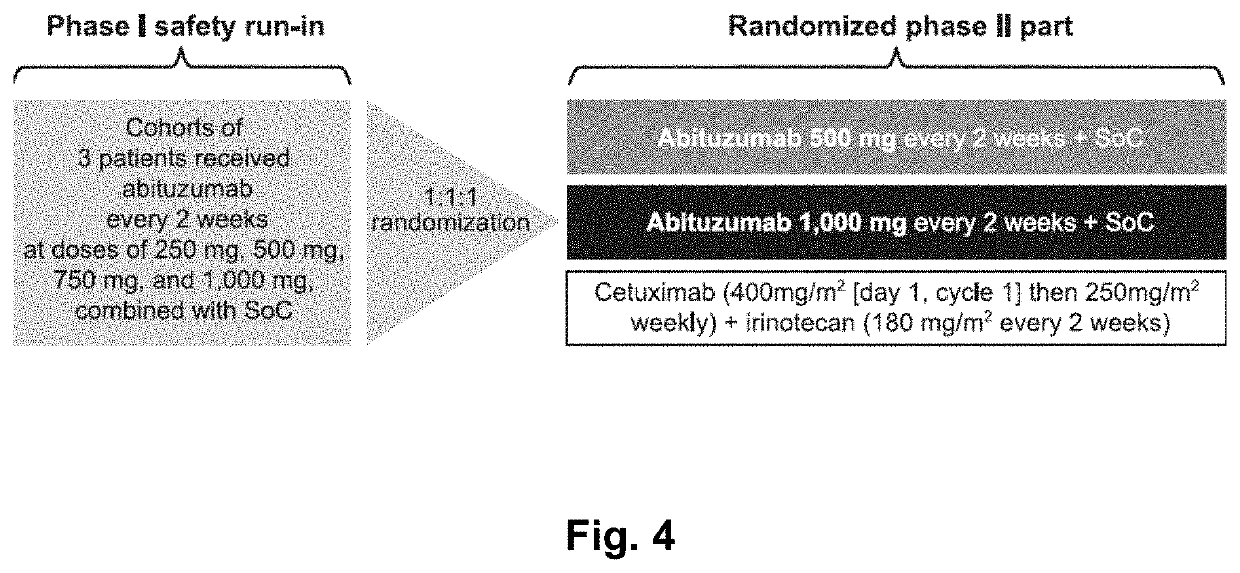
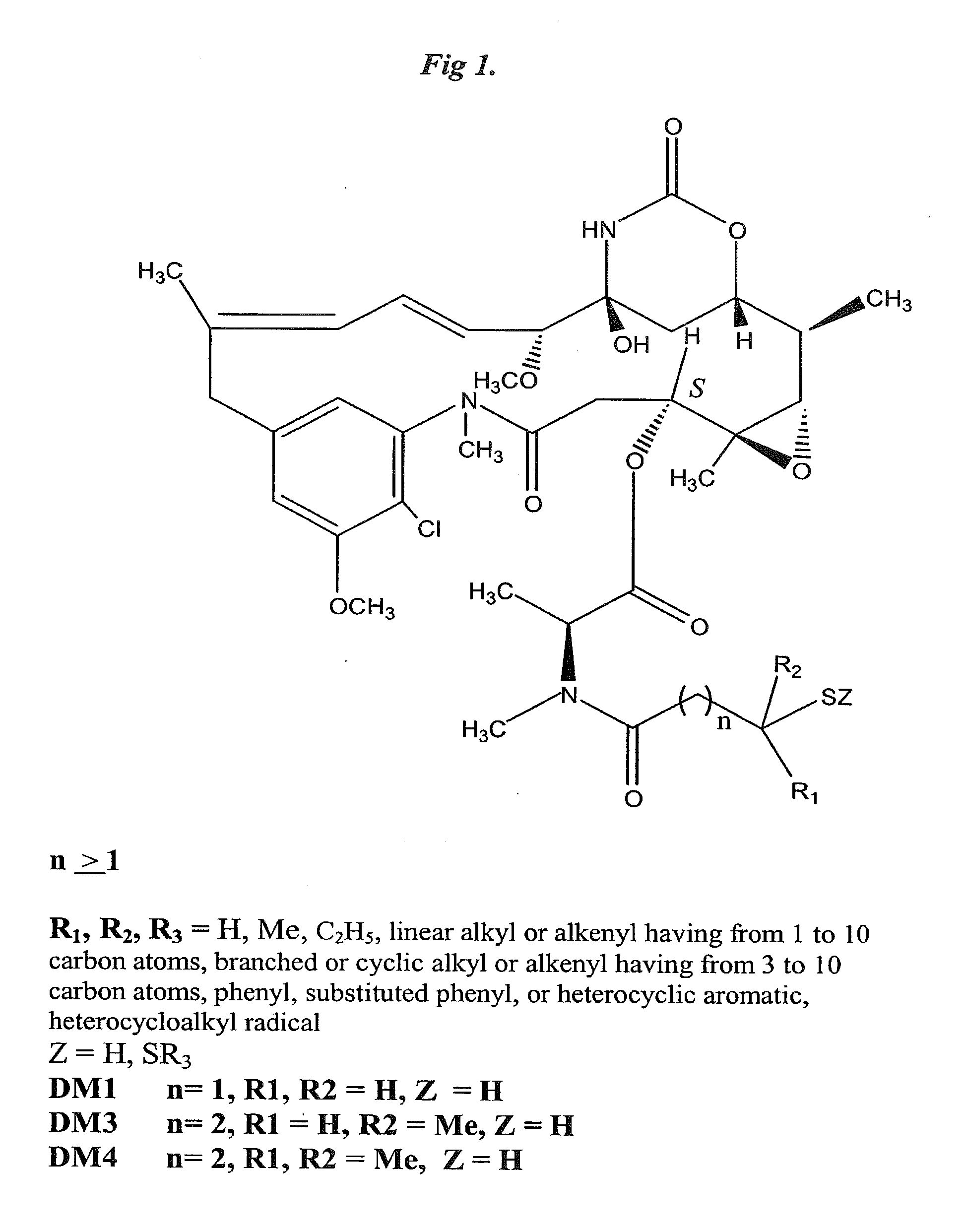
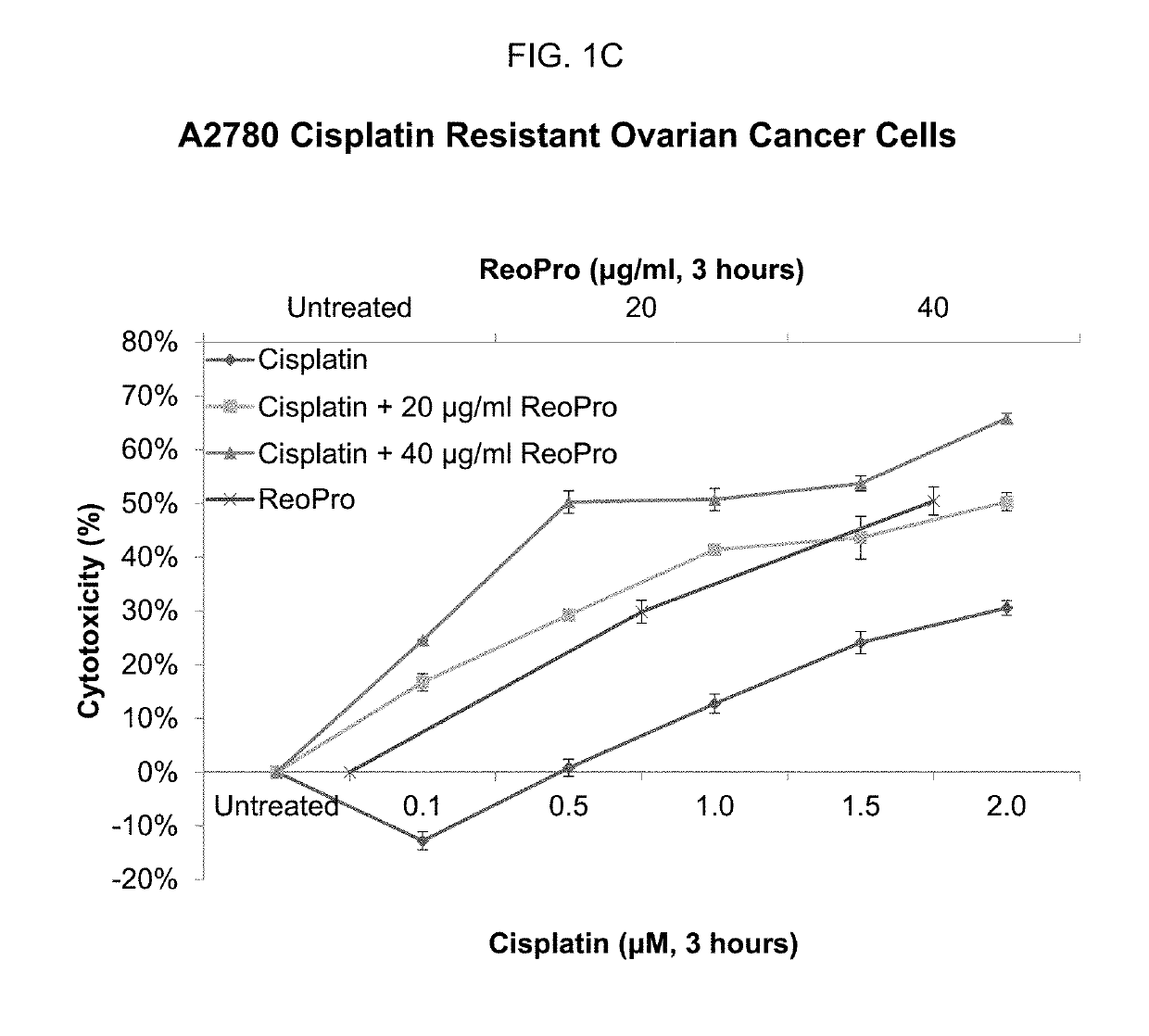
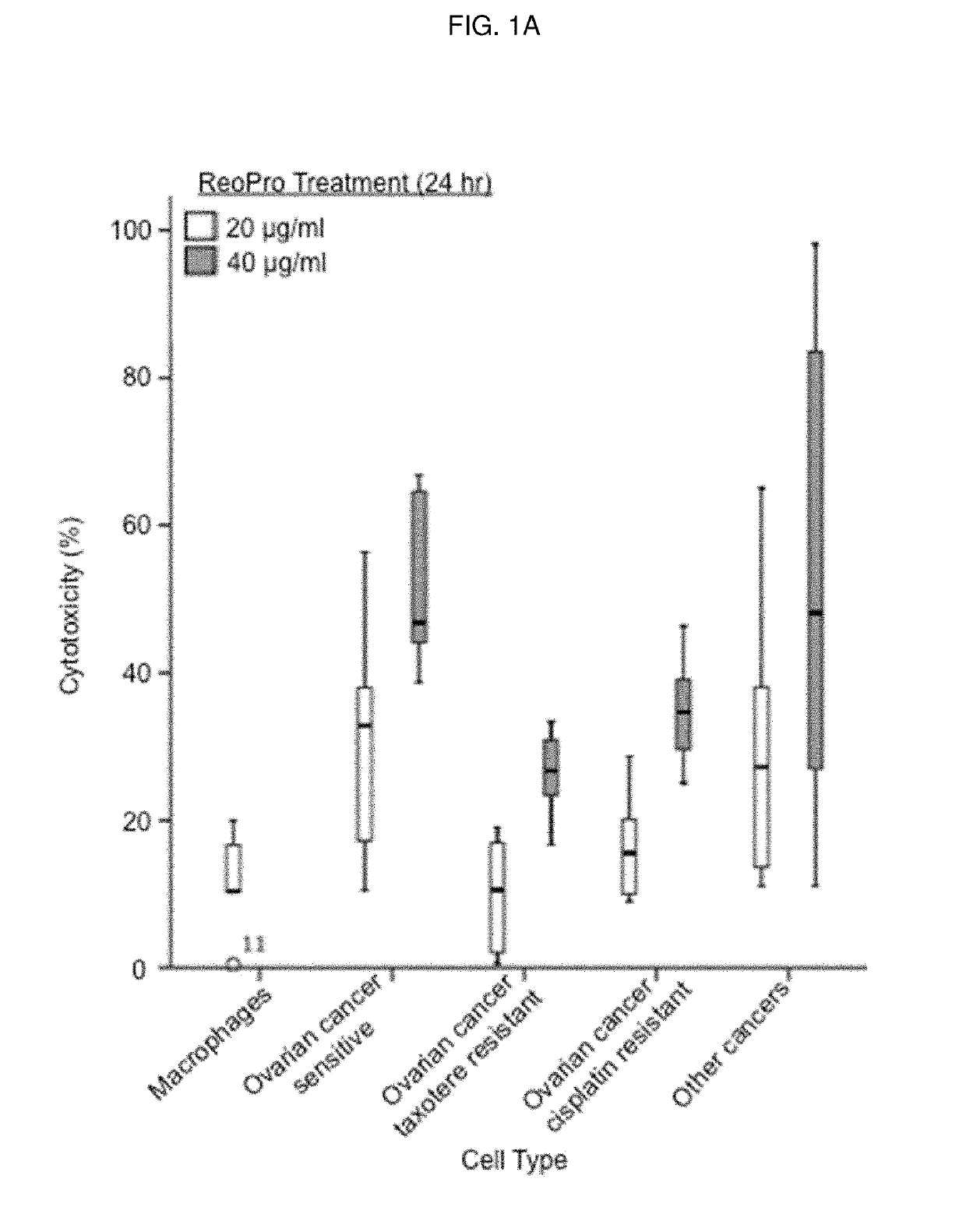
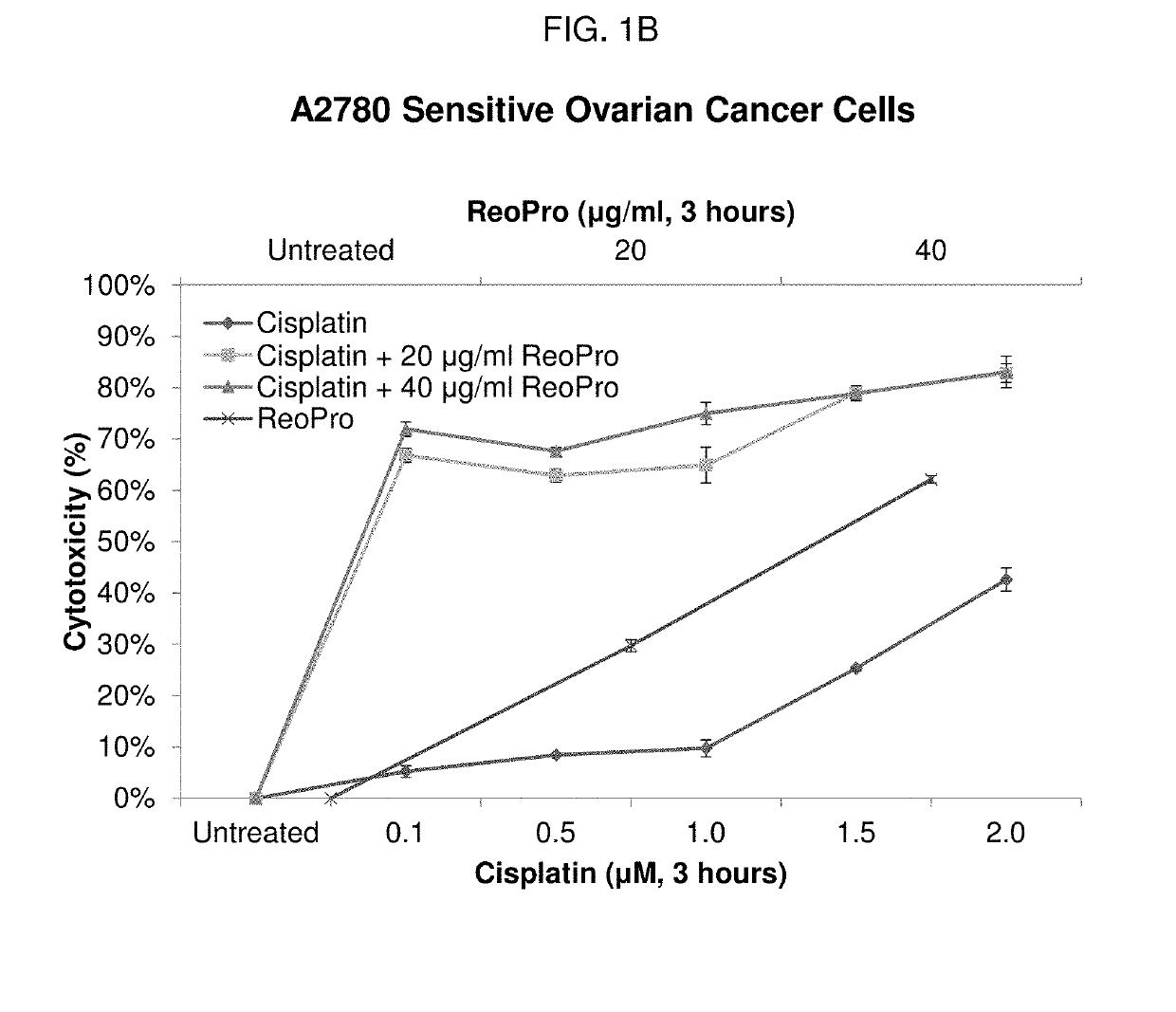
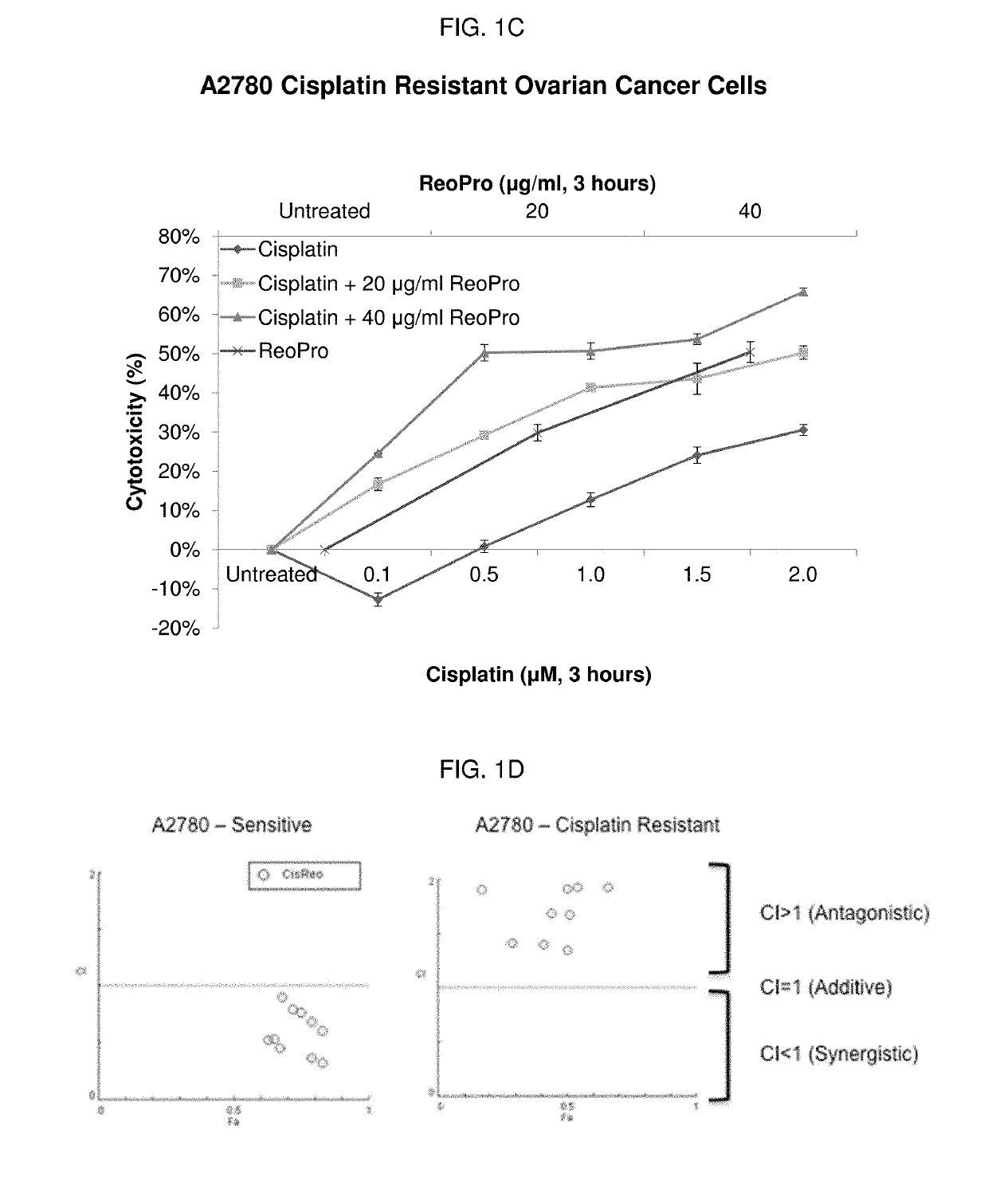
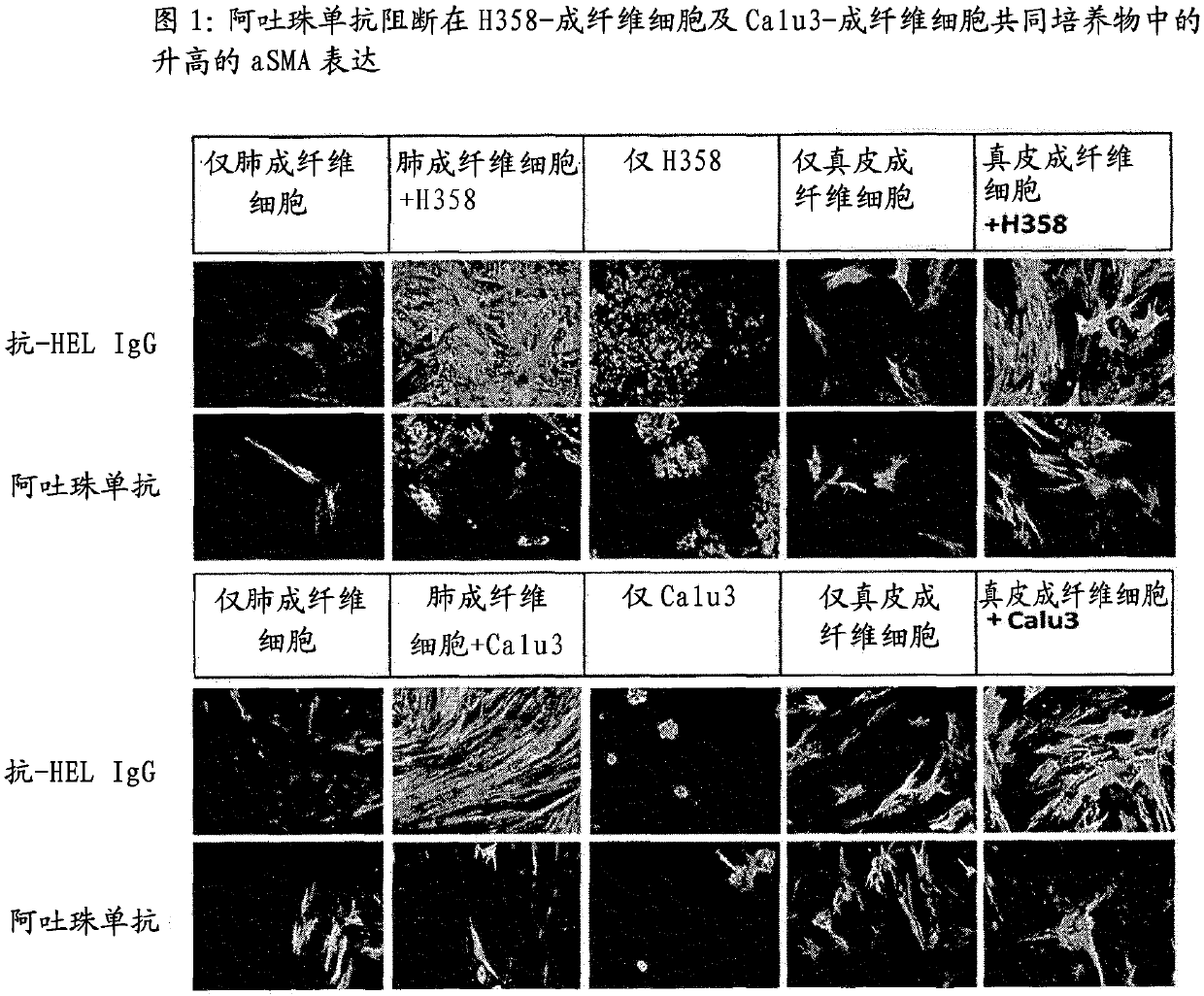
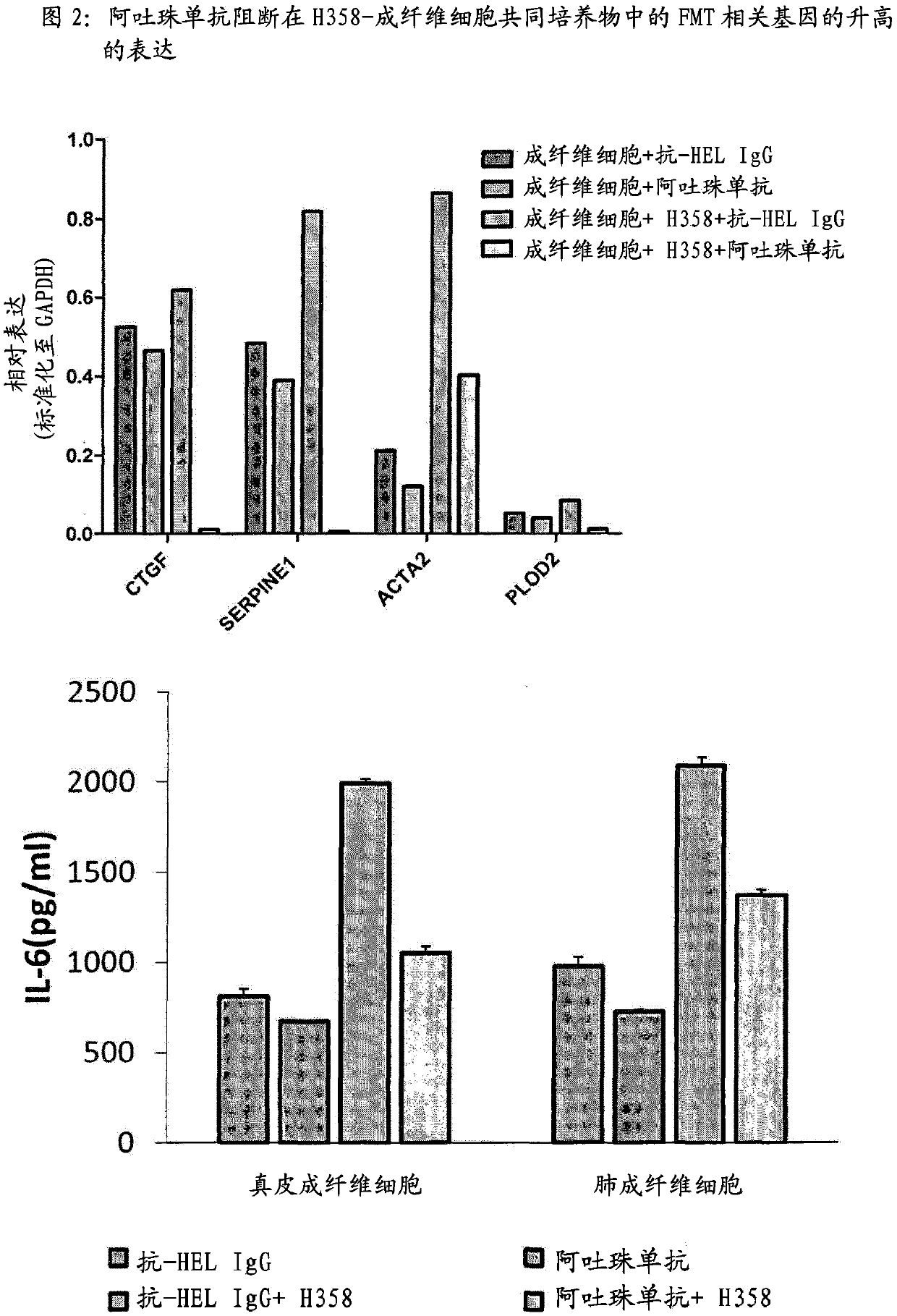
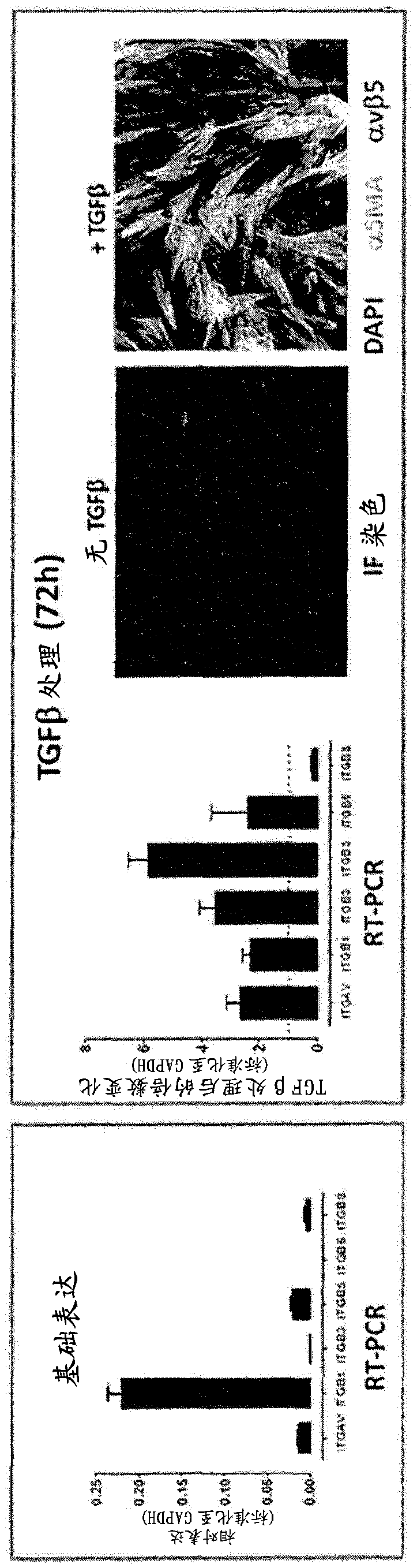
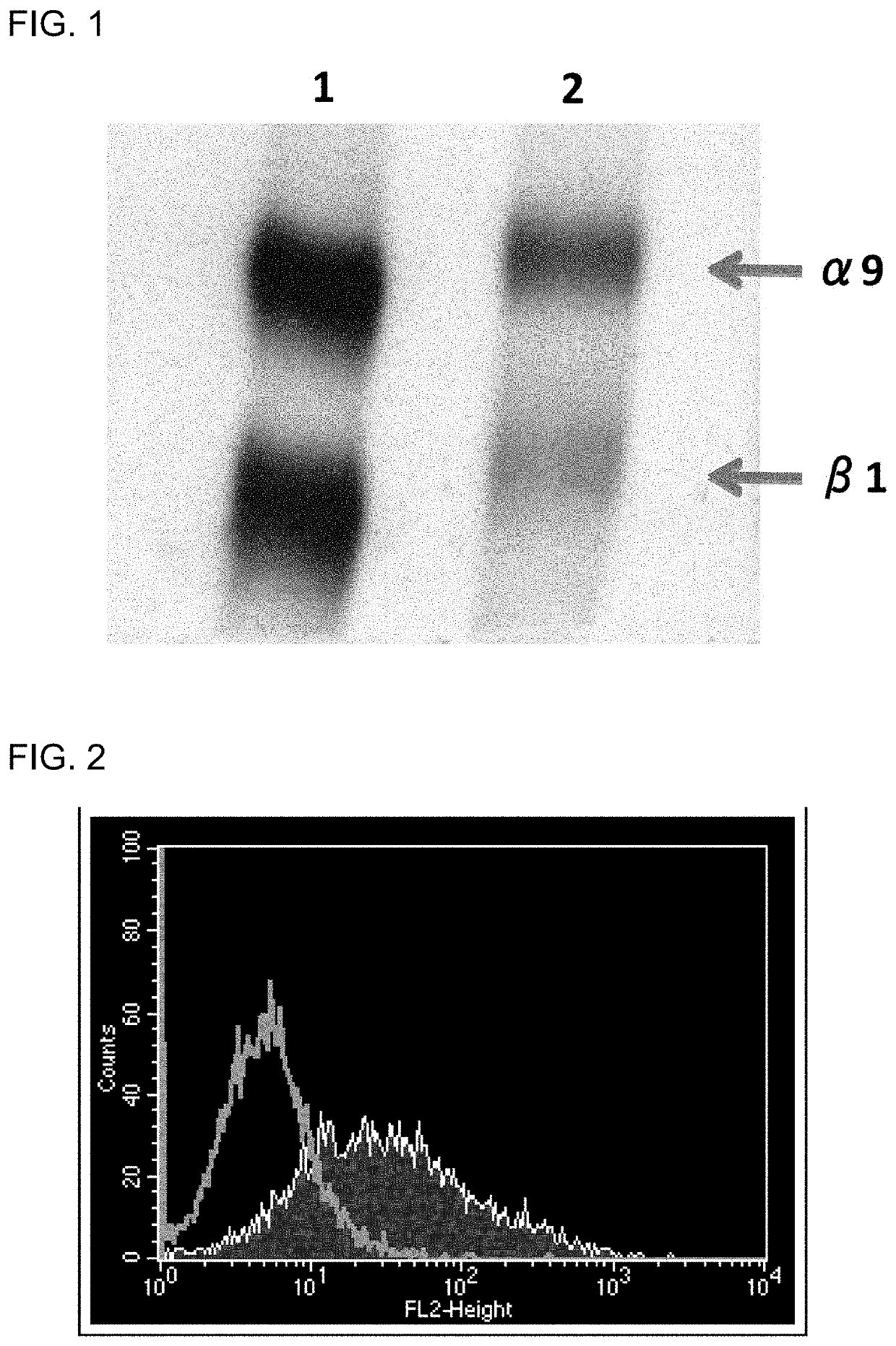
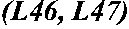Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Anti integrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human anti Integrin Alpha 4 (Natalizumab Biosimilar) Anti-Integrin Alpha 4 Antibody is a non-therapeutic biosimilar of the monoclonal antibody drug natalizumab (Tysabri) for research use. It can be used in bioanalytical assays and for studying biological pathways affected by the drug.
