A kind of compound lidocaine cream medicinal composition and preparation method thereof
A technology of compound lidocaine cream and lidocaine cream, which is applied in the field of medicine, can solve problems such as easy moisture absorption and difficult storage, and achieve the effects of reducing alkalinity, convenient medication, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Embodiment 1 The preparation of compound lidocaine cream pharmaceutical composition
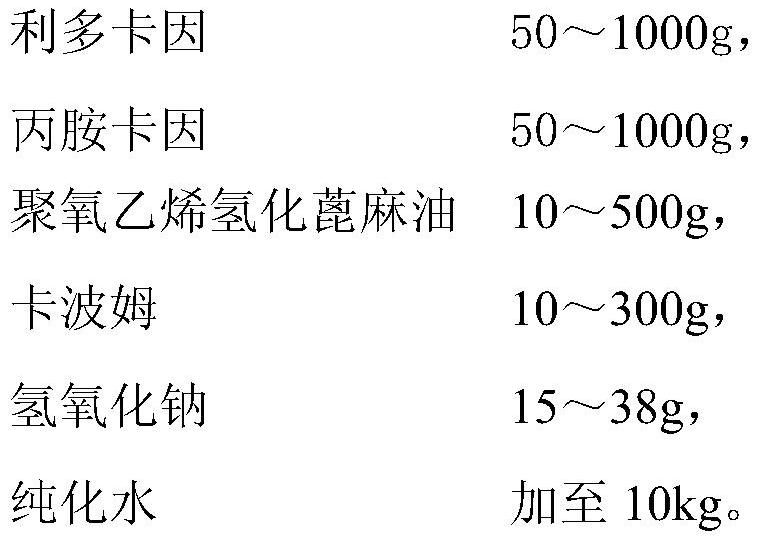
[0045] Prescription composition (preparation of 10kg cream):
[0046]
[0047] Preparation:
[0048] (1) Water phase preparation: Weigh Carbomer 974P according to the prescription amount, and measure purified water according to the prepared 1.2% Carbomer 974P aqueous solution, slowly add Carbomer 974P to the purified water, stir for more than 1 hour to dissolve completely Aqueous phase is obtained;
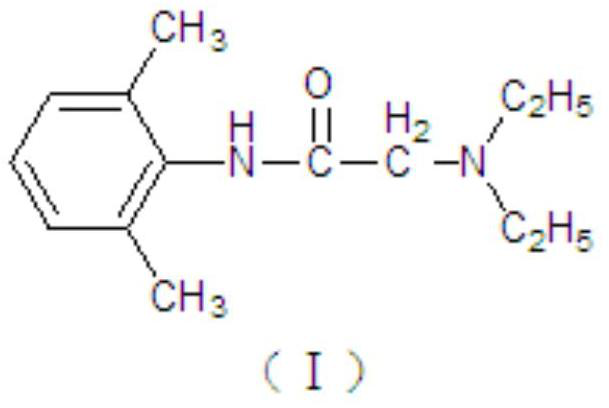
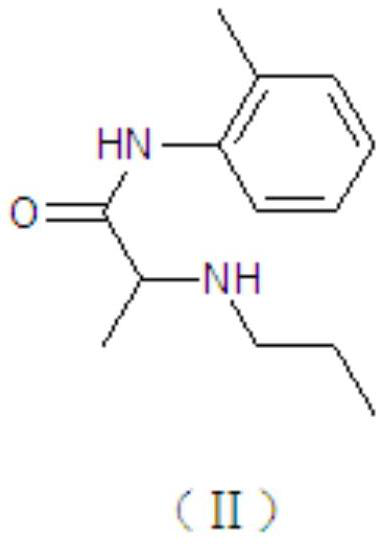
[0049] (2) Oil phase preparation: take lidocaine and prilocaine according to the prescription in the main pot, stir and mix evenly at 40-50°C, melt to a transparent liquid, and obtain lidocaine-prilocaine eutectic; The prescription amount of polyoxyethylene 40 hydrogenated castor oil is completely melted at 60°C, then added to the eutectic, and stirred evenly to prepare the oil phase for later use;
[0050] (3) Prepare 10% sodium hydroxide solution with purified water according to the...
Embodiment 2
[0059] Embodiment 2 Preparation of Compound Lidocaine Cream Pharmaceutical Composition
[0060] Prescription composition (preparation of 45kg cream):
[0061]
[0062] Preparation:
[0063] (1) Water phase preparation: slowly add the prescribed amount of Carbomer 940 into 18525 g of purified water, stir for more than 1 hour to completely dissolve to obtain the water phase;
[0064] (2) Oil phase preparation: take lidocaine and prilocaine according to the prescription in the main pot, stir and mix evenly at 40-50°C, melt to a transparent liquid, and obtain lidocaine-prilocaine eutectic; Recipe amount of polyoxyethylene 80 hydrogenated castor oil is completely melted at 60°C, then added to the eutectic, stirred evenly to prepare the oil phase for later use;
[0065] (3) Prepare 10% sodium hydroxide solution with purified water according to the prescription quantity;
[0066] (4) Emulsification: Measure the remaining amount of purified water and add it to the oil phase prep...
Embodiment 3
[0068] Example 3 Preparation of Compound Lidocaine Cream Pharmaceutical Composition
[0069] Prescription composition (preparation of 10kg cream):
[0070]
[0071] Preparation:
[0072] (1) Water phase preparation: weigh Carbomer 934 according to the prescription amount, and measure purified water according to the prepared 0.5% Carbomer 934 aqueous solution, slowly add Carbomer 934 to the purified water, stir for more than 1 hour to dissolve completely Aqueous phase is obtained;
[0073] (2) Oil phase preparation: take lidocaine and prilocaine according to the prescription in the main pot, stir and mix evenly at 40-50°C, melt to a transparent liquid, and obtain lidocaine-prilocaine eutectic; Recipe amount of polyoxyethylene 80 hydrogenated castor oil is completely melted at 60°C, then added to the eutectic, stirred evenly to prepare the oil phase for later use;
[0074] (3) Prepare 5% sodium hydroxide solution with purified water according to the prescription quantity; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com