Anti-beta 1 integrin humanized antibody, and pharmaceutical composition for treating cancer, comprising same
A technology of integrin and monoclonal antibody, applied in the direction of drug combination, antibody medical ingredients, medical preparations containing active ingredients, etc., can solve problems such as unmet needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1. Improvement and humanization of cancer cell killing ability of P5
[0083] In order to develop a humanized antibody with improved ability to kill cancer cells compared to P5 (Kim MY et al. J Biomed Res, 2016, 30(3): 217-24), the present inventors conducted the following experiments.
[0084] In order to improve P5, mutations were introduced in 4 FRs of the heavy chain variable region (HFR1, HFR2, HFR3, HFR4) and 4 FRs of the light chain variable region (LFR1, LFR2, LFR3, LFR4) in the following manner.
[0085] 1) Specifically, HFR1 uses IGHV7-4-1*03, HFR2 uses IGHV4-30-4*06, HFR3 uses IGHV1-69-2*01, HFR4 uses the sequence of IGHJ6*01, considering the physicochemical properties of amino acids Similarities and differences, parental antibody was substituted with other amino acids. The amino acids replaced by this method are I20V, T25S, S30T in IGHV7-4-1*03; R40H, H43K in IGHV4-30-4*06; K66R, A67V, F69I in IGHV1-69-2*01 , S75T, N76D, S79Y, Q81E, T83R, S87T; and...
Embodiment 2
[0098] Example 2. Complete antibody conversion and expression / purification of GP5 clones
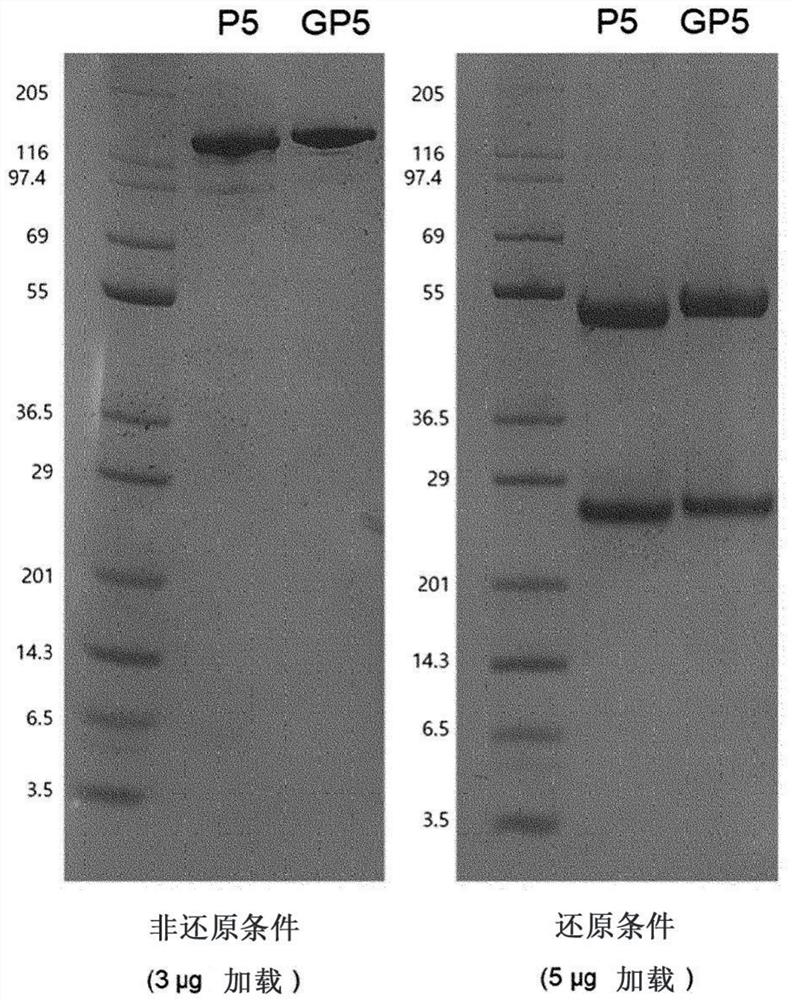
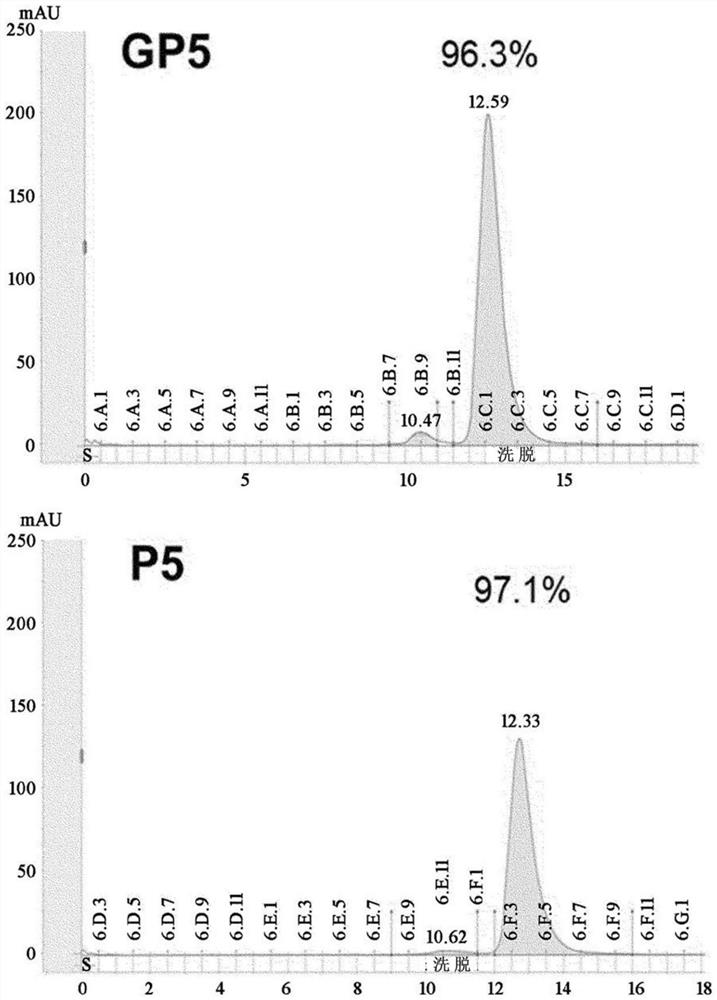