Anti-integrin antibodies, compositions, methods and uses
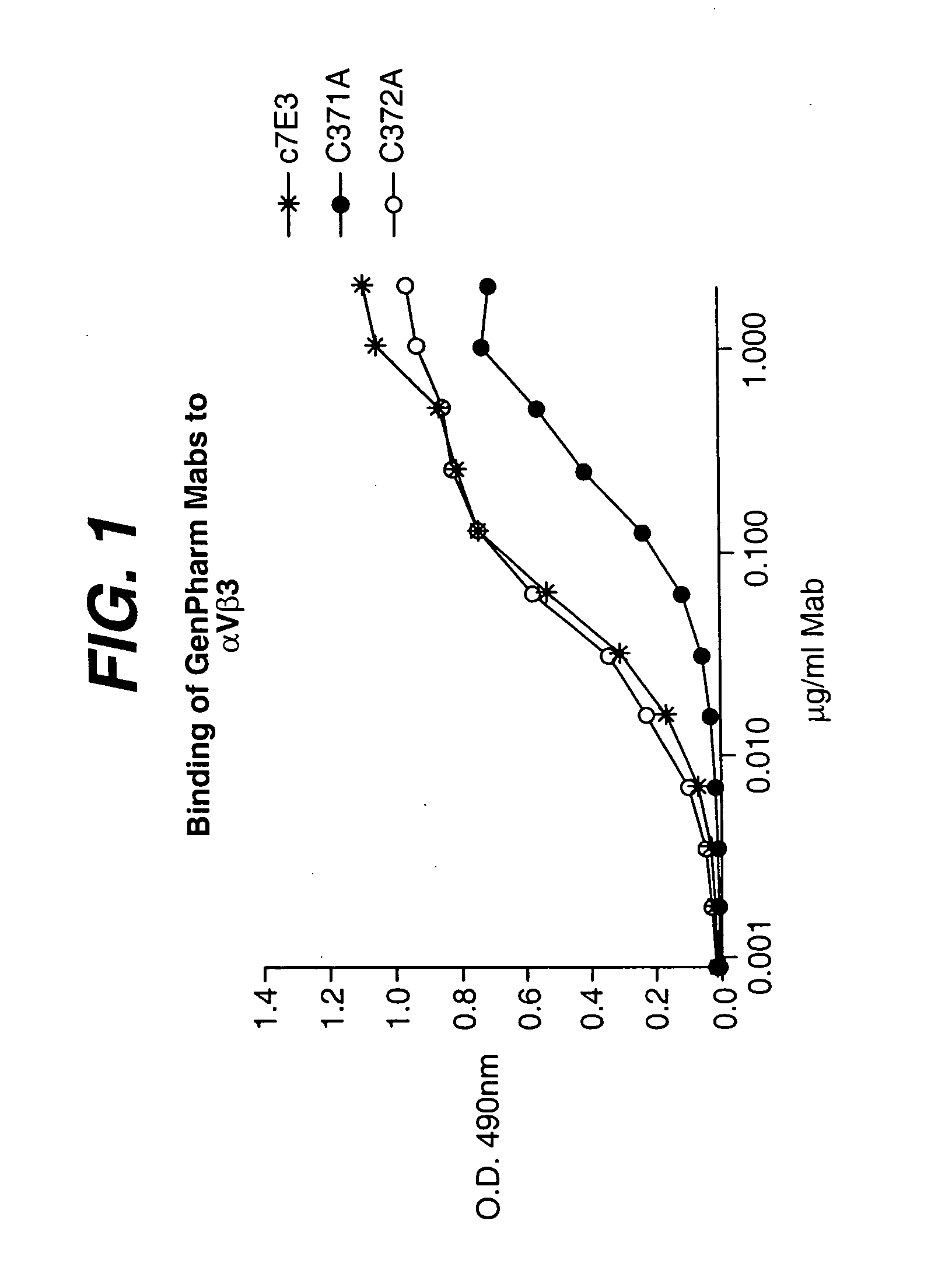
The present invention relates to at least one novel anti-alpha-V subunit antibodies, including isolated nucleic acids that encode at least one anti-alpha-V subunit antibody, alpha-V subunit, vectors, host cells, transgenic animals or plants, and methods of making and using thereof, including therapeutic compositions, methods and devices.
Owner:JANSSEN BIOTECH INC
Anti-integrin immunoconjugates, methods and uses
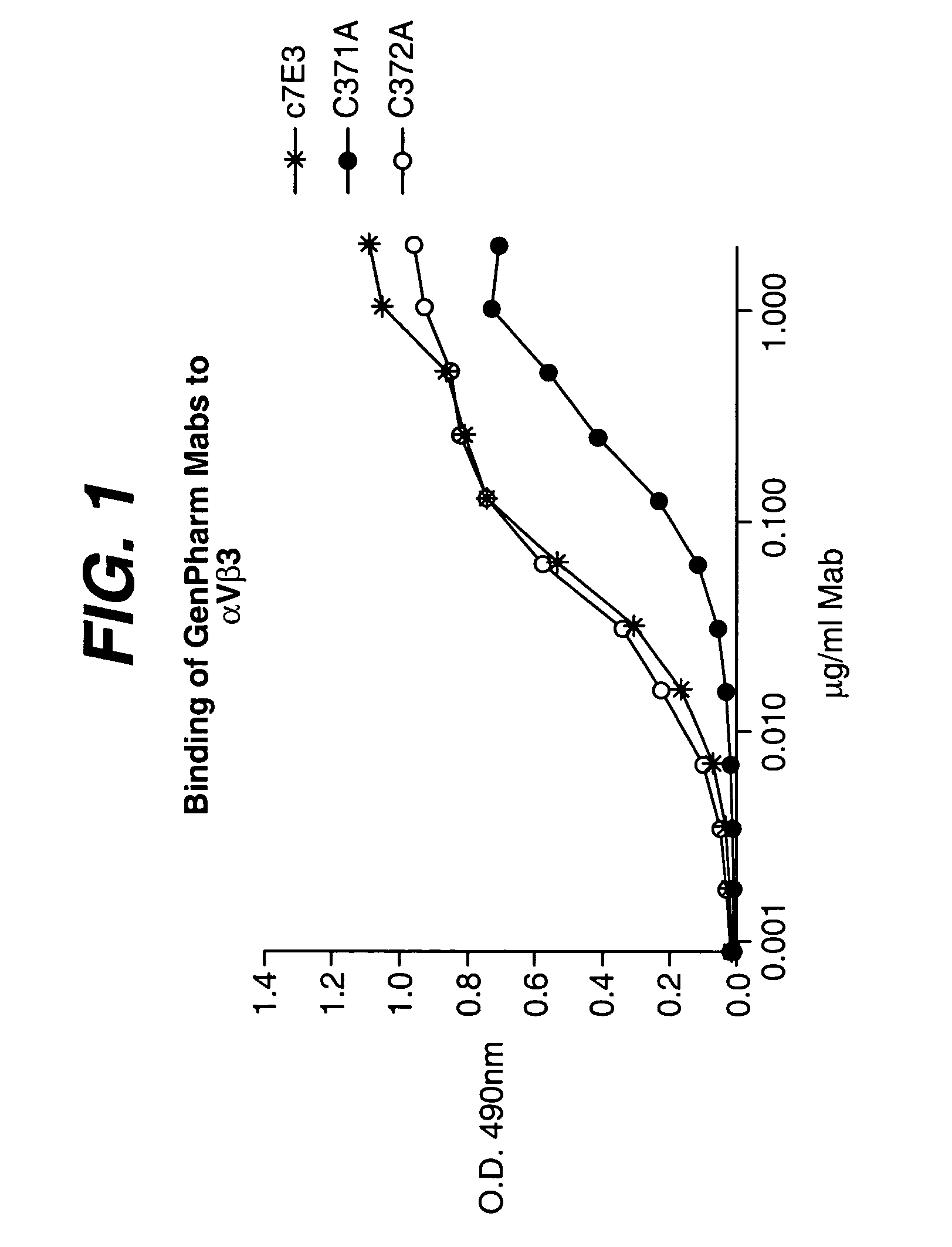
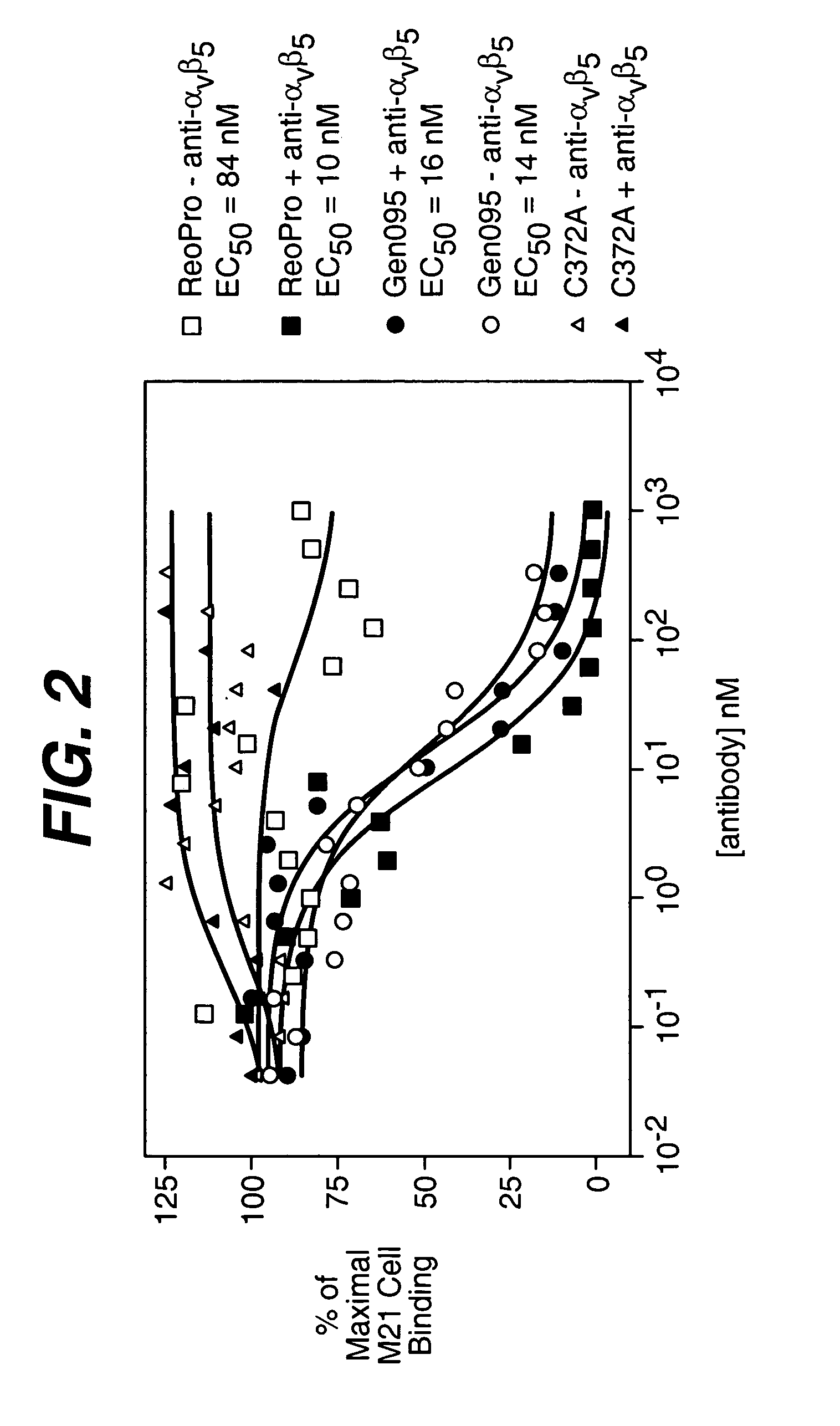
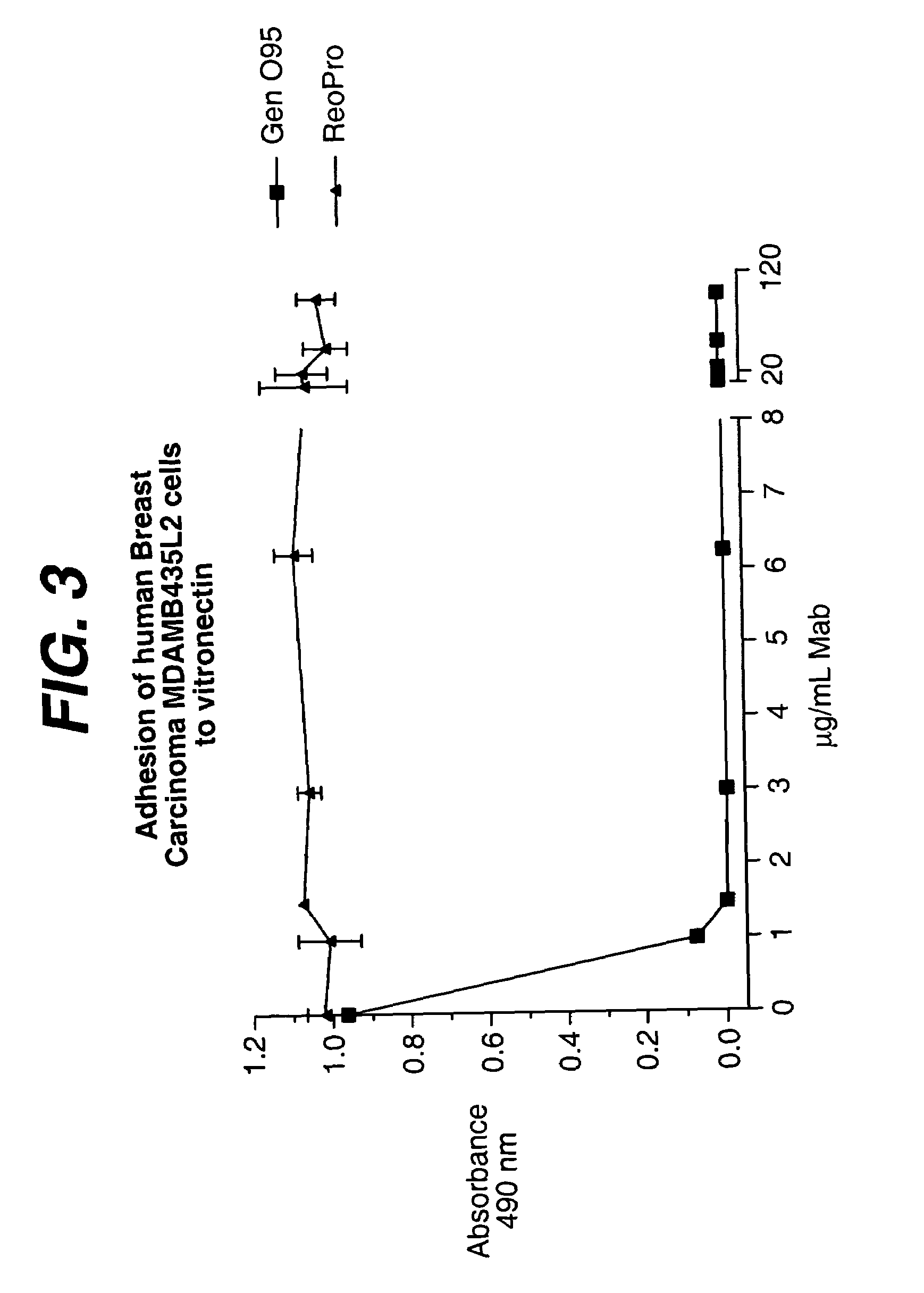
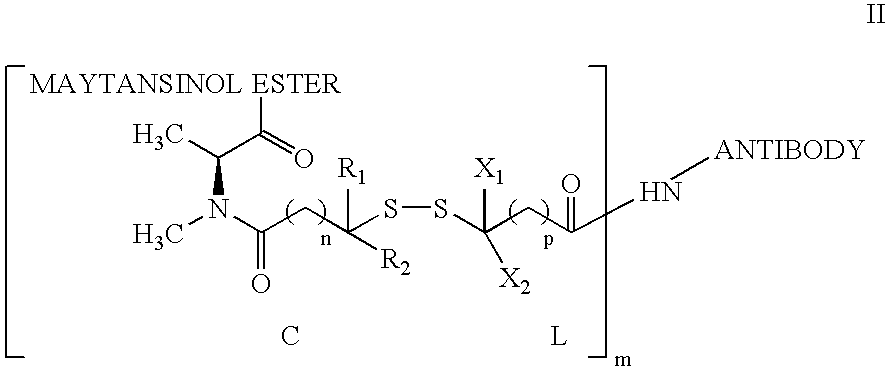
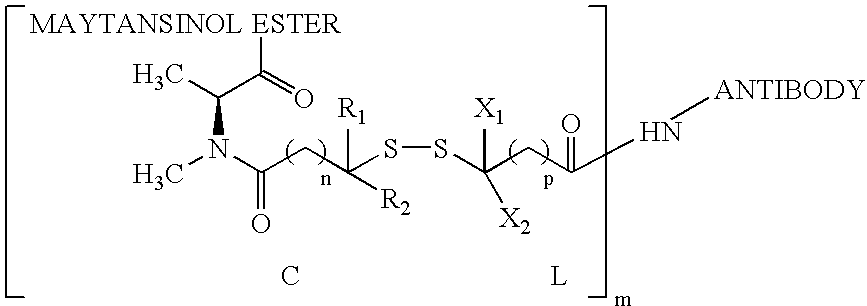
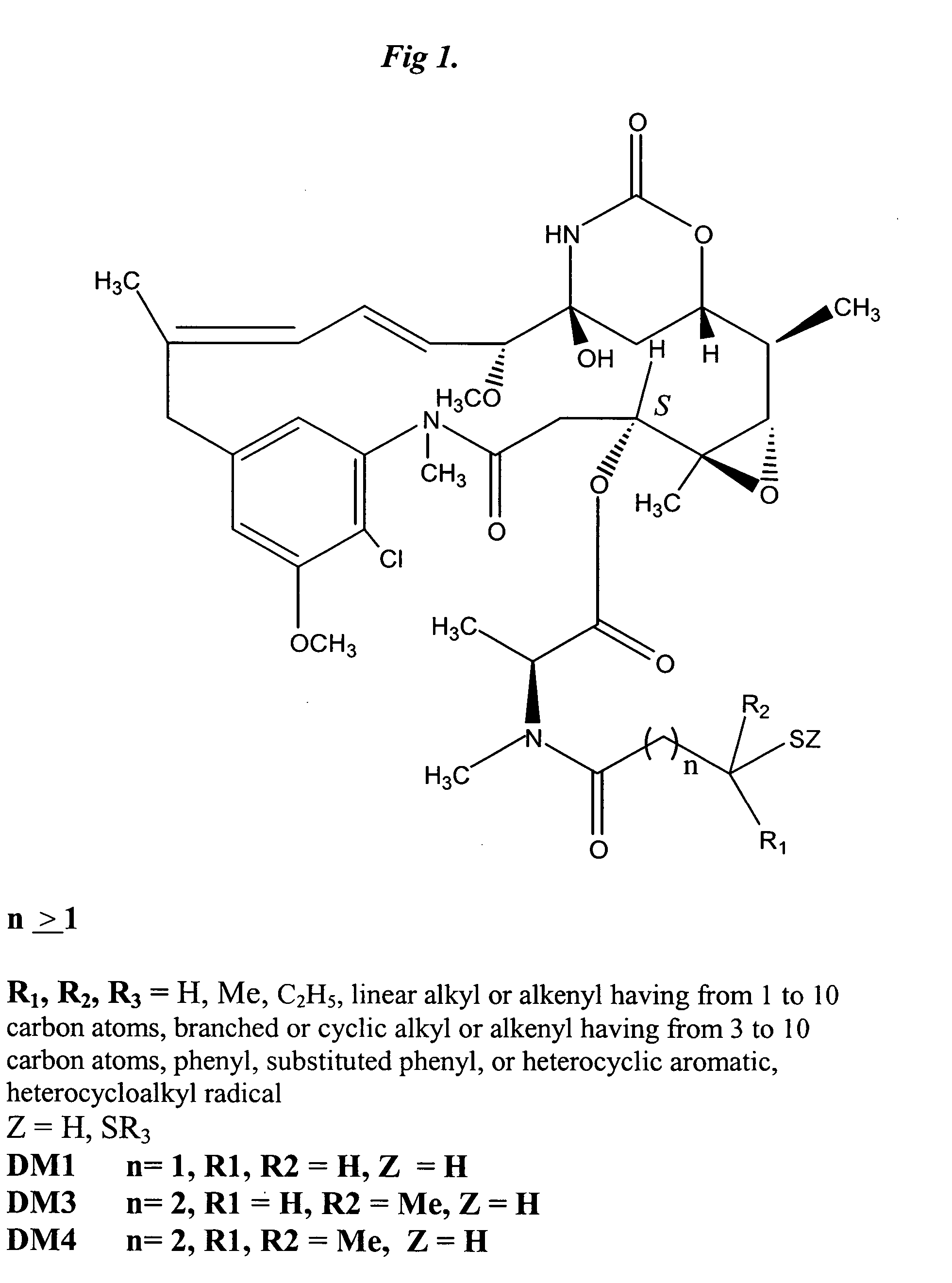
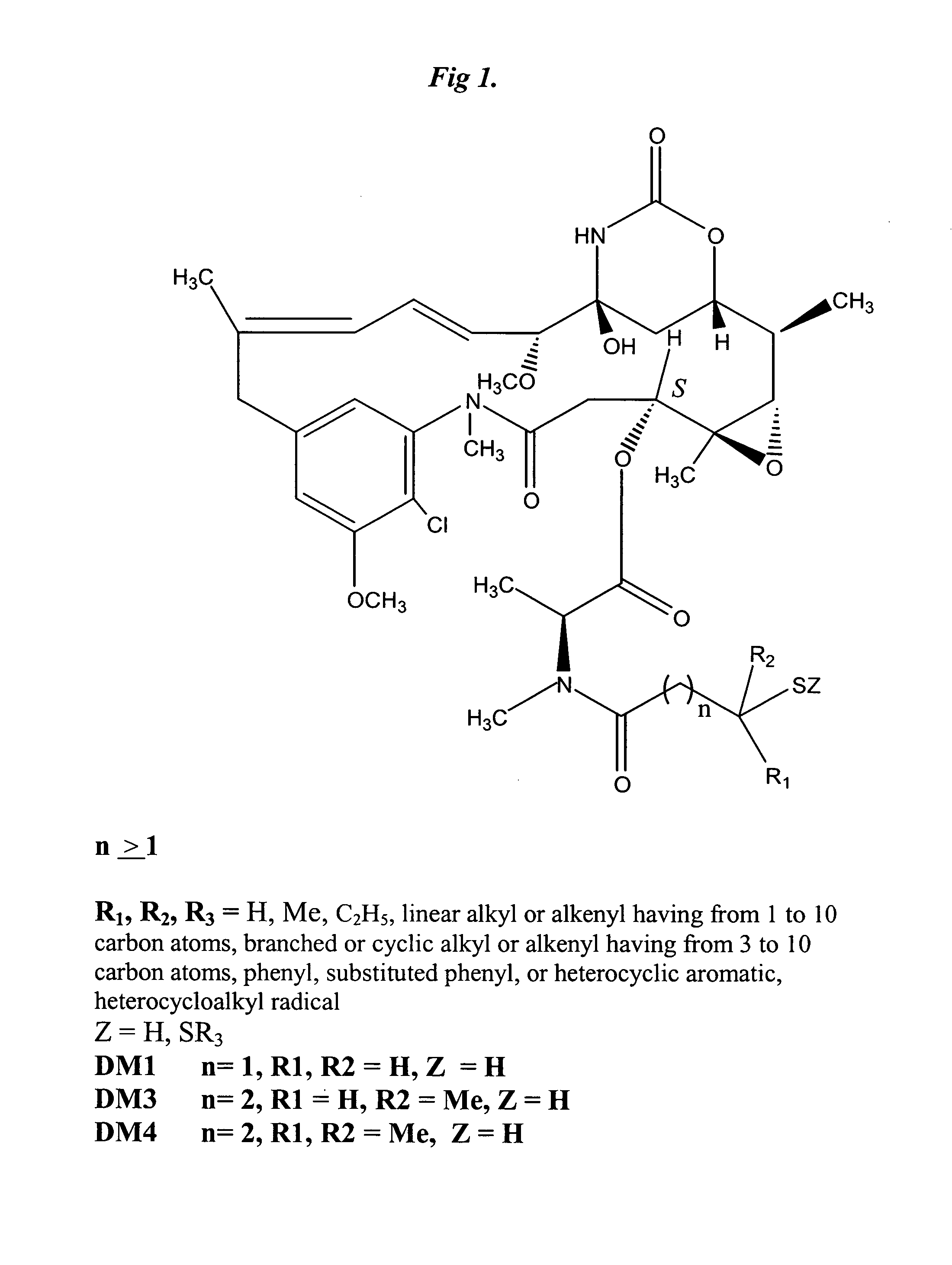
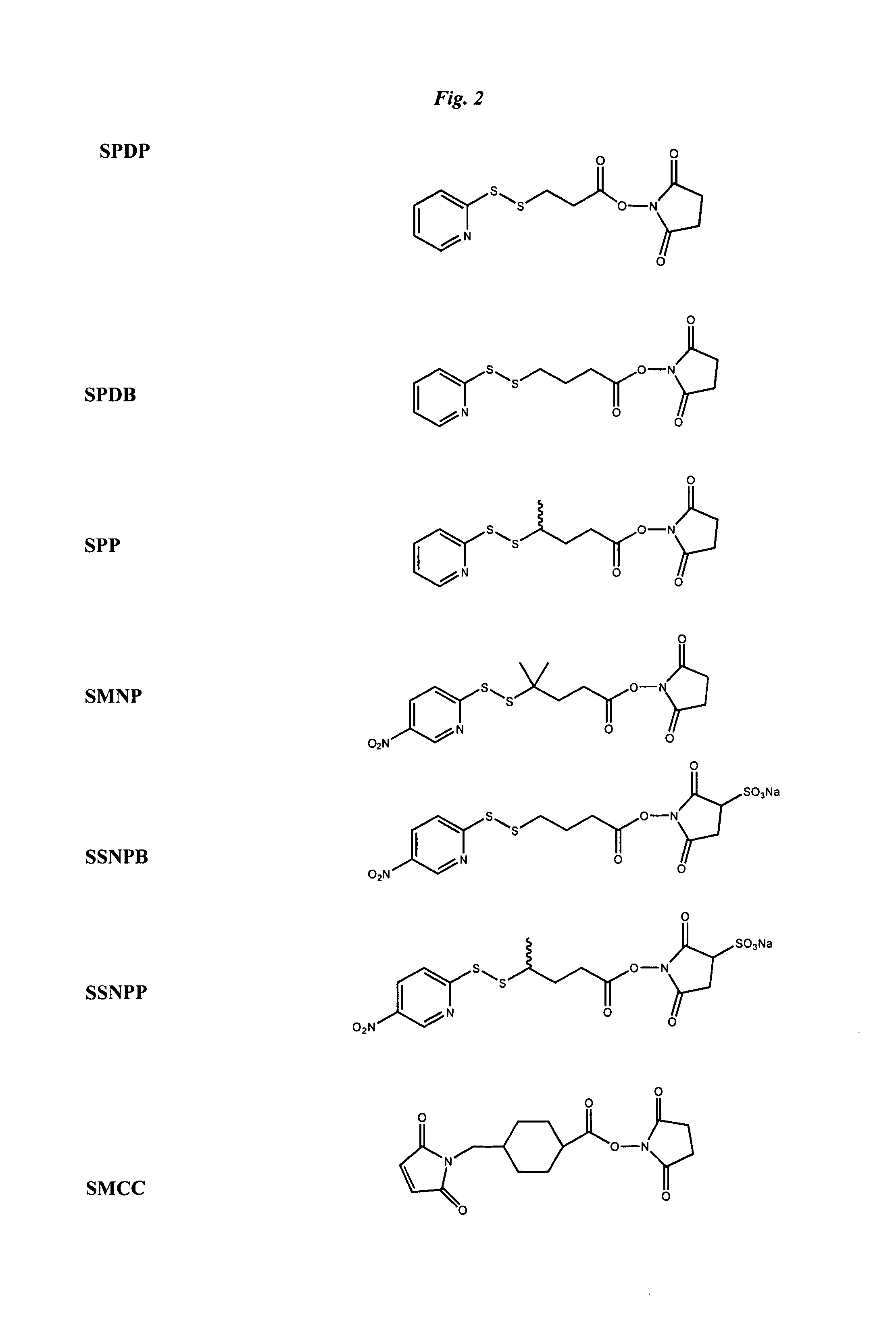
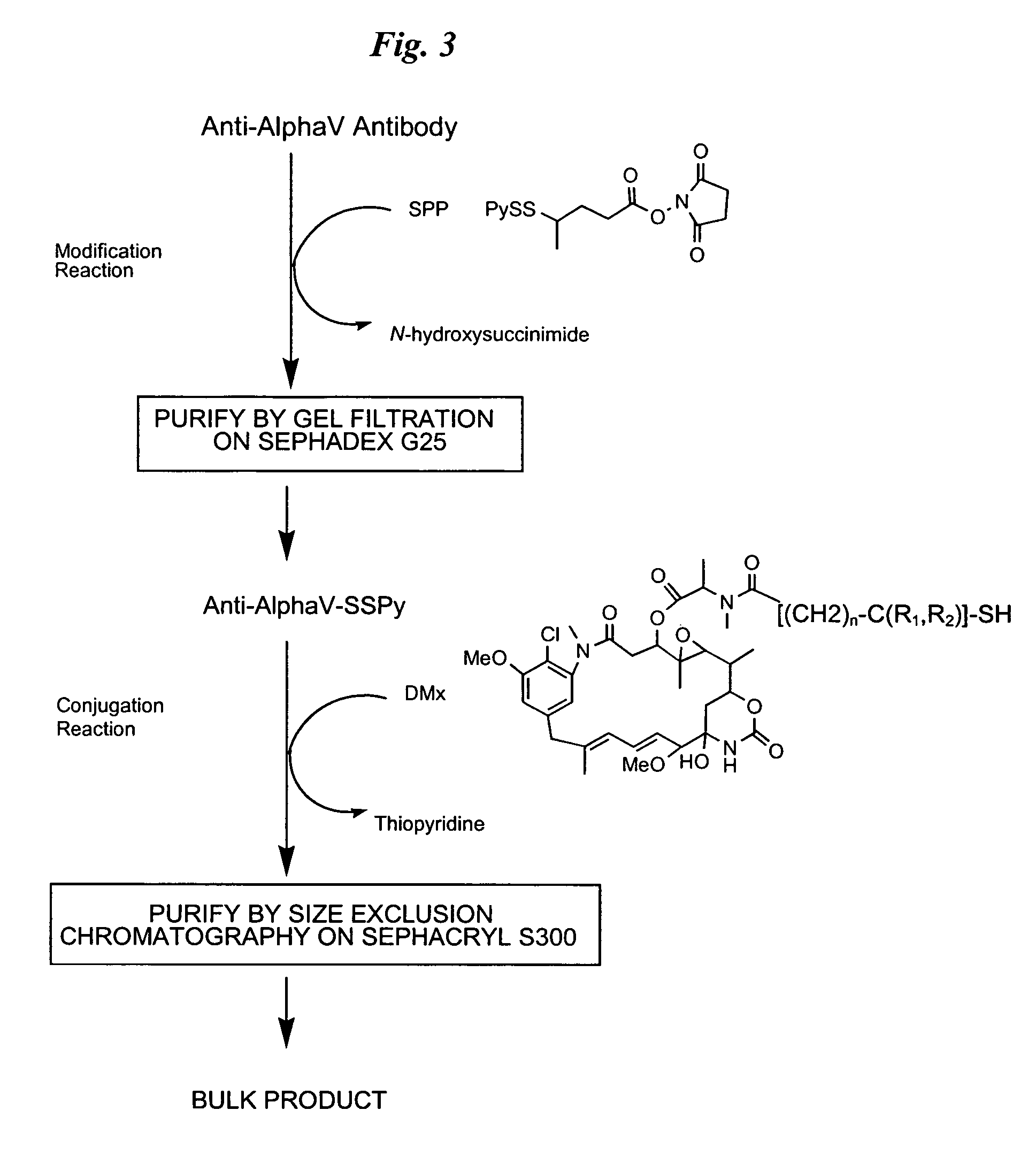
