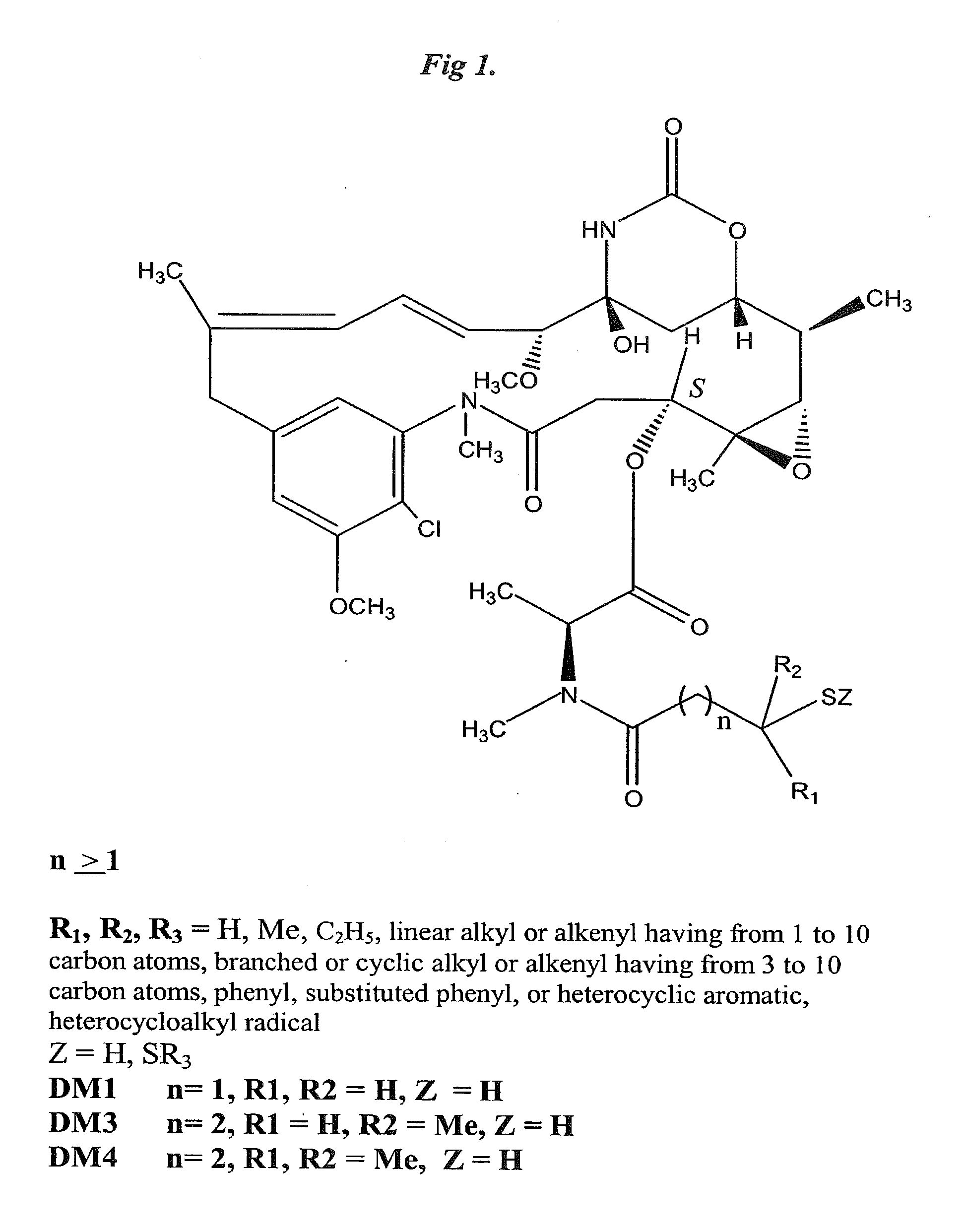
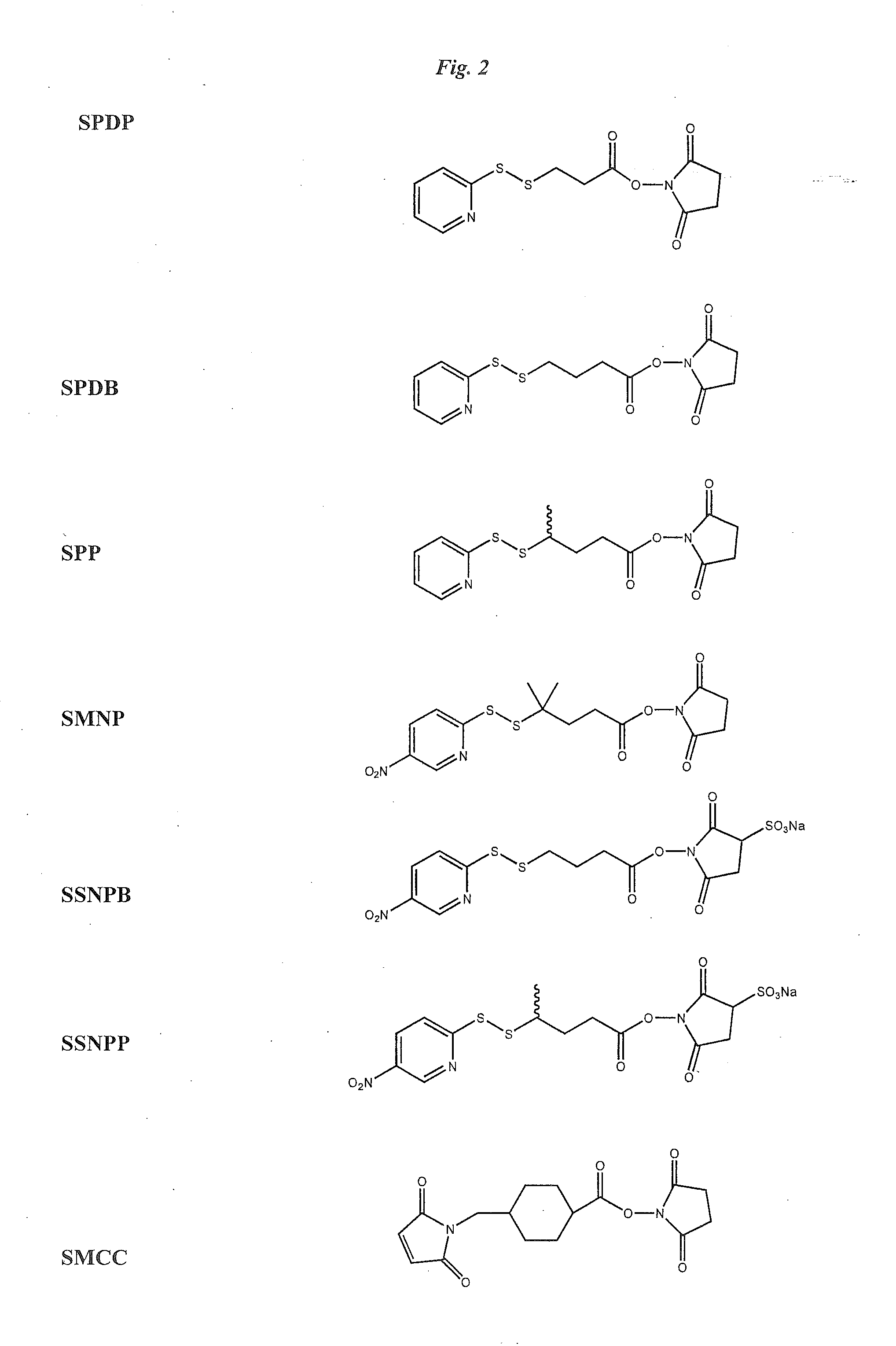
Anti-Integrin Immunoconjugates, Methods and Uses
an immunoconjugation and anti-integrin technology, applied in the field of tumor specific antibody conjugates, can solve the problems of inability to meet the needs of patients, and inability to meet the needs of patients, and achieve the effect of therapeutically effective rate of releas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production and Characterization of Monoclonal Antibody CNTO95
[0156]Preparation of the anti-alpha V integrin antibody CNTO95 is described in detail in PCT publication no. WO 02 / 12501 and in U.S. Publication No. 2003 / 040044, both incorporated by reference herein. Specifically, the human Mab CNTO 95 was generated by immunizing (CBA / J×C57 / BL6 / J, GenPharm International) F2 hybrid mice with αvβ3 integrin purified from human placenta. The antibody is composed of human variable and IgG1 kappa constant regions. The method of making and the desirable characteristics of CNTO95 have been previously described in WO0212501 and Trikha, et al. 2004, Int. J. Cancer 110 (3): 326-335.
