Stabilized High Concentration Anti-Integrin alphavbeta3 Antibody Formulations
a technology of anti-integrin and formulation, which is applied in the direction of immunoglobulins, peptides, antibody medical ingredients, etc., can solve the problems of high manufacturing cost, significant waste, and lyophilized formulations of antibodies, and achieve the effects of reducing the level of antibody fragmentation and/or aggregation, facilitating antibody administration, and reducing the risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
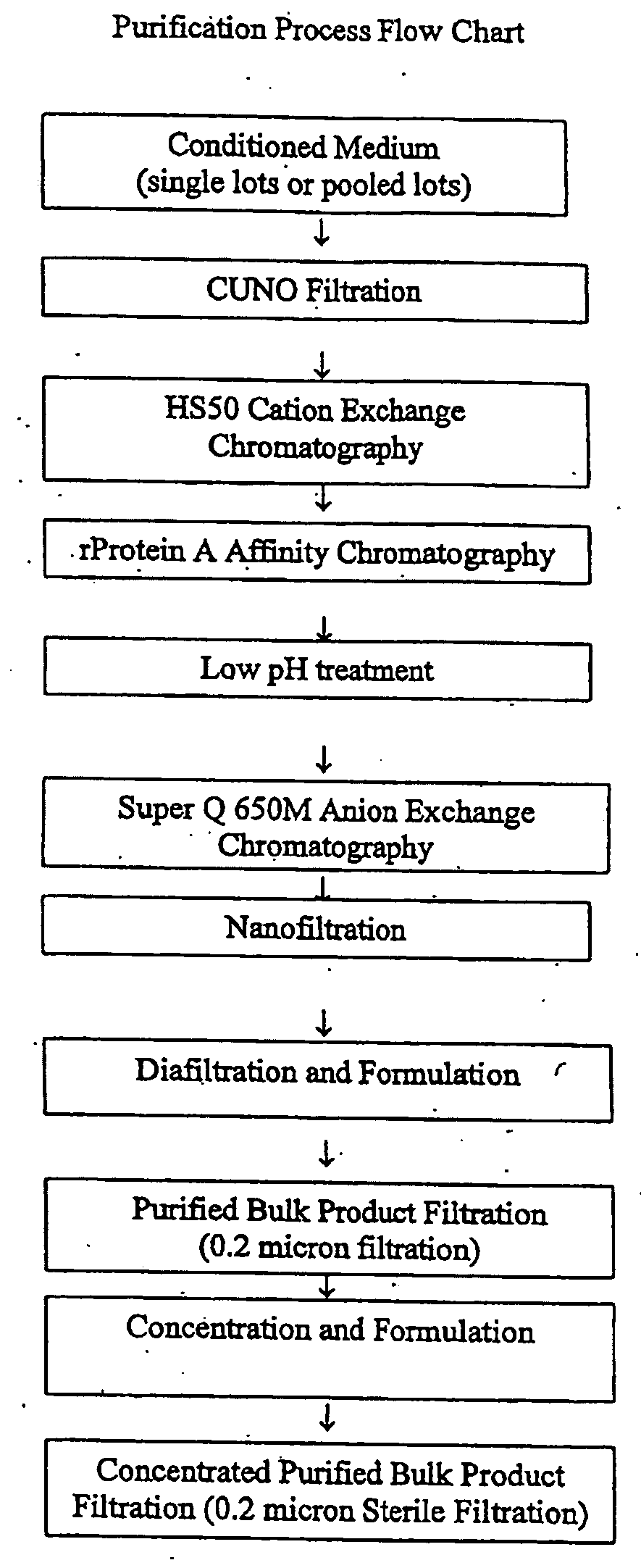
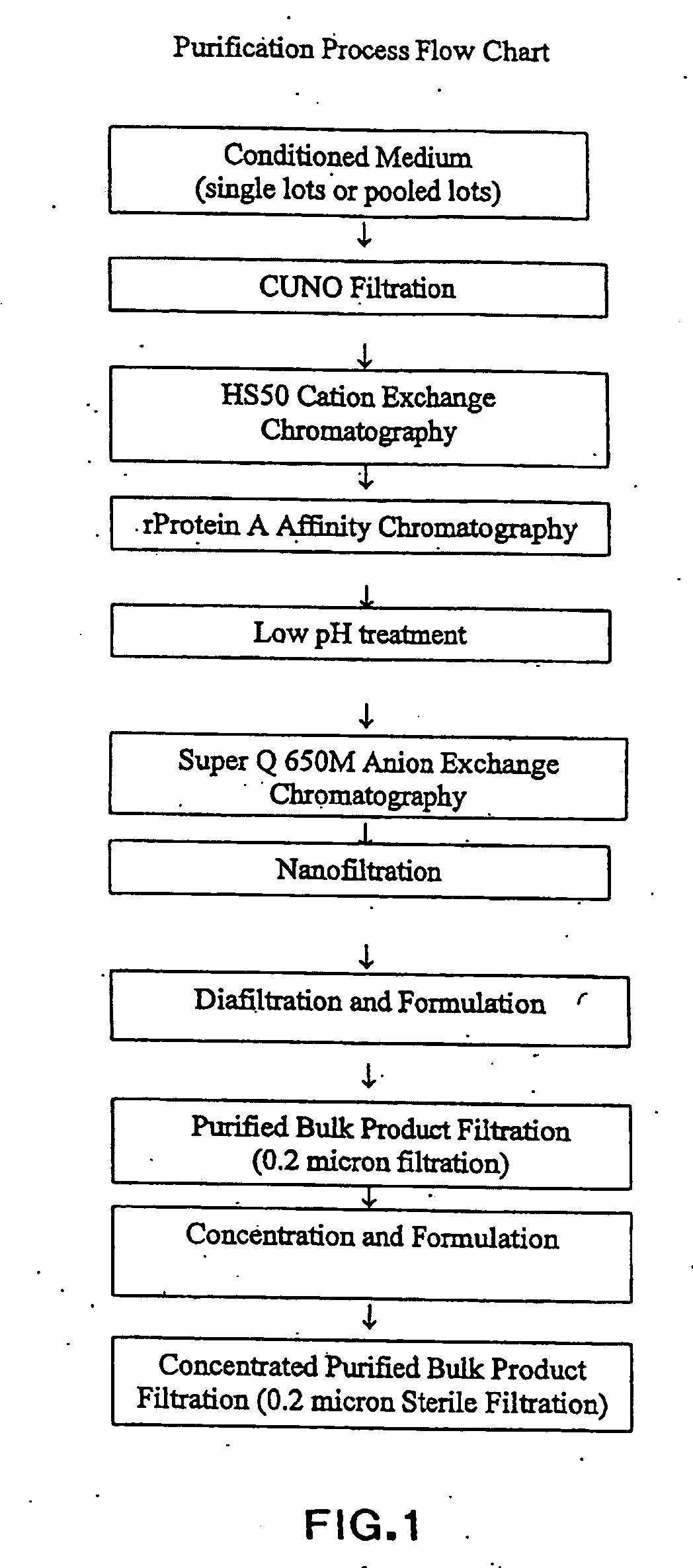
[0071]The liquid formulations of the present invention provide a ready-to-use preparation of an antibody or antibody fragment that immunospecifically binds to integrin αVβ3 for administering to a subject without having to reconstitute the preparation accurately and aseptically and waiting for a period of time until the solution clarifies before administering the formulation to the subject. It simplifies the procedure of administering the formulation to a subject for a healthcare professional. Furthermore, due to its high stability during the storage, the formulations of the present invention can contain an antibody or antibody fragment that immunospecifically binds to integrin αVβ3 at concentrations in the range of about 15 mg / ml to about 300 mg / ml without causing an adverse effect on the biological activities of the antibody or antibody fragment due to protein aggregation and / or fragmentation during a prolonged storage. Such stability not only ensures the efficacy of the antibodies...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com