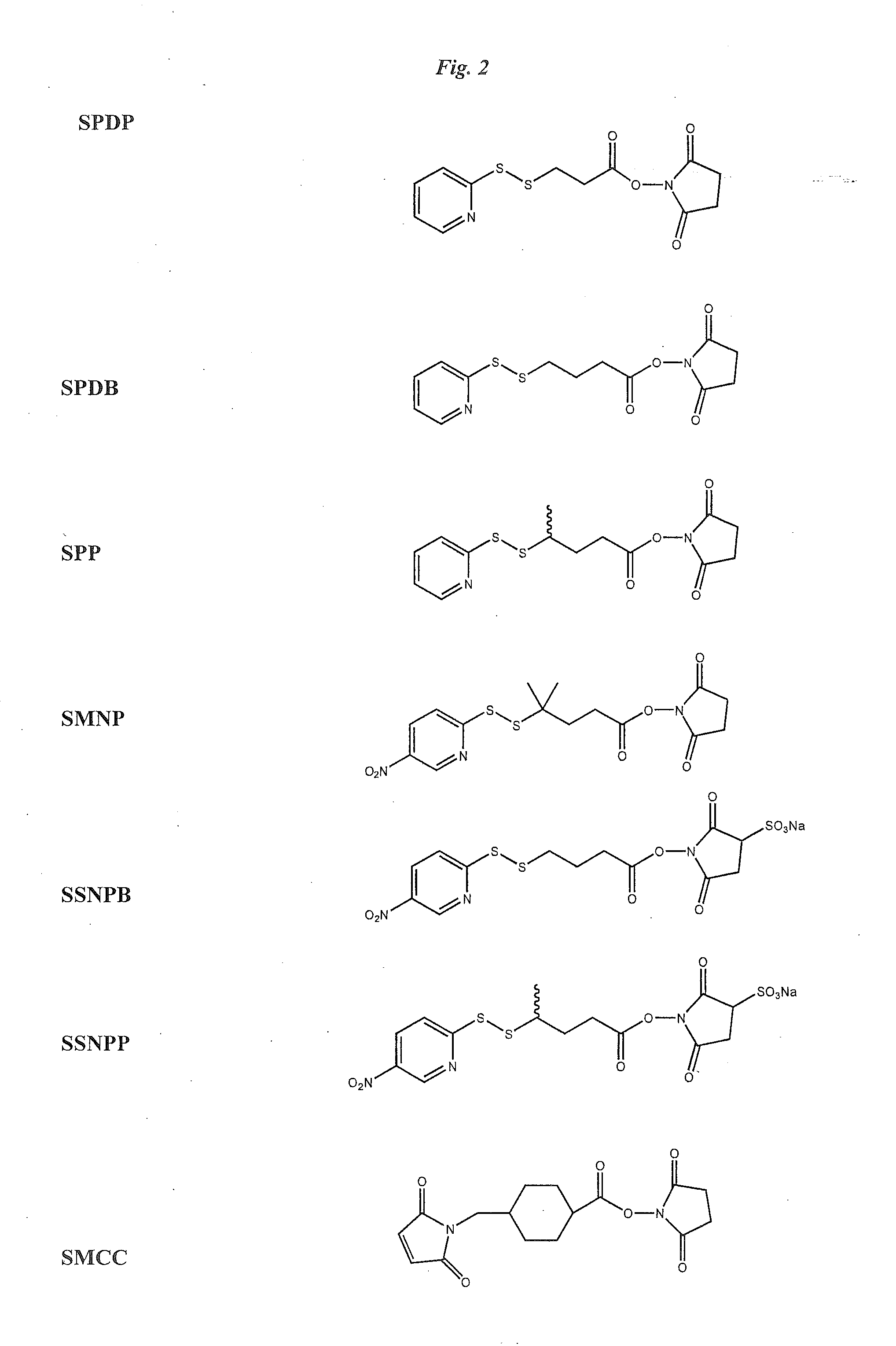
The invention relates to conjugates of anti-integrin specific antibodies with cytotoxic compounds, the synthesis, selection, and use of such conjugates for use in cancer therapy or other diseases mediated by cell proliferation, cell migration, or inflammation and which pathology involves angiogenesis or neovascularization of new tissue. In addition the invention relates to combination therapy of such diseases wherein the treatment comprises use of said conjugates in combination with one or more other treatment modalities including but not limited to: chemotherapy, surgery or radiation therapy. The preferred conjugates contain maytansinoid compounds linked to the antibody by a disulfide linkage, and preferred chemotherapeutic agents are doxorubicin, a taxane, a camptothecin, a podophyllotoxin, a nucleoside analog, or a pyrimidine analog.