[0099] The DNA of the GP5 variable region developed in Example 1 was synthesized into a scFv format (Cosmogenetech, Korea), and converted into a full antibody (full IgG) using the PCR method. First, from a pUC vector (Cosmogenetech, Korea) including scFv, V H 、C H and V L 、C KPrimer combinations, sections of the variable and constant regions of the heavy and light chains were obtained by PCR. For the variable region and constant region of the obtained antibody, PCR was performed using the HC and LC primer combinations in Table 2 below to obtain the heavy chain and light chain of GP5. The heavy chain was treated with EcoRI and NotI (New England Biolab, UK) enzymes, and ligated to the same restriction enzyme-treated pCMV vector (Thermo Fisher SCIENTIFIC, USA), which is a vector for animal cell expression. And, the light chain was treated with XbaI (New England Biolab, UK) enzyme, and ...
Embodiment 3
[0105] Example 3. Analysis of the binding ability of GP5 monoclonal antibody to β1 integrin
[0106] The direct ELISA method confirmed the binding ability of the GP5 monoclonal antibody prepared in Example 2 above to β1 integrin. Since the GP5 monoclonal antibody is a humanized antibody, and P5 is a mouse antibody, a peroxidase labeling kit (Peroxidase Labeling)-NH 2 (Dojindo, Japan) labeled each antibody with HRP for direct comparison of binding. In direct ELISA, recombinant human β1 integrin (Sino bio, China) and recombinant mouse β1 integrin (MyBioSource, USA) were diluted at 1 μg / ml in 50 μl PBS, and placed in a 96-well immunoplate (Corning, USA), stored overnight at 4°C for adsorption. After reacting with buffer containing 3% bovine serum albumin (Milipore, USA) for 1 hour at 37°C, dilute to serial concentrations (0.01, 0.03, 0.1, 0.3, 1, 3, 100, 300, 1000 nM) each HRP-labeled antibody was treated at 50 μl per well. After reacting at 37° C. for 2 hours, after the anti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com