[0157]Transgenic mice from GenPharm International express human immunoglobulins but not mouse IgM or Igκ were used. These mice contain human sequence transgenes that undergo V(D)J joining, heavy-chain class switching and somatic mutation to generate a repertoire of human sequence immunoglobulins (Taylor et al., Internatio...
example 2
Preparation of CNTO 95-Maytansine Conjugates
[0171]Antibody conjugates of thiolated maytansines were prepared for further biological testing starting using bifunctional linkers as described.
[0172]CNTO 95 antibody was supplied by Centocor for conjugation. CNTO 95 was supplied at approximately 20 mg / ml (260 mg) total. The antibody was dialysed into Buffer A (50 mM KPi, 50 mM NaCl, 2 mM EDTA pH6.5), then brought to 8 mg / ml in 95% Buffer A, 5% ETOH. The antibody was modified with 6.5 fold molar excess of SPP to introduce the linker for drug conjugation, forming CNTO 95-SS-Py where S-Py is 2-mercaptopyridine. Residual SPP was removed by G25 gel filtration chromatography. The linker to Ab ratio was measured as 4.7. The Ab-SS-Py conjugate was modified with 1.7 fold molar excess of DM1 (MW=737.5 g / mole) to linker, using an antibody concentration of 3.2 / mlin 97% Buffer A, 3% dimethylacetamide. Following conjugation, the conjugate was rechromatographed on G25 using PBS, pH 6.5 as the buffer. T...
example 3
CNTO 95-Maytansine Conjugate Binding to Tumor Cells
[0204]The ability and affinity of CNTO95-Maytansinoid conjugate binding to living cells was tested.
[0205]Materials and Methods. CNTO 95, Centocor lot #95-VF30A03-1, 20 mg / ml in PBS; CNTO 364 (CNTO 95-SPP-DM1), ImmunoGen lot #1806-164, 37.6 mg / ml of DM1, 2.74 mg / ml of conjugated antibody, Endotoxin level <0.1 EU / mg; CNTO 365 (CNTO 95-SPDB-DM4), ImmunoGen lot #2020-78, 41.2 mg / ml of DM4, 2.36 mg / ml of conjugated antibody, Endotoxin level <0.1 EU / mg; CNTO 366 (CNTO95-SPP-DM4), ImmunoGen lot #2020-48, 3.1.5 mg / ml of DM4, 2.19 mg / ml of conjugated antibody, Endotoxin level <0.1 EU / mg.
[0206]Cells: HT29 human colon carcinoma and A549 human lung carcinoma cells were from ATCC and maintained in alphaMEM supplemented with 10% fetal bovine serum (FBS). A2780 human ovarian carcinoma cells were obtained from National Cancer Institute. A2780 cells were cultured in RPMI 1640 medium containing 10% FBS. Cells were harvested, rinsed, suspended in seru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com