Owner:IMMUNOGEN INC +1
Stabilized high concentration anti-integrin alphanubeta3 antibody formulations
InactiveUS20040208870A1Low to undetectable levelEasy to manageBiocideOrganic active ingredientsHigh concentrationAntibody fragments
The present invention provides liquid formulations of antibodies or antibody fragments that immunospecifically bind to integrin alphaVbeta3, which formulations exhibit stability, low to undetectable levels of aggregation, and very little to no loss of the biological activities of the antibodies or antibody fragments, even during long periods of storage. In particular, the present invention provides liquid formulations of antibodies or fragments thereof that immunospecifically bind to integrin alphaVbeta3, which formulations are substantially free of surfactant, inorganic salts, and / or other common excipients. Furthermore, the invention provides methods of preventing, treating or ameliorating an inflammatory disorder, an autoimmune disorder, a disorder associated with aberrant expression and / or activity of integrin alphaVbeta3, a disorder associated with abnormal bone metabolism, a disorder associated with aberrant angiogenesis or cancer utilizing the liquid formulations of the present invention.
Owner:MEDIMMUNE LLC
Engineered anti-alpha V-integrin hybrid antibodies
ActiveUS8562986B2Reduce in quantityEliminate and reduce immune responseAntibody ingredientsImmunoglobulinsHeavy chainBinding site
Owner:MERCK PATENT GMBH
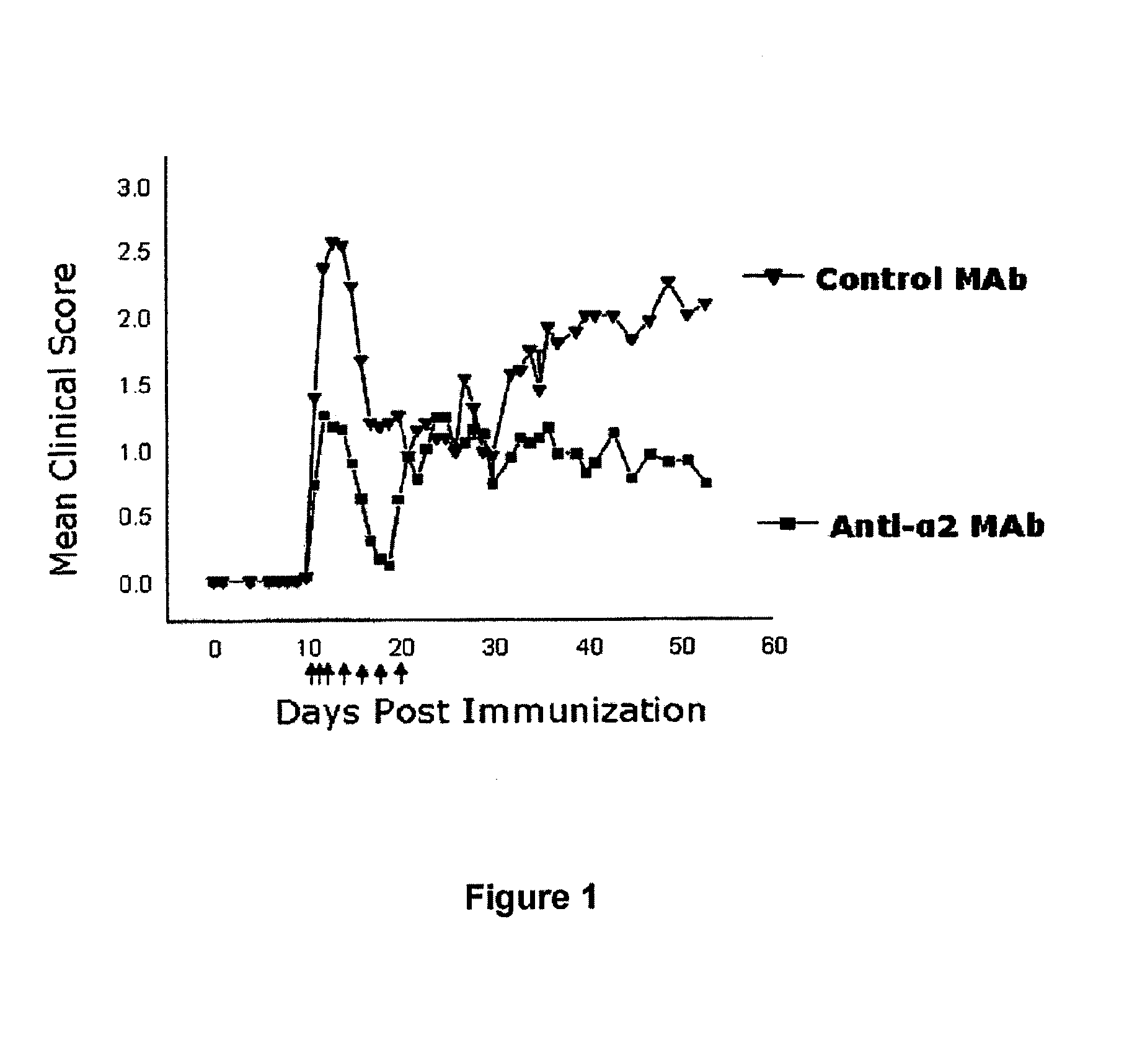
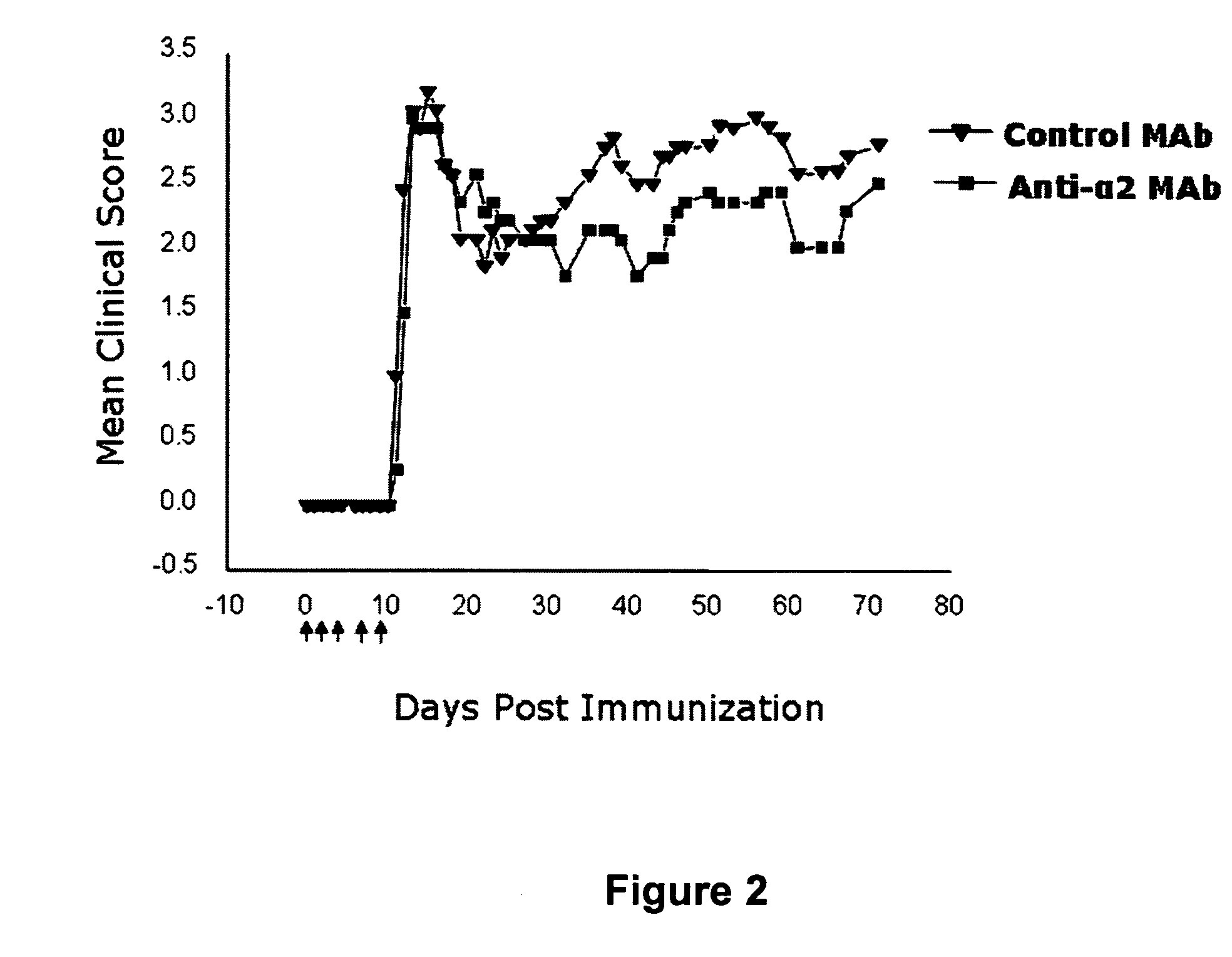
Anti-alpha2 integrin antibodies and their uses
InactiveUS20070128190A1Increased transgene transfer efficiencyEffective absorptionSenses disorderNervous disorderDiseaseAntiendomysial antibodies
The invention relates to anti-α2 integrin antibodies and their uses. Humanized antibodies are disclosed that bind to the I domain of α2 integrin and inhibit the interaction of α2β1 integrin with collagen. Also disclosed are therapeutic uses of anti-α2 integrin antibodies in treating α2β1-mediated disorders, including anti-α2 integrin antibodies that bind to α2 integrin without activating platelets.
Owner:ICHNOS SCI SA
Methods of production and use of anti-integrin antibodies for the control of tissue granulation
InactiveUS20050002930A1MinimizingReducing deleterious granulationSenses disorderAntipyreticWound siteAnti integrin
The present invention provides methods that enable the user to identify inhibitors of tissue granulation in and around a wound site, thereby limiting excessive scar formation as the wounded tissue heals. The some granulation inhibitors identified using the methods of the invention inhibit granulation in and around a wound site up to five fold, with a corresponding decrease in the formation of scar tissue when tested on retinal injuries. Granulation inhibitors that can be identified using the methods of the present invention include antibodies, peptides, nucleic acids (aptamers), and non-peptide small molecules.
Owner:FACET BIOTECH CORP
Engineered Anti-alpha v-integrin hybrid antibodies
ActiveUS20100254977A1Reduce in quantityEliminate and reduce immune responseAnimal cellsSugar derivativesHeavy chainBinding site
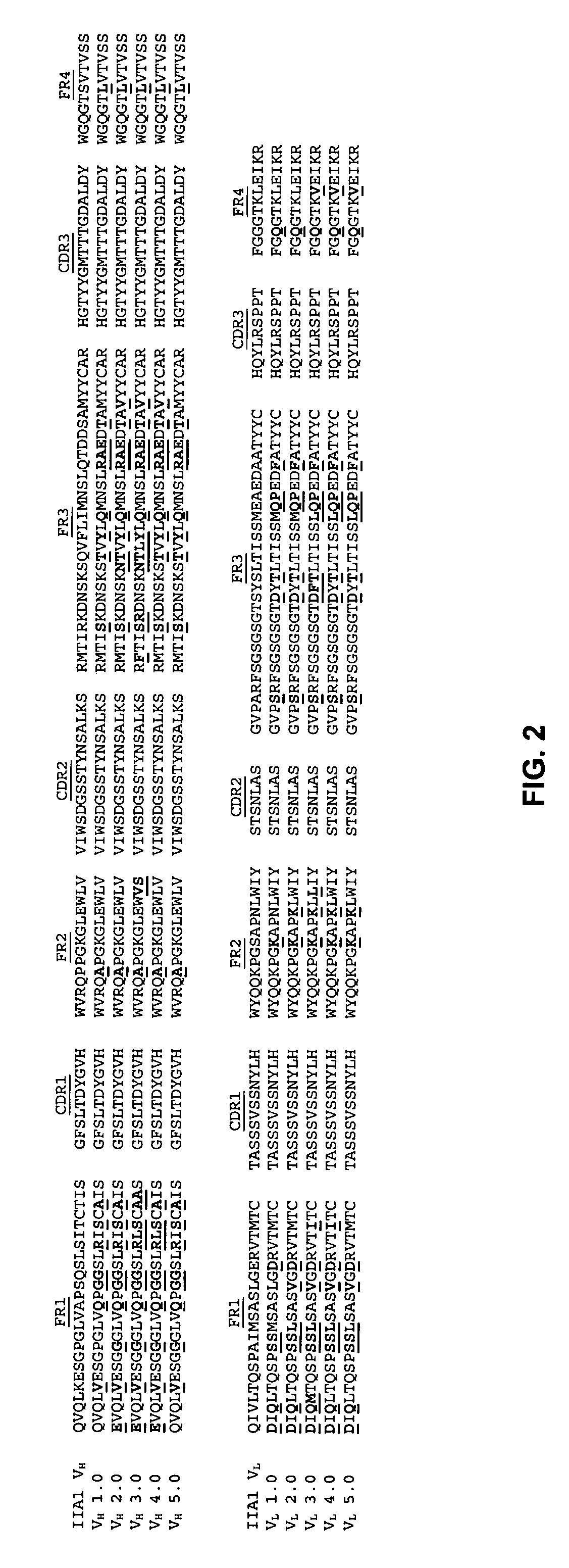
The invention relates to engineered antibodies which specifically bind to integrin receptors, especially the alpha V integrin receptor subunit. The antibodies comprise the antigen binding sites (CDRs) of a known mouse anti-integrin antibody, as well as hybrid light chain variable sequences, mutated heavy chain variable sequences (Frs) and modified heavy chain constant sequences. The novel antibodies have improved immunogenic and expression properties and elicit excellent anti-angiogenic as well as anti-tumor activities in humans in monotherapy but also and above all in combination with other angiogenesis and tumor inhibiting agents.
Owner:MERCK PATENT GMBH
Anti-integrin antibodies, compositions, methods and uses
The present invention relates to at least one novel anti-alpha-V subunit antibodies, including isolated nucleic acids that encode at least one anti-alpha-V subunit antibody, alpha-V subunit, vectors, host cells, transgenic animals or plants, and methods of making and using thereof, including therapeutic compositions, methods and devices.
Owner:JANSSEN BIOTECH INC
Stabilized High Concentration Anti-Integrin alphavbeta3 Antibody Formulations
InactiveUS20090053238A1Low to undetectable levelLittle to no loss of the biological activitiesOrganic active ingredientsAntibody ingredientsHigh concentrationAntibody fragments
The present invention provides liquid formulations of antibodies or antibody fragments that immunospecifically bind to integrin αVβ3, which formulations exhibit stability, low to undetectable levels of aggregation, and very little to no loss of the biological activities of the antibodies or antibody fragments, even during long periods of storage. In particular, the present invention provides liquid formulations of antibodies or fragments thereof that immunospecifically bind to integrin αVβ3, which formulations are substantially free of surfactant, inorganic salts, and / or other common excipients. Furthermore, the invention provides methods of preventing, treating or ameliorating an inflammatory disorder, an autoimmune disorder, a disorder associated with aberrant expression and / or activity of integrin αVβ3, a disorder associated with abnormal bone metabolism, a disorder associated with aberrant angiogenesis or cancer utilizing the liquid formulations of the present invention.
Owner:MEDIMMUNE LLC
Anti-α2 integrin antibodies and their uses
InactiveUS7807794B2Effective absorptionSpecific “homing” propertiesSenses disorderNervous disorderDiseaseAntiendomysial antibodies
The invention relates to anti-α2 integrin antibodies and their uses. Humanized antibodies are disclosed that bind to the I domain of α2 integrin and inhibit the interaction of α2β1 integrin with collagen. Also disclosed are therapeutic uses of anti-α2 integrin antibodies in treating α2β1-mediated disorders, including anti-α2 integrin antibodies that bind to α2 integrin without activating platelets.
Owner:ICHNOS SCI SA
Use of Anti-integrin antibodies for reducing scar tissue formation
InactiveUS20090041785A1Minimize damageReducing granulationSenses disorderAntipyreticInjury mouthWound site
The present invention provides methods that enable the user to identify inhibitors of tissue granulation in and around a wound site, thereby limiting excessive scar formation as the wounded tissue heals. The some granulation inhibitors identified using the methods of the invention inhibit granulation in and around a wound site up to five fold, with a corresponding decrease in the formation of scar tissue when tested on retinal injuries. Granulation inhibitors that can be identified using the methods of the present invention include antibodies, peptides, nucleic acids (aptamers), and non-peptide small molecules.
Owner:FACET BIOTECH CORP
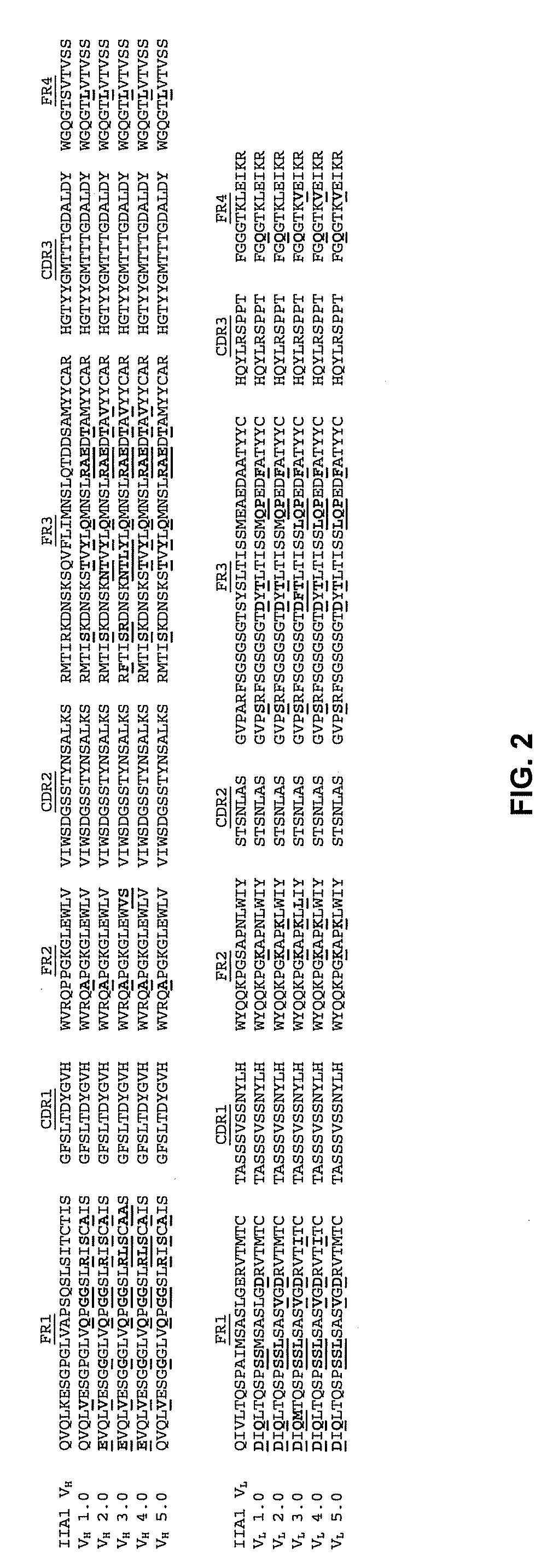
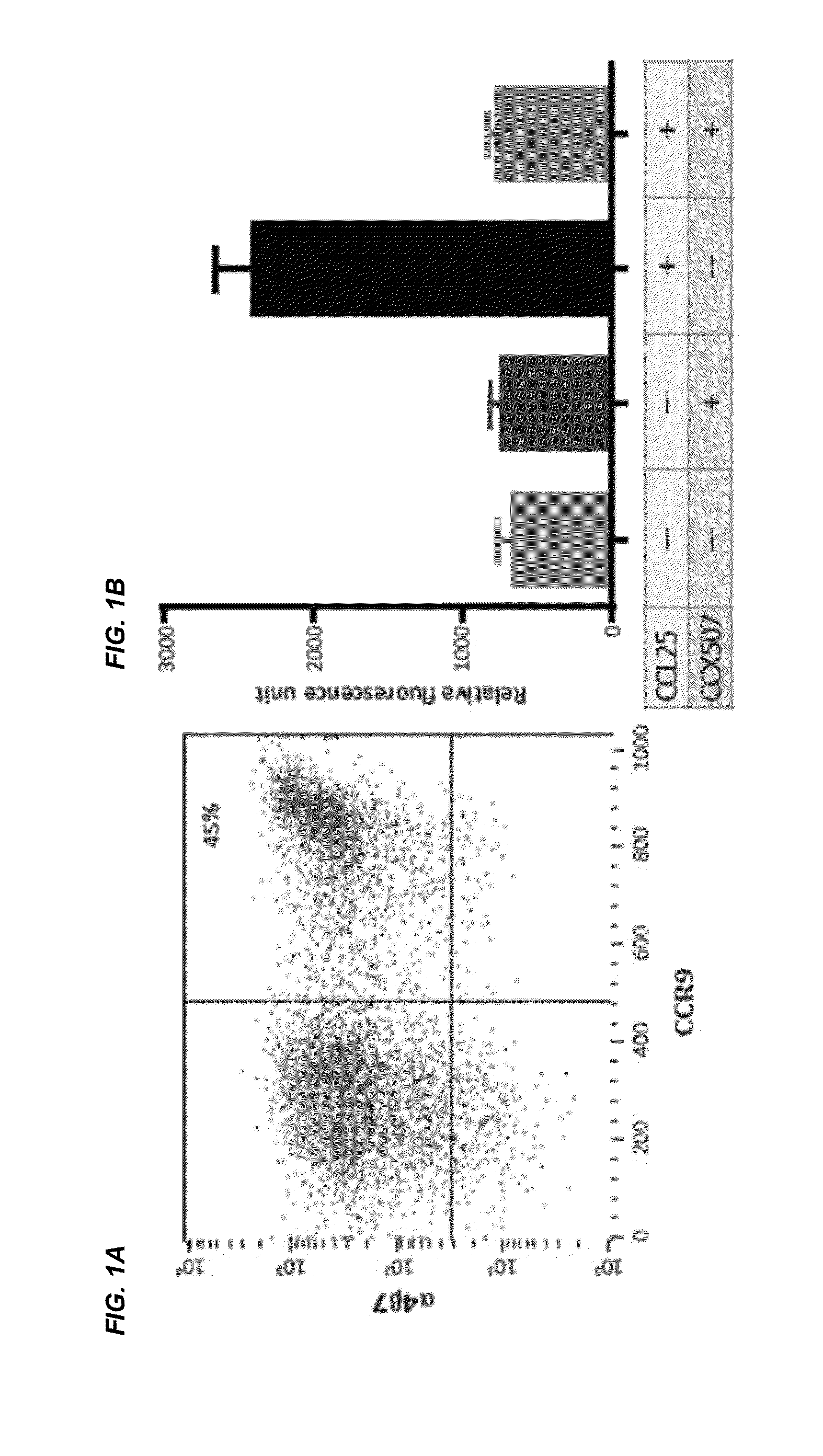
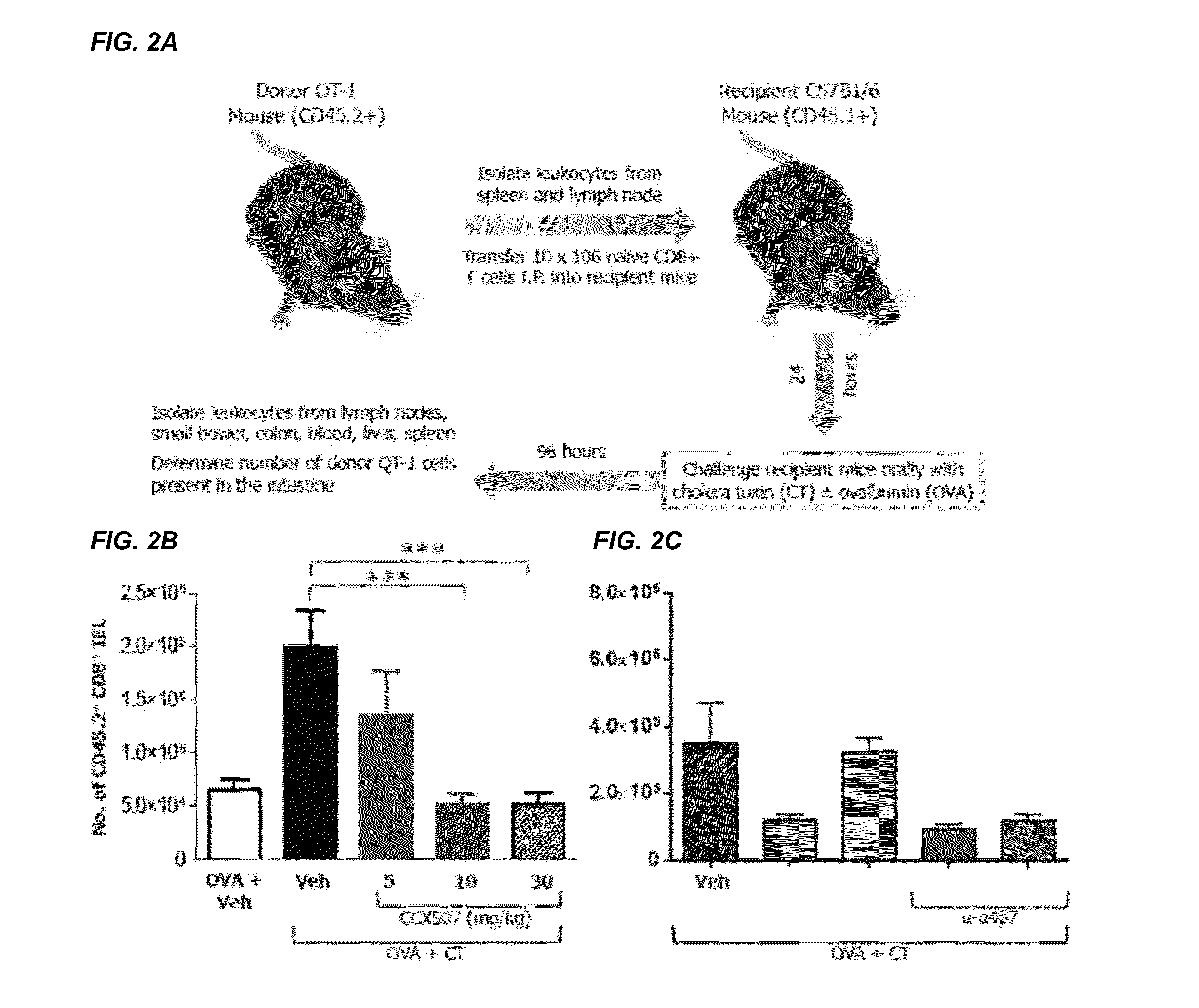
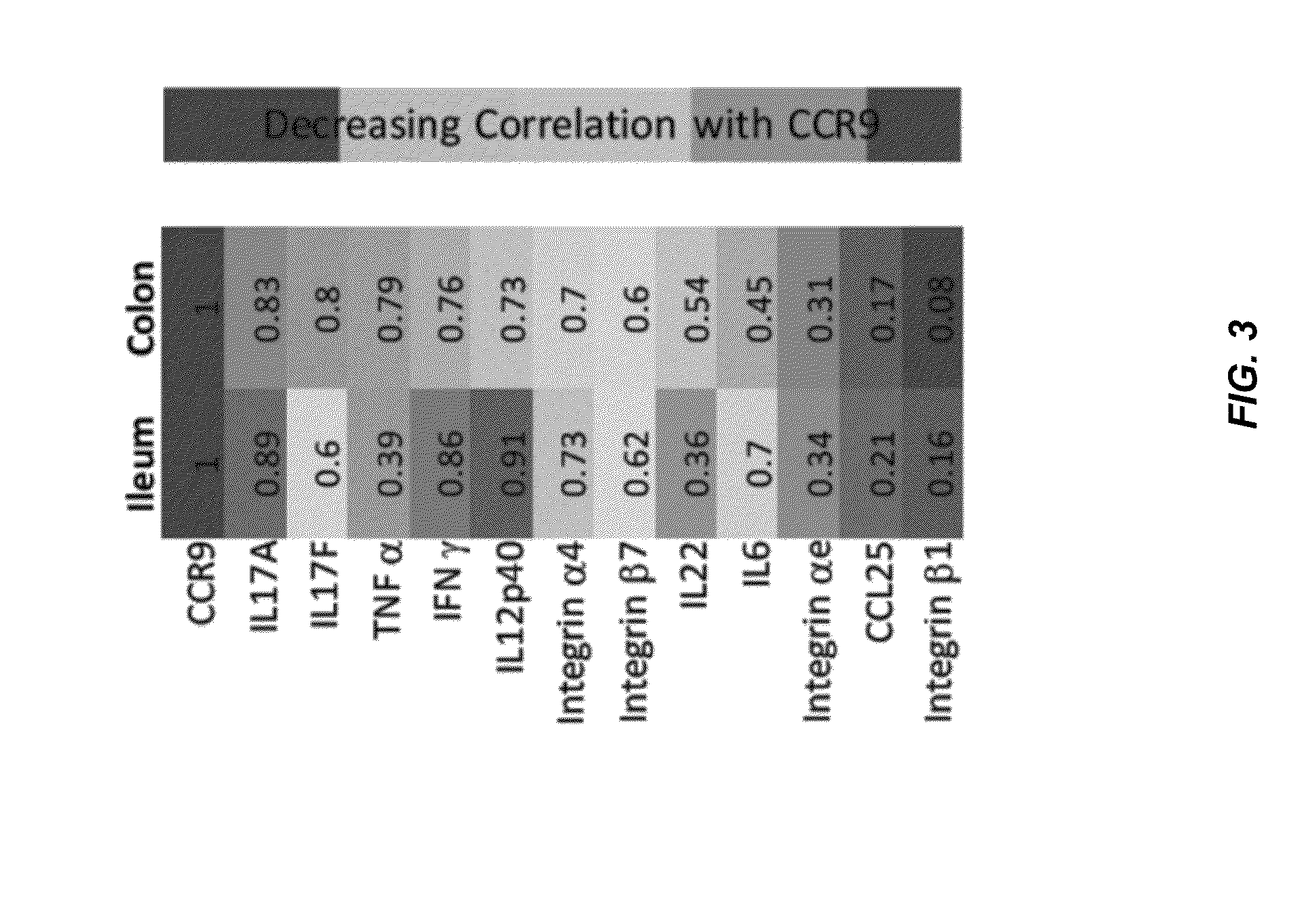
Combinations and Methods for Treating Inflammatory Bowel Disease Using a Combination Therapy of Small Molecule Inhibitors of C-C Chemokine Receptor 9 (CCR9) and Anti-alpha4beta7 Integrin Blocking Antibodies
ActiveUS20160095921A1Reduce developmentOrganic active ingredientsDigestive systemInflammatory Bowel DiseasesCCR9
Provided herein are compositions, methods and kits for treating inflammatory bowel disease (IBD) such as Crohn's disease and ulcerative colitis in a mammal in need thereof. The method include administering to a subject with IBD a combination therapy containing a therapeutically effective amount of a chemokine receptor 9 (CCR9) inhibitor compound and a therapeutically effective amount of an anti-α4β7 integrin antibody such as vedolizumab. Also provided herein is a kit containing the CCR9 inhibitor compound and anti-α4β7 integrin antibody.
Owner:CHEMOCENTRYX INC
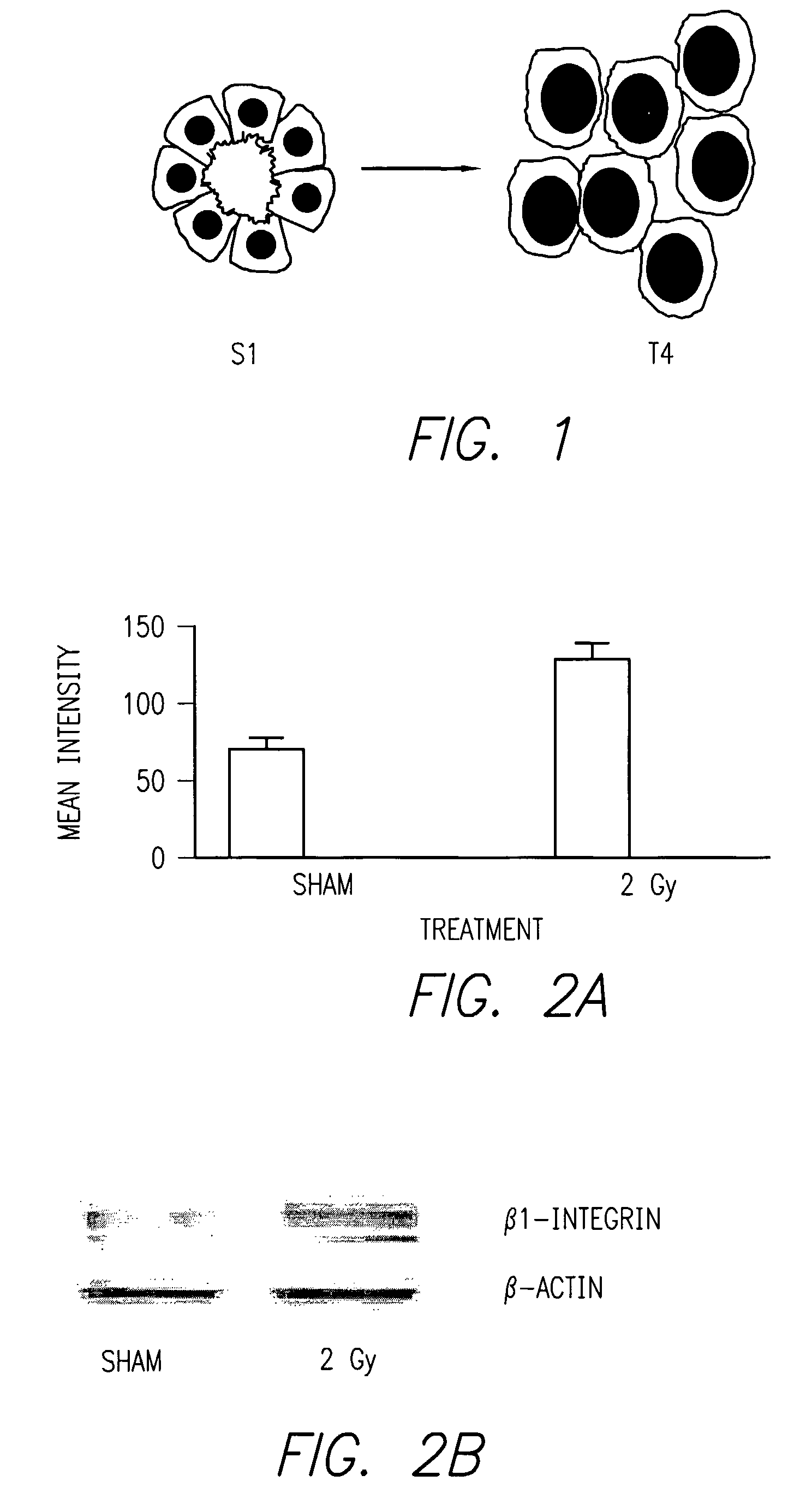
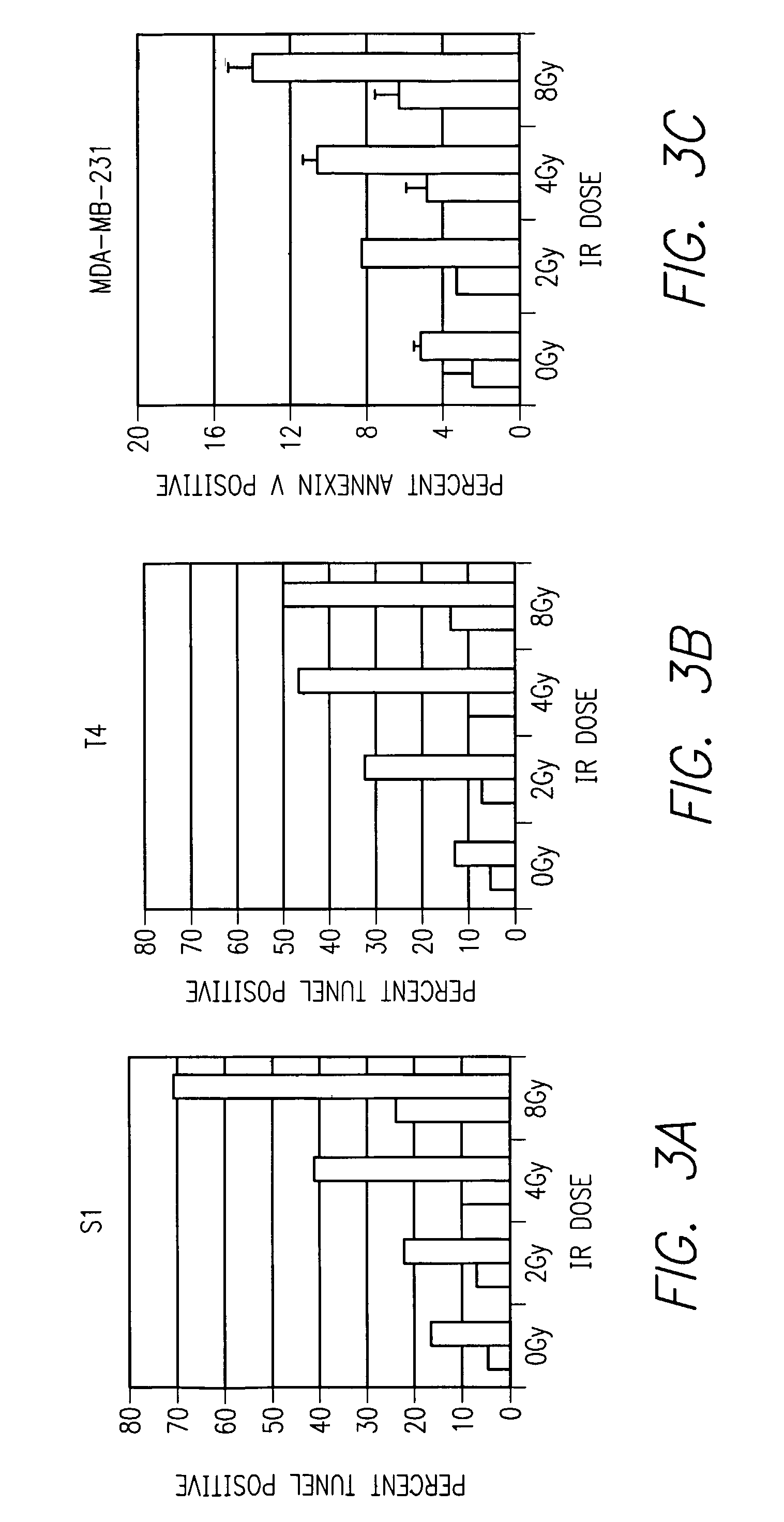
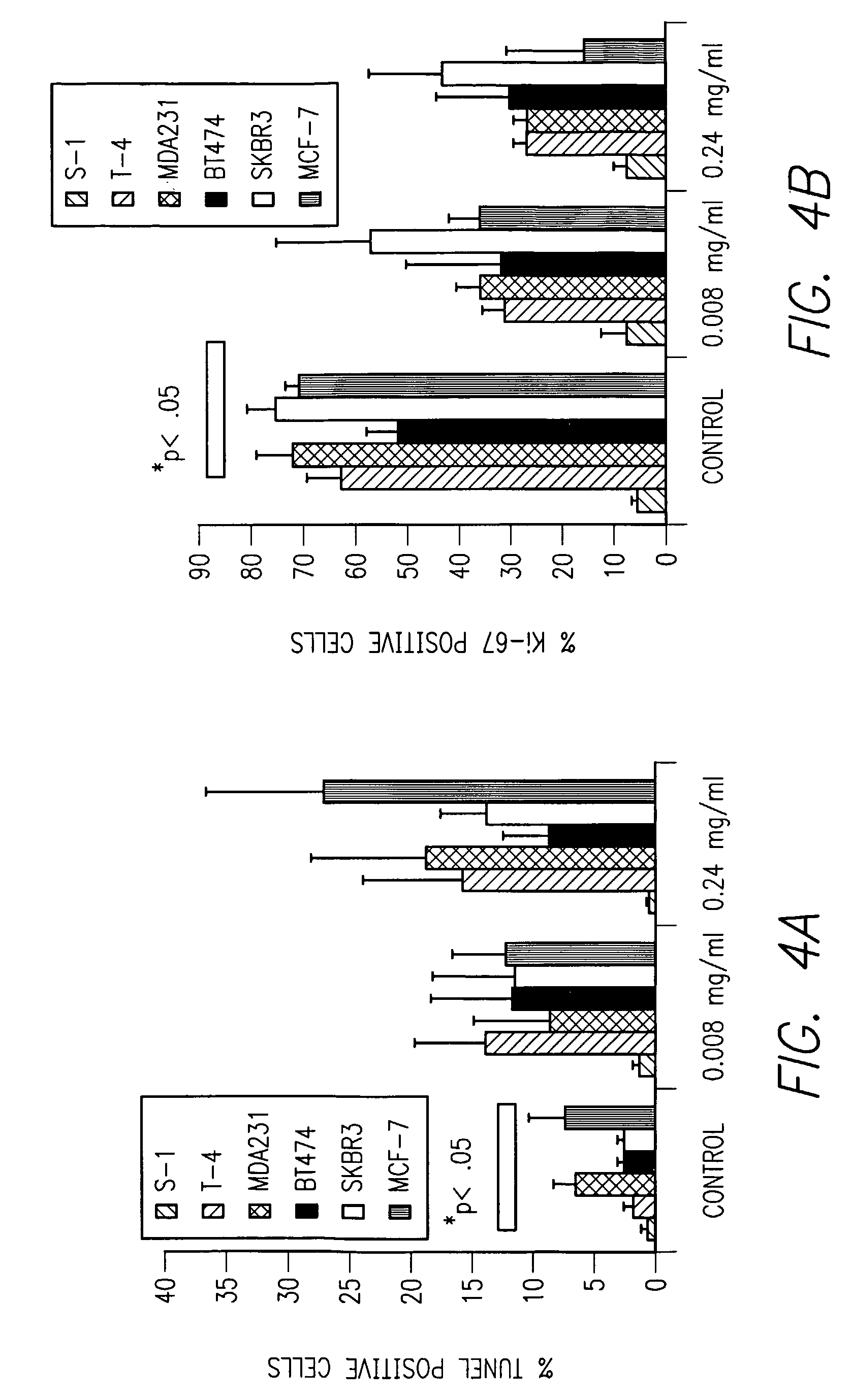
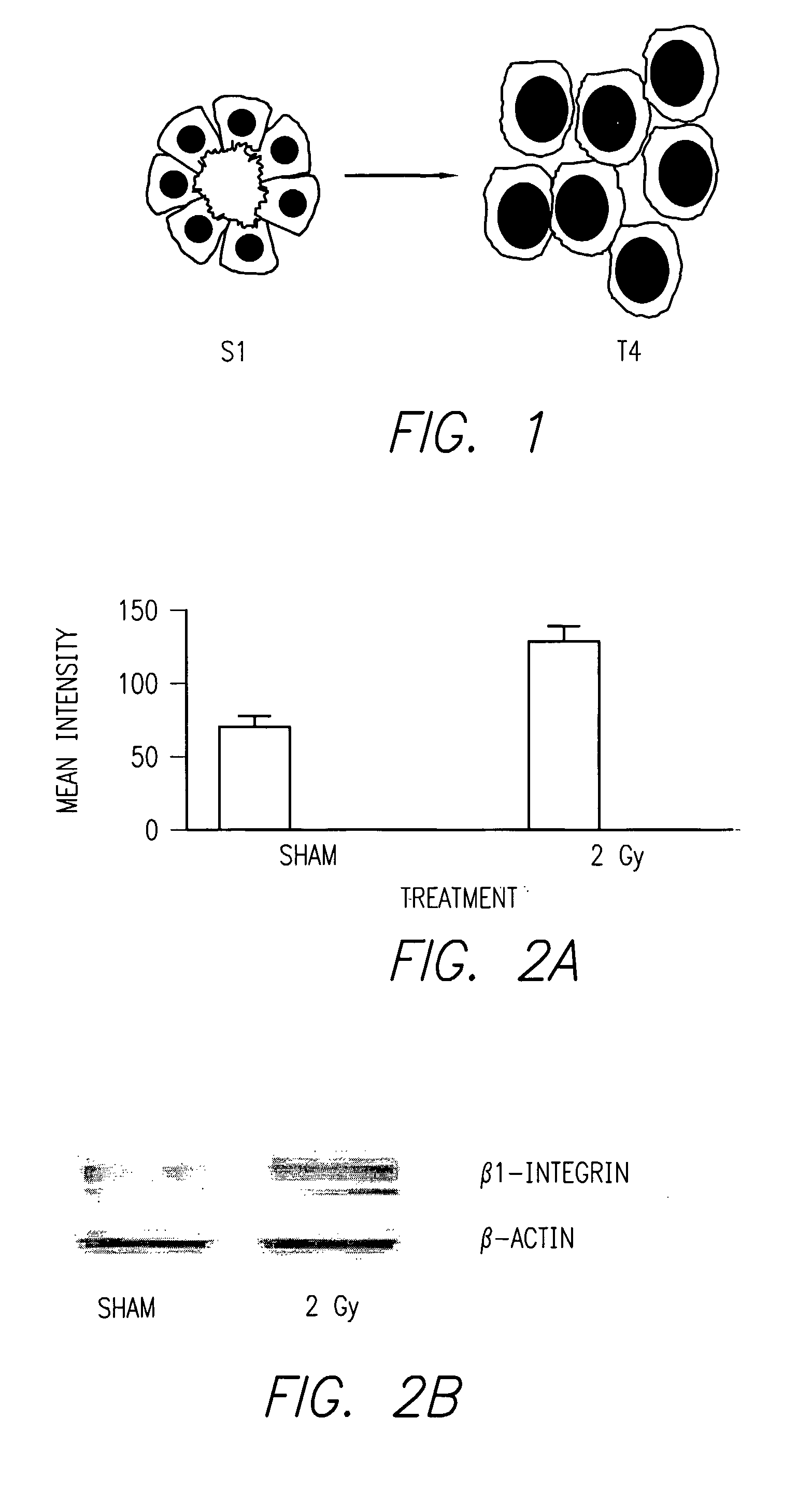
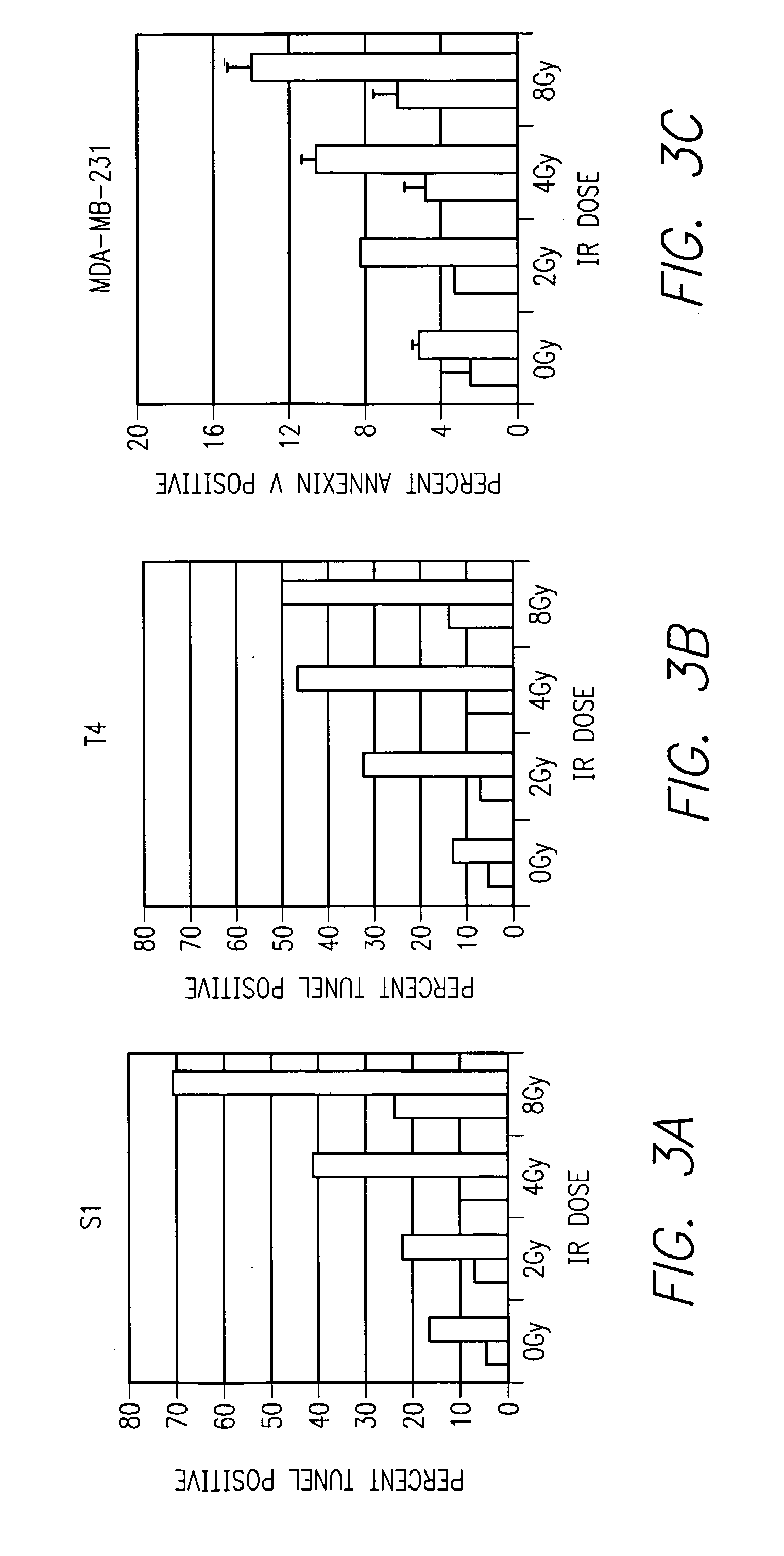
Method of increasing radiation sensitivity by inhibition of beta one integrin
ActiveUS7618627B2In-vivo radioactive preparationsPeptide/protein ingredientsPresent methodCo administration
A method for increasing or monitoring apoptosis in tumor cells by the co-administration of ionizing radiation and an anti-integrin antibody. Increasing apoptosis reduces tumor growth in vivo and in a cell culture model. The antibody is directed against the beta-1 integrin subunit and is inhibitory of beta-1 integrin signaling. Other molecules having an inhibitory effect on beta-1 integrin, either in signaling or in binding to its cognate extracellular receptors may also be used. The present method is particularly of interest in treatment of tumor cells associated with breast cancer, wherein radiation is currently used alone. The present method further contemplates a monoclonal antibody suitable for human administration that may further comprise a radioisotope attached thereto.
Owner:RGT UNIV OF CALIFORNIA
Anti-integrin immunoconjugates, methods and uses
InactiveUS8603483B2Effective rate of releaseSenses disorderAntipyreticDiseaseAntiendomysial antibodies
The invention relates to conjugates of anti-integrin specific antibodies with cytotoxic compounds, the synthesis, selection, and use of such conjugates for use in cancer therapy or other diseases mediated by cell proliferation, cell migration, or inflammation and which pathology involves angiogenesis or neovascularization of new tissue. In addition the invention relates to combination therapy of such diseases wherein the treatment comprises use of said conjugates in combination with one or more other treatment modalities including but not limited to: chemotherapy, surgery or radiation therapy. The preferred conjugates contain maytansinoid compounds linked to the antibody by a disulfide linkage, and preferred chemotherapeutic agents are doxorubicin, a taxane, a camptothecin, a podophyllotoxin, a nucleoside analog, or a pyrimidine analog.
Owner:IMMUNOGEN INC +1
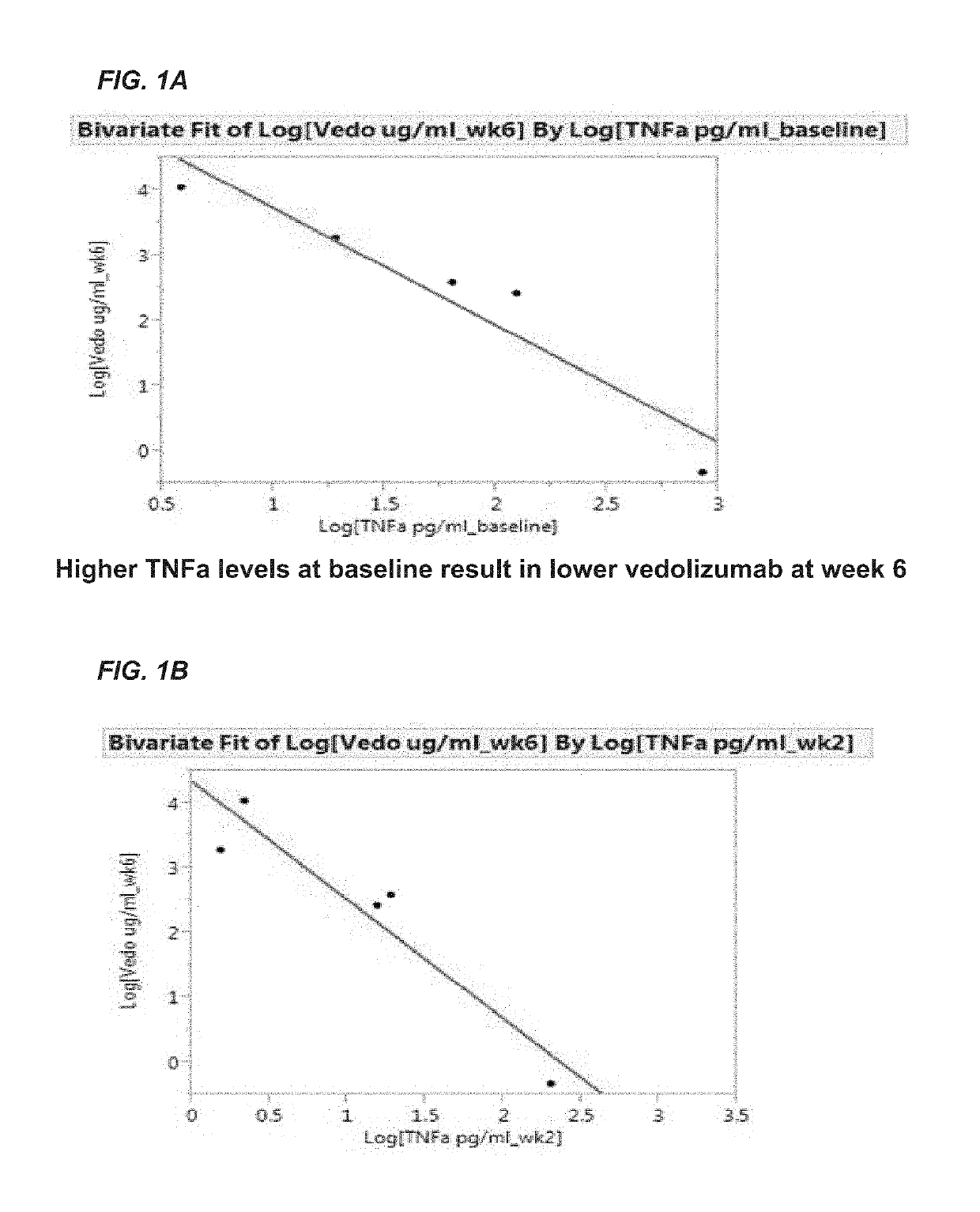
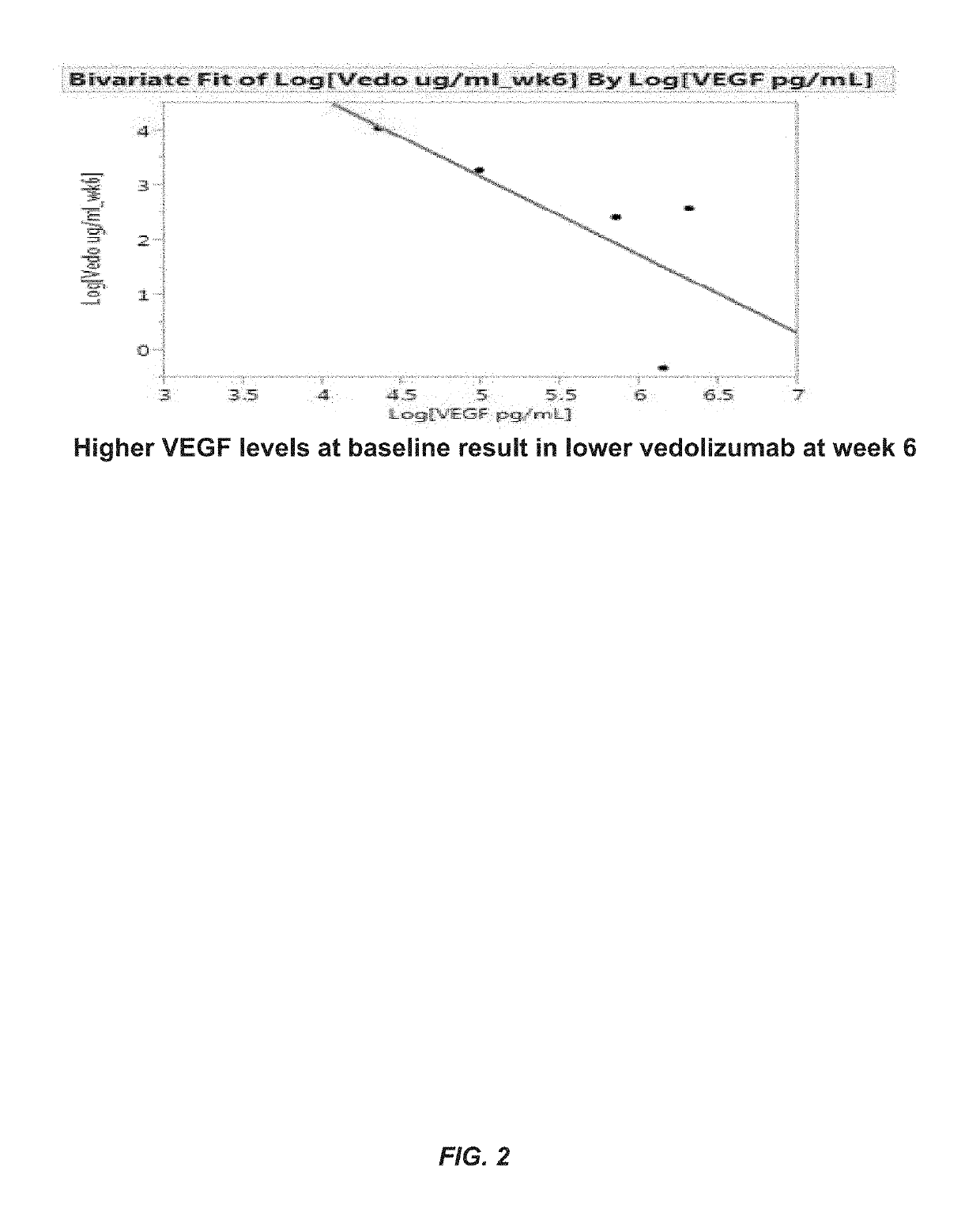
Methods for establishing a vedolizumab dosing regimen to treat patients with irritable bowel disease
The present invention provides methods for predicting whether an individual having inflammatory bowel disease (IBD) is likely to respond to vedolizumab treatment. Also provided are methods for predicting whether an individual with IBD such as Crohn's disease or ulcerative colitis will develop autoantibodies against vedolizumab. The present invention also provides a treatment regimen for an IBD patient which includes measuring the level of one or more predictive markers of response to vedolizumab prior to administering the anti-α4β7 integrin drug.
Owner:PROMETHEUS LAB
Method of Increasing Radiation Sensitivity by Inhibition of Beta One Integrin
ActiveUS20070237711A1Increased apoptosisReduce cell proliferationIn-vivo radioactive preparationsPeptide/protein ingredientsPresent methodCo administration
A method for increasing or monitoring apoptosis in tumor cells by the co-administration of ionizing radiation and an anti-integrin antibody. Increasing apoptosis reduces tumor growth in vivo and in a cell culture model. The antibody is directed against the beta-1 integrin subunit and is inhibitory of beta-1 integrin signaling. Other molecules having an inhibitory effect on beta-1 integrin, either in signaling or in binding to its cognate extracellular receptors may also be used. The present method is particularly of interest in treatment of tumor cells associated with breast cancer, wherein radiation is currently used alone. The present method further contemplates a monoclonal antibody suitable for human administration that may further comprise a radioisotope attached thereto.
Owner:RGT UNIV OF CALIFORNIA
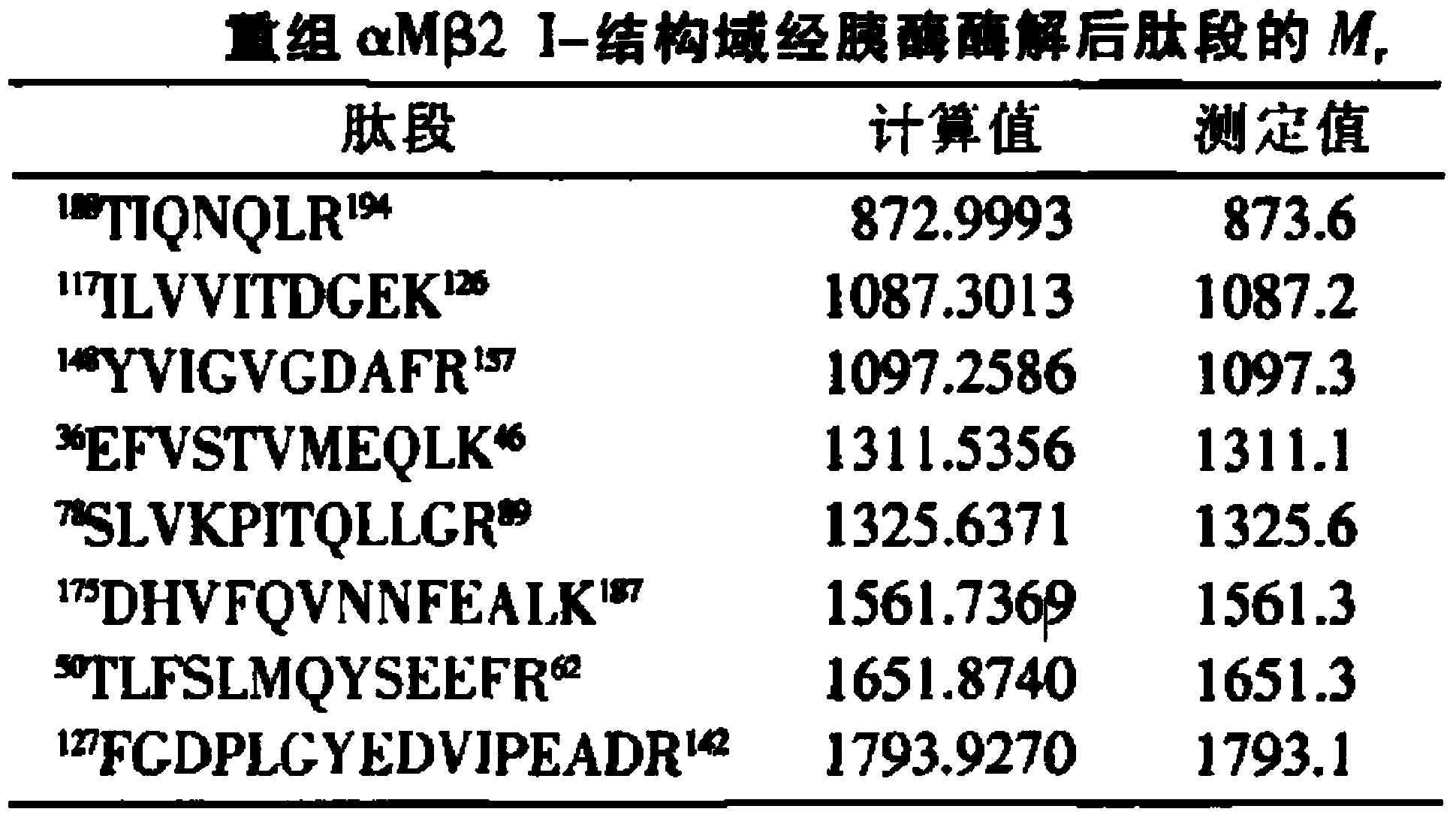
Application of antagonist of integrin alpha M beta2 to preparation of medicine for treating blood platelet quantity related diseases
InactiveCN103977408AClear inhibitionStrong specificityAntibody ingredientsBlood disorderDiseaseSecondary thrombocytopenia
The invention discloses application of an antagonist of integrin alpha M beta2 to preparation of a medicine for treating blood platelet quantity related diseases. The blood platelet quantity related diseases include idiopathic or secondary thrombocytopenia and idiopathic or secondary thrombocytosis. The antagonist of the integrin alpha M beta2 can be an anti-integrin alpha M beta2 antibody, amino acid sequence polypeptide and oligopeptide of the integrin alpha M beta2, derivatives and analogues of the amino acid sequence polypeptide and oligopeptide of the integrin alpha M beta2, and a small-molecular compound antagonist of the integrin alpha M beta2. The invention discloses the application of the antagonist of the integrin alpha M beta2 to the preparation of the medicine for treating the blood platelet quantity related diseases for the first time.
Owner:SUZHOU UNIV
Peptides for treating non-exudative macular degeneration and other disorders of the eye
PendingUS20220031800A1Improves color visionReduce expressionSenses disorderPeptide/protein ingredientsOphthalmologyExudative age-related macular degeneration
Methods of using anti-integrin peptides for a) improving best corrected visual acuity of an eye of a subject suffering from non-exudative age related macular degeneration and / or b) improving color vision in an eye of a subject suffering from impaired color vision and / or for treatment of other disorders.
Owner:ALLEGRO PHARMA
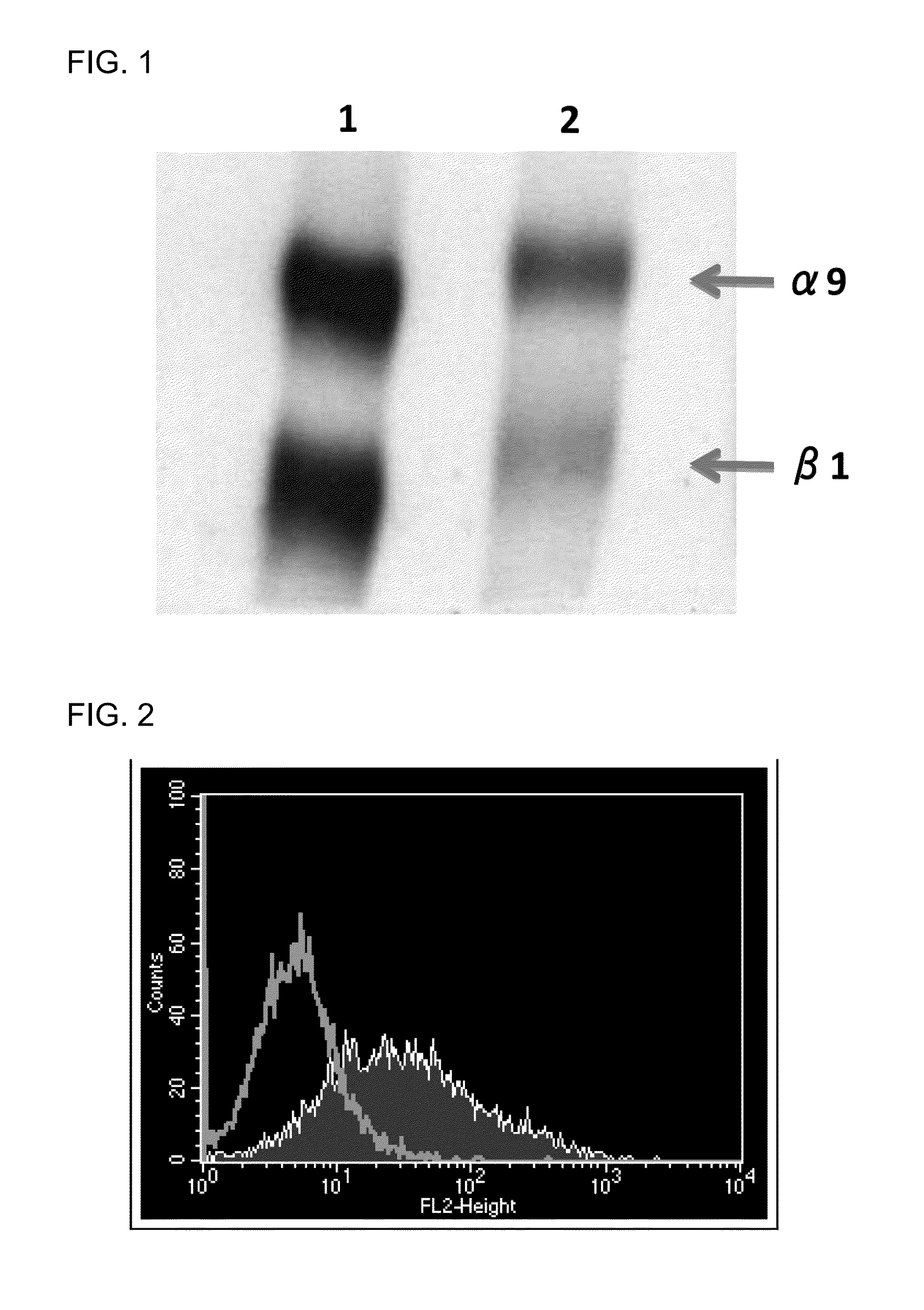
Integrin alpha 9 blockade suppresses lymphatic valve formation and promotes transplant survival
In certain embodiments, the present invention provides methods of suppressing valvulogenesis (VG) in a lymphatic vessel in an inflamed or transplanted tissue or organ in a mammal in need thereof comprising administering an effective amount of an anti-integrin alpha 9 (Itga-9) therapeutic agent to the mammal, and optionally by administering VEGFR-3.
Owner:RGT UNIV OF CALIFORNIA
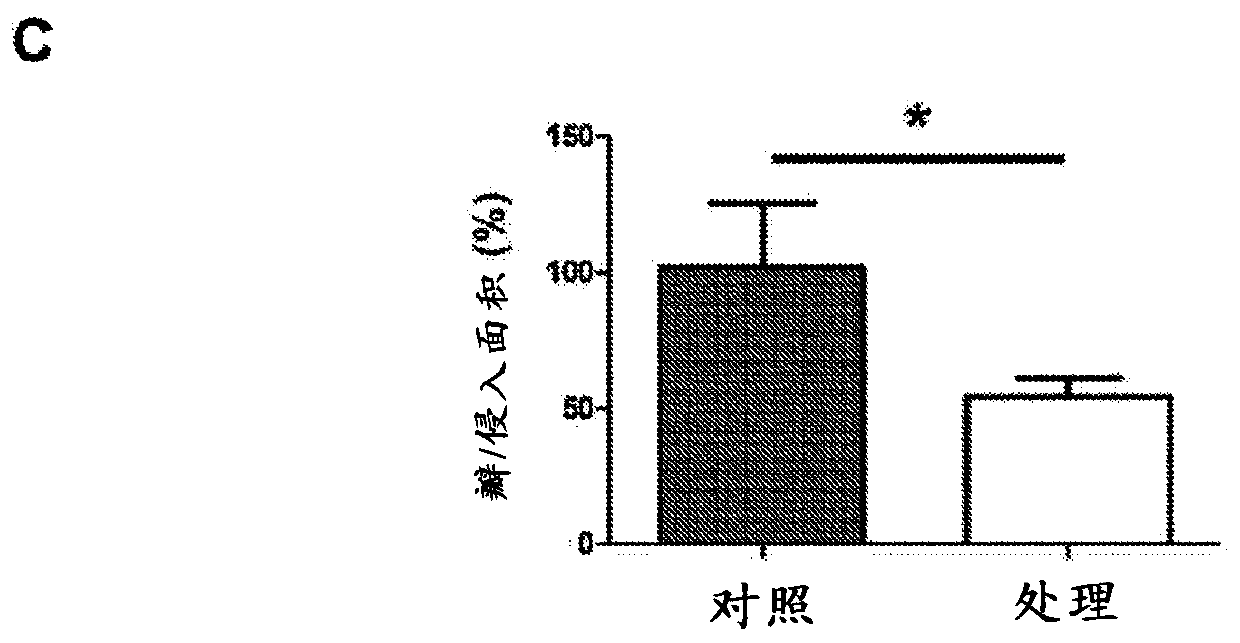
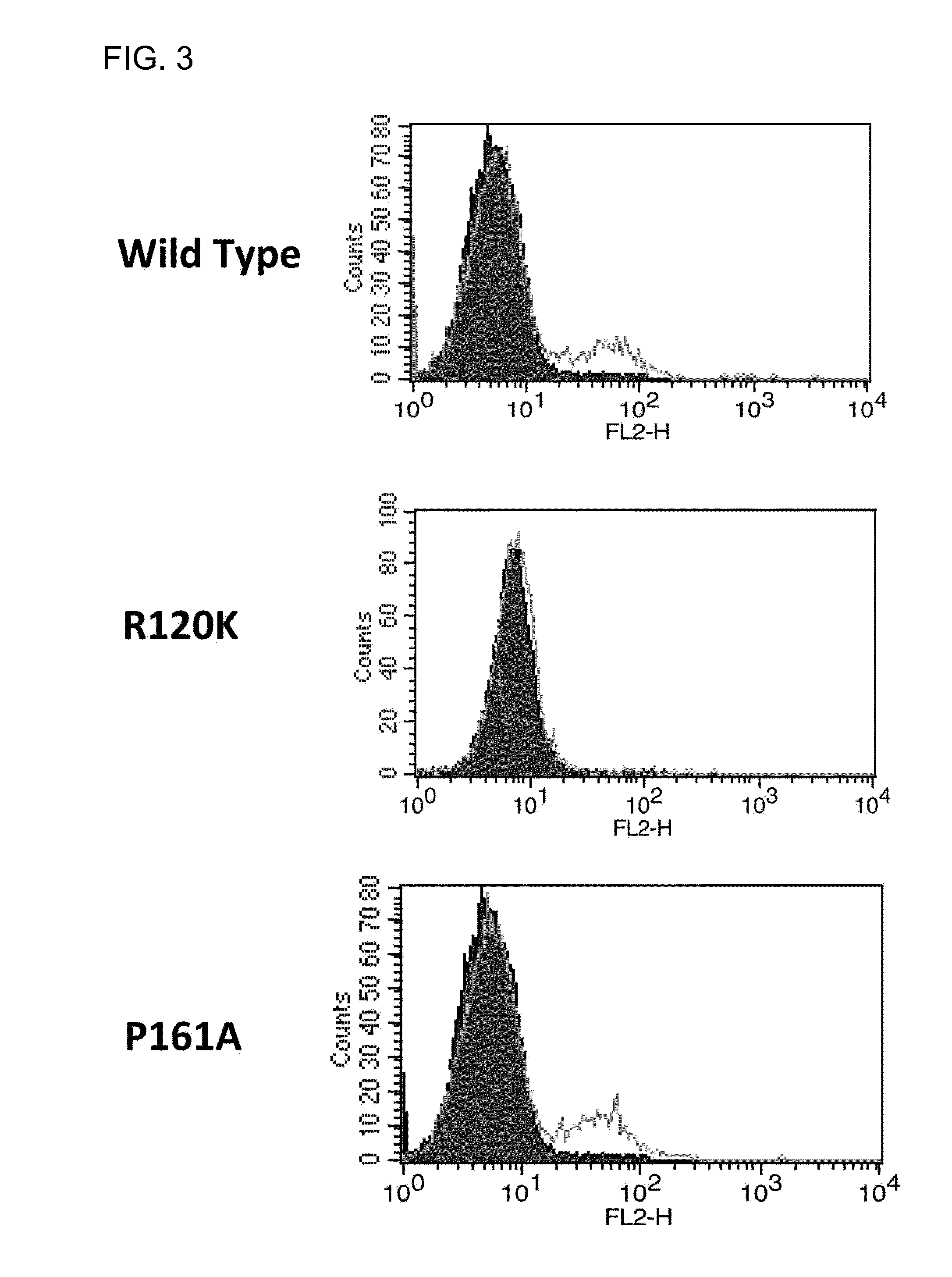
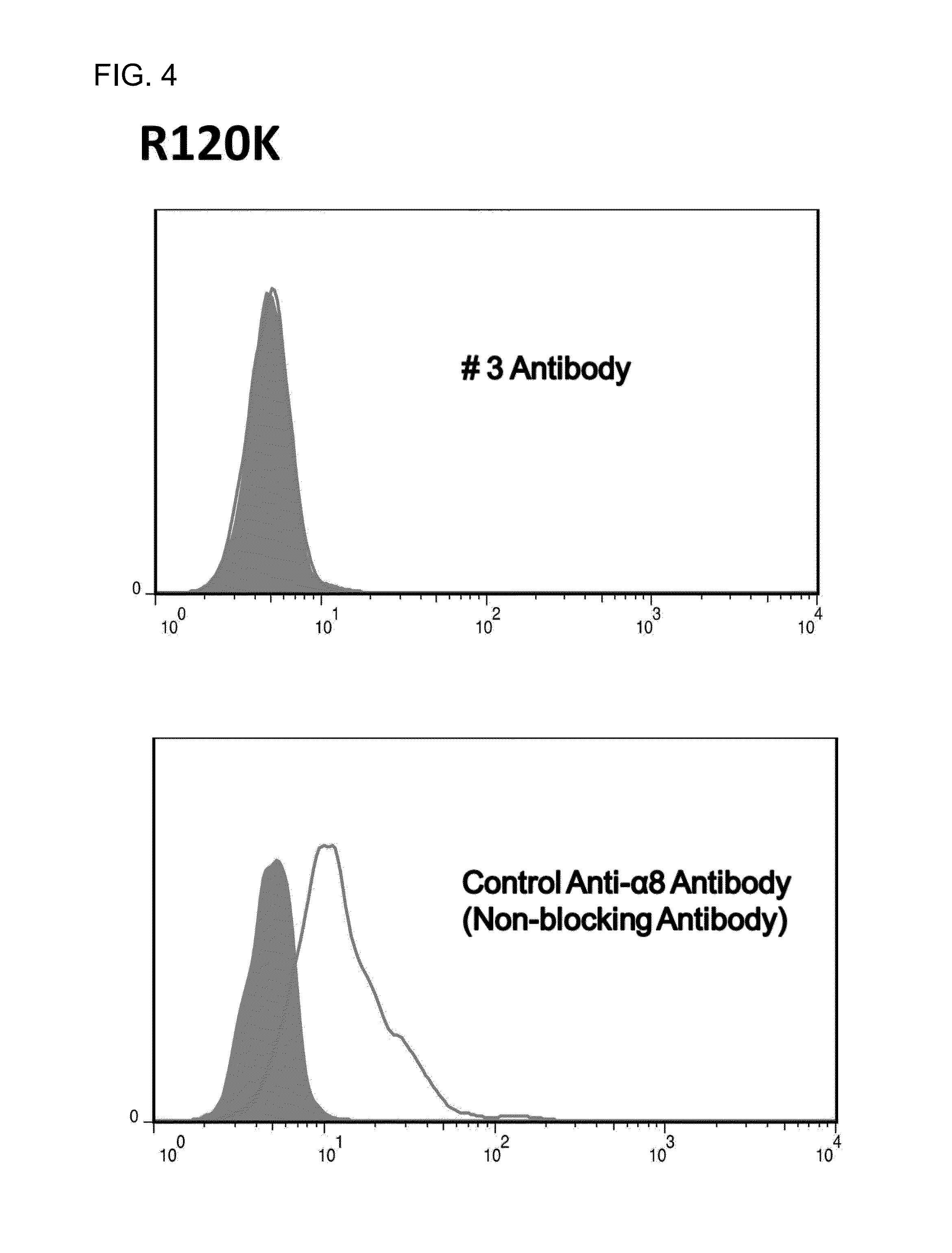
Fibrosis suppression by inhibiting integrin alpha-8 beta-1 function
ActiveUS20150064187A1Prevent fibrosisDigestive systemImmunoglobulins against cell receptors/antigens/surface-determinantsInvolucrinFibrosis
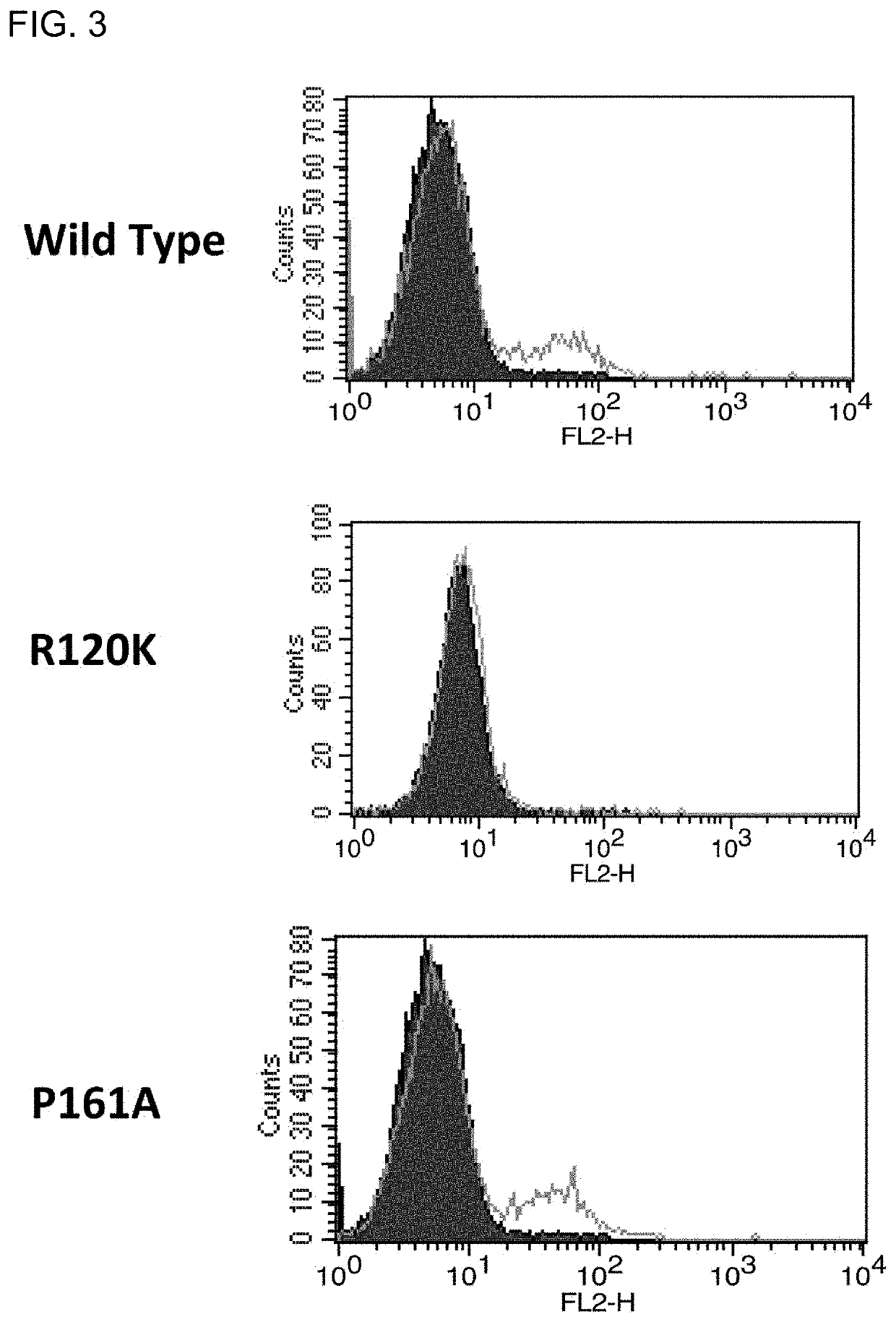
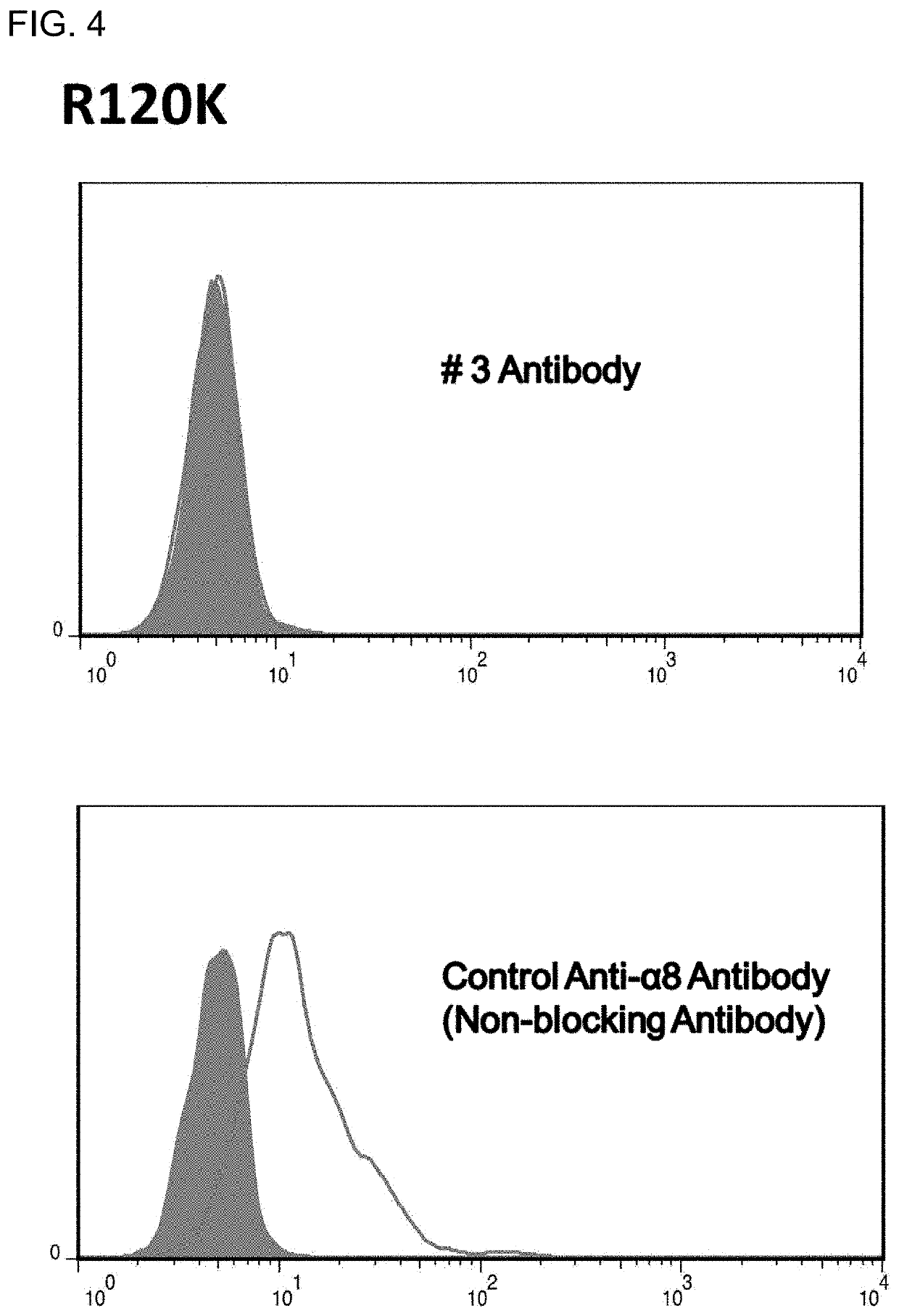
Novel and effective anti-fibrosis agents are obtained. An anti-fibrosis agent containing an antagonist for integrin α8β1 is used. In addition, used is an antagonist containing an anti-integrin α8β1 antibody that specifically binds to at least one amino acid in a cap subdomain of an integrin α8 chain and a periphery thereof. Also, used is an anti-fibrosis agent containing an anti-integrin α8β1 antibody that specifically binds to R120 of an integrin α8 chain and a periphery thereof or S132 and a periphery thereof. Note that the above antagonists may each be an anti-integrin α8β1 antibody capable of binding to any of integrins α8β1 derived from a human, mouse, and rat.
Owner:HIROSHIMA UNIVERSITY
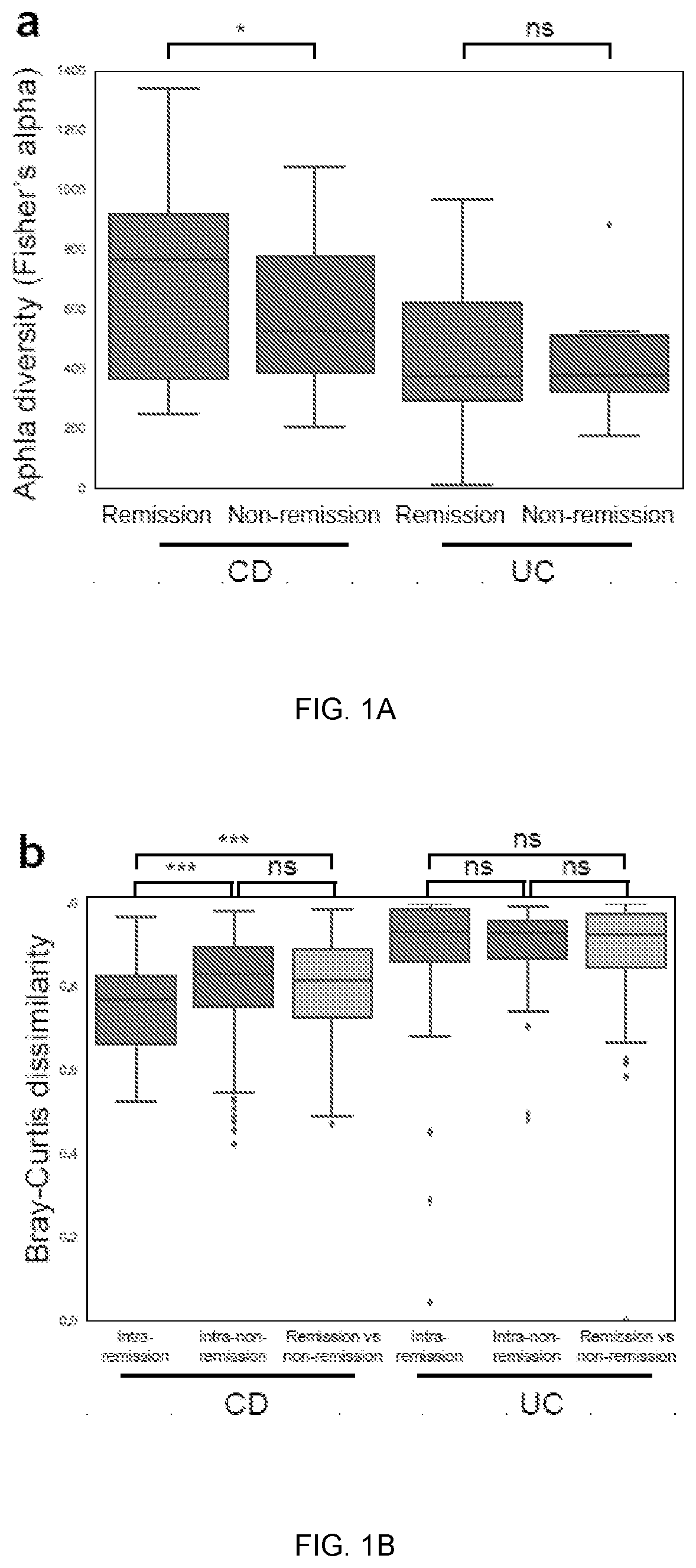
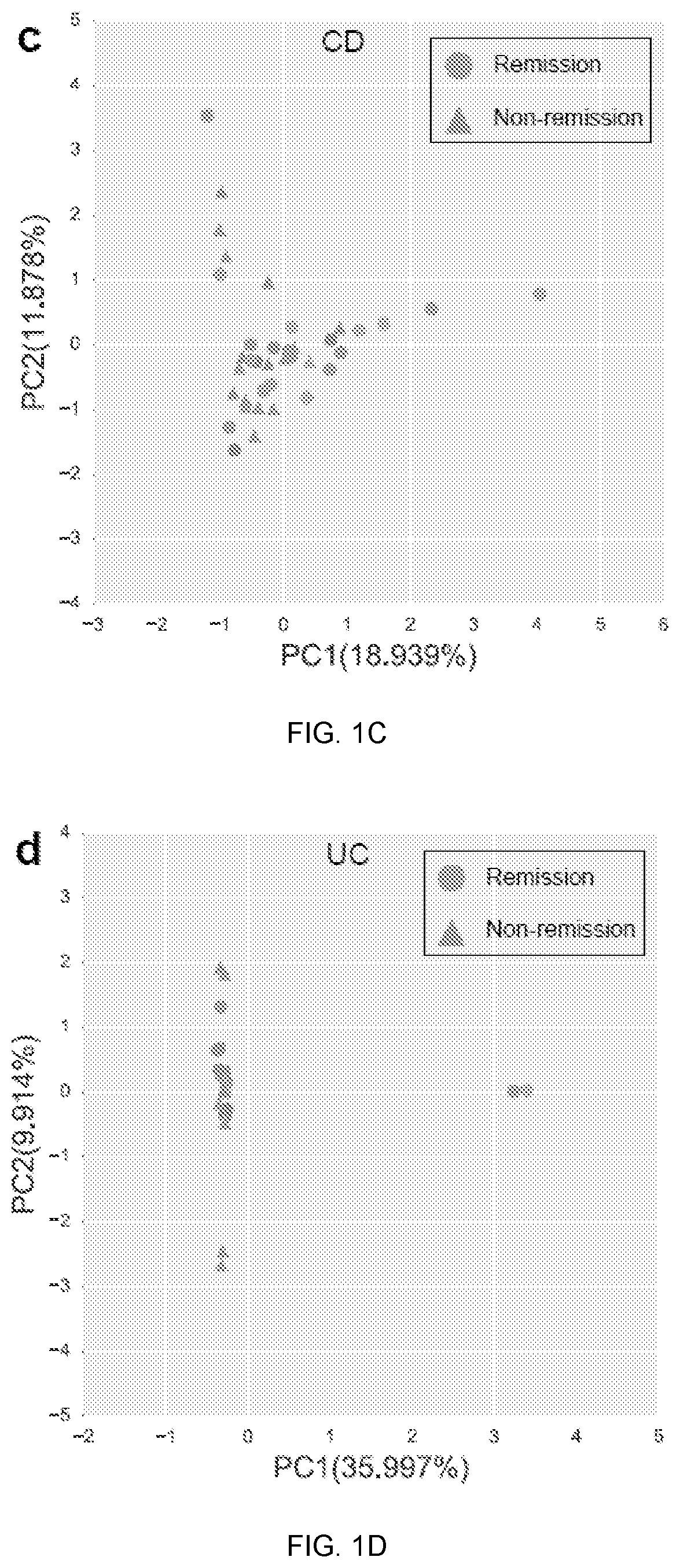
Gut microbiome function predicts response to Anti-integrin biologic therapy in inflammatory bowel diseases
PendingUS20210278416A1Accurate responseHigh levelDisease diagnosisBiological testingMicroorganismBowels diseases
The present invention relates to a relationship between microbial metagenomic structure and function and clinical remission with anti-integrin therapy induction; longitudinal trajectory of changes in the microbiome with maintenance treatment; and a comprehensive predictive model incorporating clinical and microbiome-related data to accurately classify treatment response.
Owner:THE BROAD INST INC +1
Anti-beta 1 integrin humanized antibody, and pharmaceutical composition for treating cancer, comprising same
PendingCN113330035ABiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAntiendomysial antibodies
The present invention relates to a monoclonal antibody or a fragment thereof which recognizes beta 1 integrin as an antigen and thus binds specifically to same. In addition, the present invention relates to a pharmaceutical composition comprising the monoclonal antibody or a fragment thereof for preventing or treating cancer. The monoclonal antibody of the present invention suppresses the proliferation of cancer cells and angiogenesis and effectively induces apoptosis, and thus can be usefully used in preventing or treating cancer.
Owner:SG医疗株式会社
Abituzumab for the treatment of colorectal cancer
PendingUS20200121788A1Peptide/protein ingredientsInorganic active ingredientsAntiendomysial antibodiesChemotherapy combinations
Methods of treatment of colorectal cancer can include the administration of the anti-alpha-v integrin (receptor) antibody Abituzumab. Preferably, the methods of treating colorectal cancer can include treating Stage II-IV colorectal cancer, metastatic colorectal cancer, left-sided colorectal cancer and / or left-sided metastatic colorectal cancer, involving the administration of said Abituzumab to patients in need thereof. Abituzumab is also useful for the manufacture of a medicament for treating colorectal cancer, preferably colorectal cancer as defined herein. Abituzumab is further useful for the manufacture of a medicament for treating colorectal cancer in combination with suitable targeted therapy concepts, such as growth factor or growth factor receptor targeting monoclonal antibodies, and / or chemotherapy.
Owner:MERCK PATENT GMBH +1
Anti-Integrin Immunoconjugates, Methods and Uses
InactiveUS20140093523A1Effective rate of releaseSenses disorderHybrid immunoglobulinsDiseaseCytotoxicity
The invention relates to conjugates of anti-integrin specific antibodies with cytotoxic compounds, the synthesis, selection, and use of such conjugates for use in cancer therapy or other diseases mediated by cell proliferation, cell migration, or inflammation and which pathology involves angiogenesis or neovascularization of new tissue. In addition the invention relates to combination therapy of such diseases wherein the treatment comprises use of said conjugates in combination with one or more other treatment modalities including but not limited to: chemotherapy, surgery or radiation therapy. The preferred conjugates contain maytansinoid compounds linked to the antibody by a disulfide linkage, and preferred chemotherapeutic agents are doxorubicin, a taxane, a camptothecin, a podophyllotoxin, a nucleoside analog, or a pyrimidine analog.
Owner:IMMUNOGEN INC +1
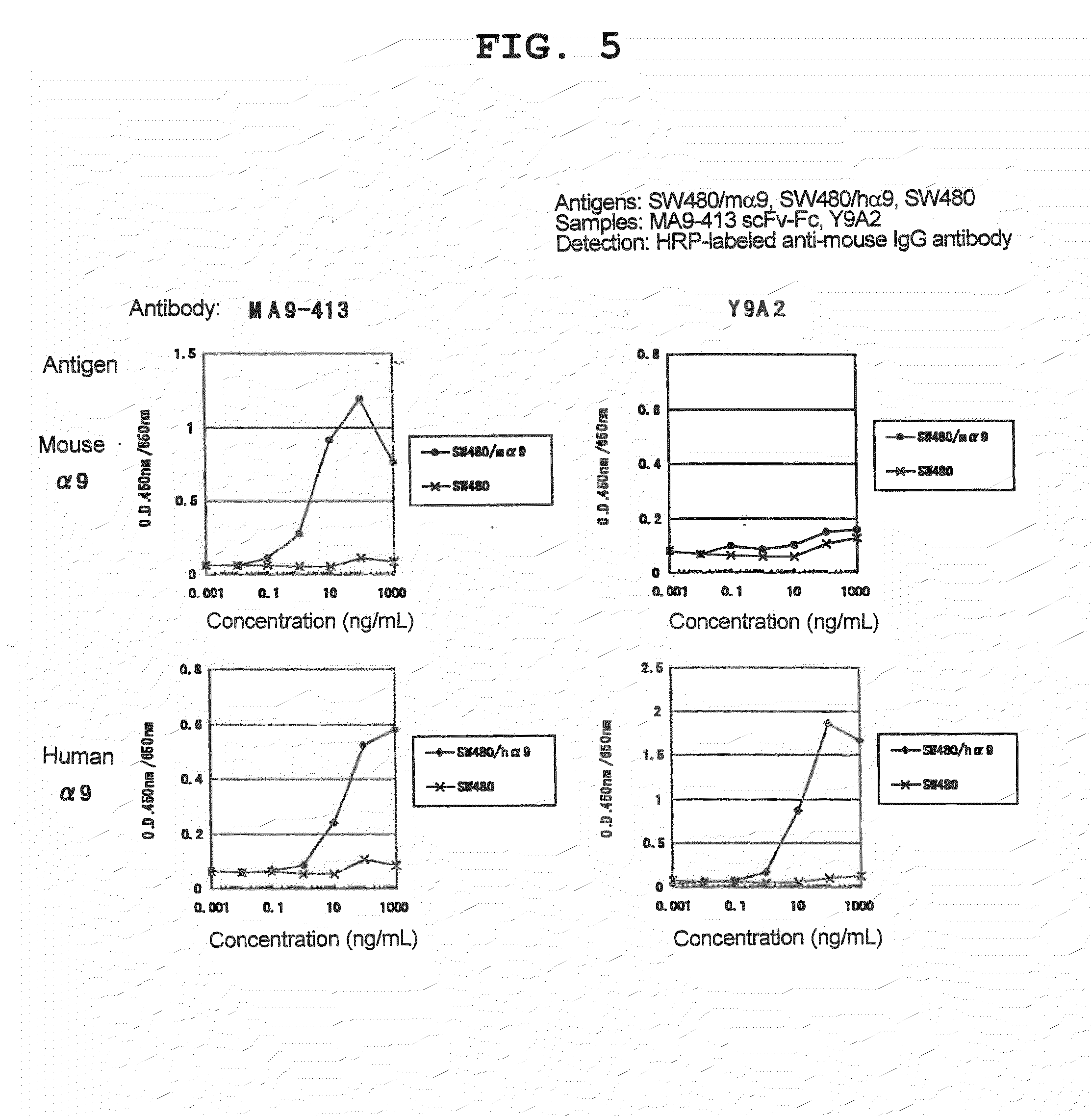
Human Anti-alpha 9 integrin antibody
InactiveUS20110014213A1Specific reactivitySuppressive actionAntibody mimetics/scaffoldsAntipyreticAntibody fragmentsIntegrin
The present invention provides a human anti-α9 integrin antibody or an antibody fragment which specifically recognize human α9 integrin and mouse α9 integrin, inhibit interaction with their ligands, particularly, the antibody or antibody fragment which recognize loop regions of human and mouse α9 integrins, a gene encoding the antibody or antibody fragment, a recombinant expression vector containing the gene, a transformant harboring the gene, production method of human anti-α9 integrin antibody or antibody fragment using the transformant, and an agent for the prophylaxis or treatment of rheumatoid arthritis which contains the antibody or antibody fragment.
Owner:KM BIOLOGICS CO LTD
Compositions and methods to treat solid tumors
ActiveUS10336827B2Reduce doseEffective therapyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAbciximabNeutrophil granulocyte
Owner:WAYNE STATE UNIV
Compositions and methods to treat solid tumors
ActiveUS20190309073A1Reduce doseEffective therapyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAbciximabNeutrophil granulocyte
Compositions and methods that utilize anti-CD11b antibodies, anti-CD18 antibodies, anti-myeloperoxidase (MPO) antibodies, anti-integrin αV antibodies, anti-integrin β1 antibodies, Abciximab, neutrophil inhibitory factor (NIF) protein, and / or combinations thereof to treat solid tumors are described.
Owner:WAYNE STATE UNIV
Tumor targeted radionuclide therapy and molecular imaging of her2+ cancers and other neoplasms
ActiveUS20200085981A1Reduce spreadModulate pathologic angiogenesisImmunoglobulins against growth factorsRadioactive preparation carriersImmunooncologyTumor targeting
Methods and compositions for treating, diagnosing and staging cancers, in particular overexpressing the Human Epidermal growth factor Receptor 2 protein (HER2+) given rise to in breast, gastric, gastroesophageal, ovarian, pancreatic cancer and brain tumors, which may be metastatic to the brain or other site. More specifically, the invention provides for Targeted Radionuclide Therapy (TRNT) with a compound of the invention having a peptide that targets the HER2+ cells, a second component for combining metals into complexes through a ring structure (DOTA), and a third radioisotope component, Lu-177 and Ga-68, in which embodiments further include a companion diagnostic, and in which embodiments further include anti-integrin precision medicines for cancers expressing αvβ3 and αvβ5 integrins, HER2+, vascular endothelial growth factor, vitronectin, fibronectin, tenascin, reelin, kindlin and talin. TRNT may be administered alone or in combination with standard-of-care; an immunooncologic and / or chemotherapeutic, adjuvantly or neoadjuvantly.
Owner:SATZ STANLEY
Anti-alpha-v integrin antibody for the treatment of fibrosis and/or fibrotic disorders
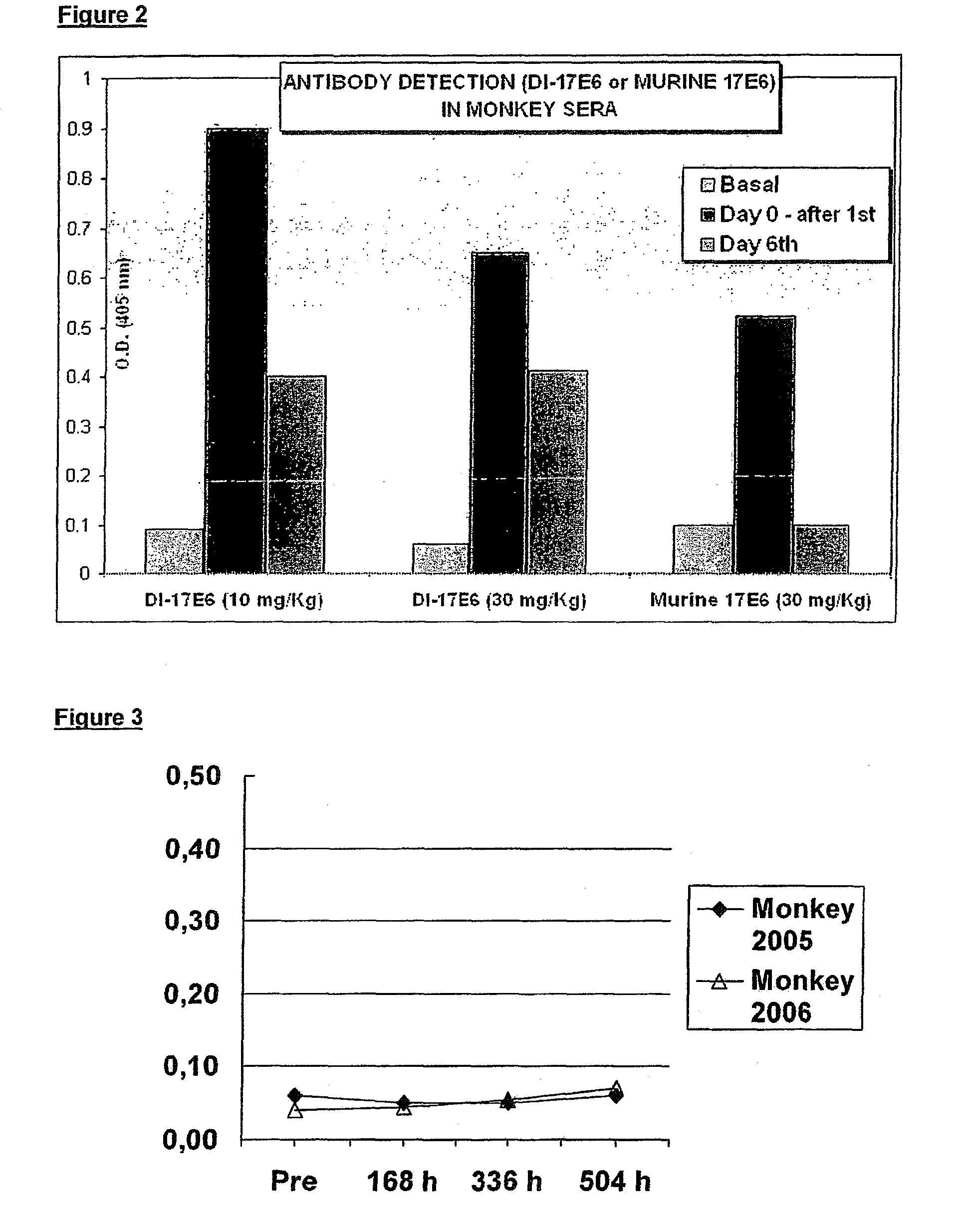
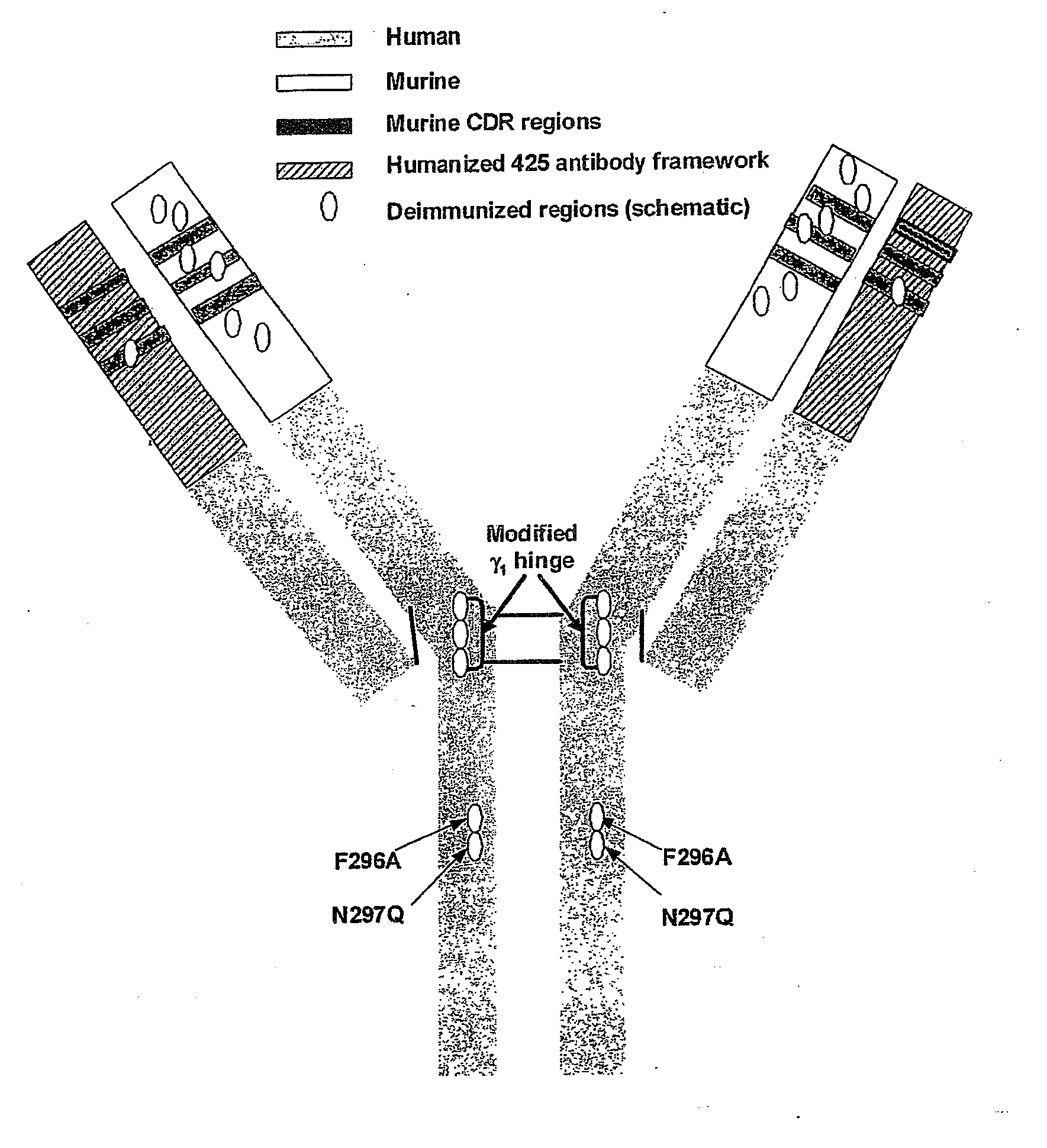
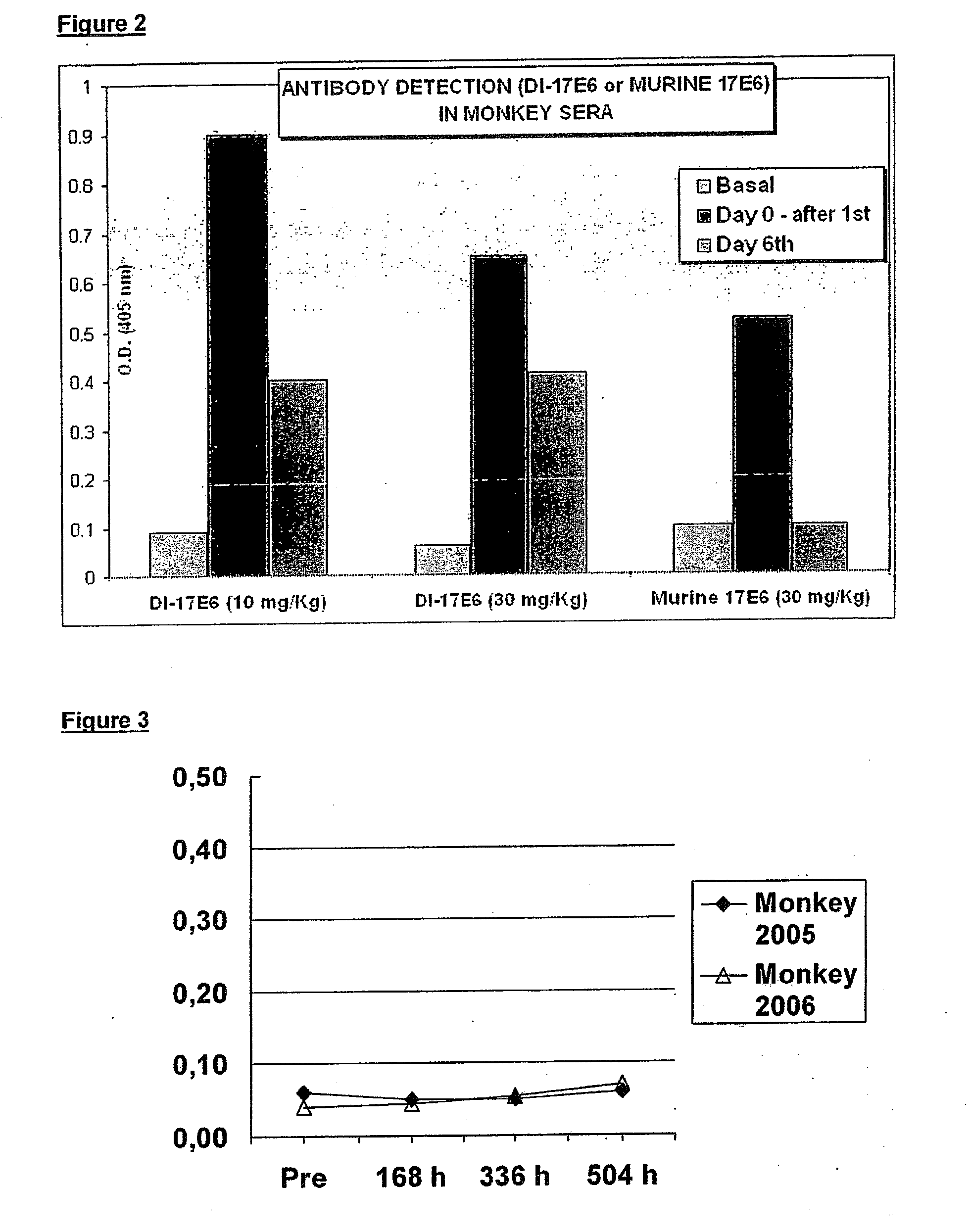
The invention relates to the prophylaxis and / or treatment of fibrosis and / or fibrotic diseases by means of antibodies. Above all, the invention relates to the administration of an anti-alpha-v integrin (receptor) antibody to patients suffering from fibrosis and / or fibrotic diseases, including but not limited to systemic sclerosis (SSc). More specifically, the instant invention relates to the treatment of fibrotic diseases of the skin, lung, heart, liver and / or kidney by means of said antibody. Even more specifically, the instant invention relates to the administration of a recombinant, de- immunized monoclonal antibody targeting av-integrins patients suffering from systemic sclerosis, including, but not limited to systemic sclerosis of the skin, lung, heart and / or kidney by means of the anti-alpha-v integrin antibody DI17E6 and structural mutants or modifications thereof.
Owner:MERCK PATENT GMBH
Fibrosis suppression by inhibiting integrin α-8 β-1 function
ActiveUS10822418B2Prevent fibrosisDigestive systemImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesIntegrin antagonist
Novel and effective anti-fibrosis agents are obtained. An anti-fibrosis agent containing an antagonist for integrin α8β1 is used. In addition, used is an antagonist containing an anti-integrin α8β1 antibody that specifically binds to at least one amino acid in a cap subdomain of an integrin α8 chain and a periphery thereof. Also, used is an anti-fibrosis agent containing an anti-integrin α8β1 antibody that specifically binds to R120 of an integrin α8 chain and a periphery thereof or S132 and a periphery thereof. Note that the above antagonists may each be an anti-integrin α8β1 antibody capable of binding to any of integrins α8β1 derived from a human, mouse, and rat.
Owner:HIROSHIMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com