Novel topiramate compositions and an escalating dosing strategy for treating obesity and related disorders
a technology of compositions and topiramate, applied in the direction of drug compositions, antibacterial agents, metabolic disorders, etc., can solve the problems of obesity, shortness of breath, and substantial increase in the risk of morbidity from hypertension, so as to reduce the side effects of bupropion and reduce the exposure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
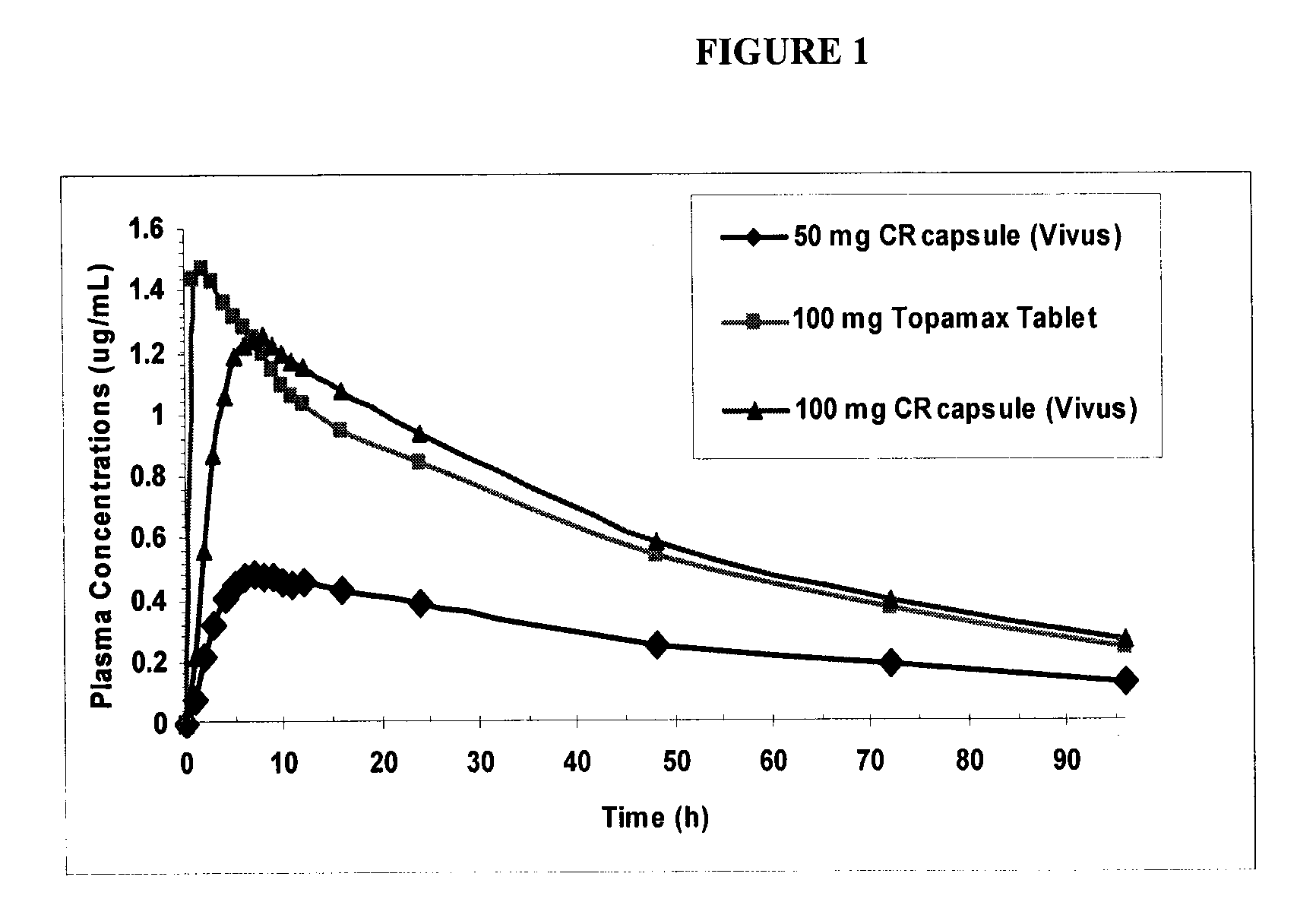
[0151]The controlled release topiramate beads were made using an extrusion spheronization process to produce a matrix core comprised of topiramate: 40.0% w / w; microcrystalline cellulose, Avicel® PH102: 56.5% w / w; and Methocel™ A15 LV: 3.5% w / w. The topiramate cores were then coated with ethyl cellulose: 5.47% w / w and Povidone K30: 2.39% w / w.
[0152]The final coated beads % w / w were as follows:
Ingredient% w / wtopiramate36.85microcrystalline cellulose,52.05Avicel ® PH102Methocel ™ A15 LV3.22ethyl cellulose5.47Povidone K302.39
[0153]The phentermine beads were straight immediate release drug coated onto sugar spheres. Both sets of beads were then encapsulated into one capsule.
example 2
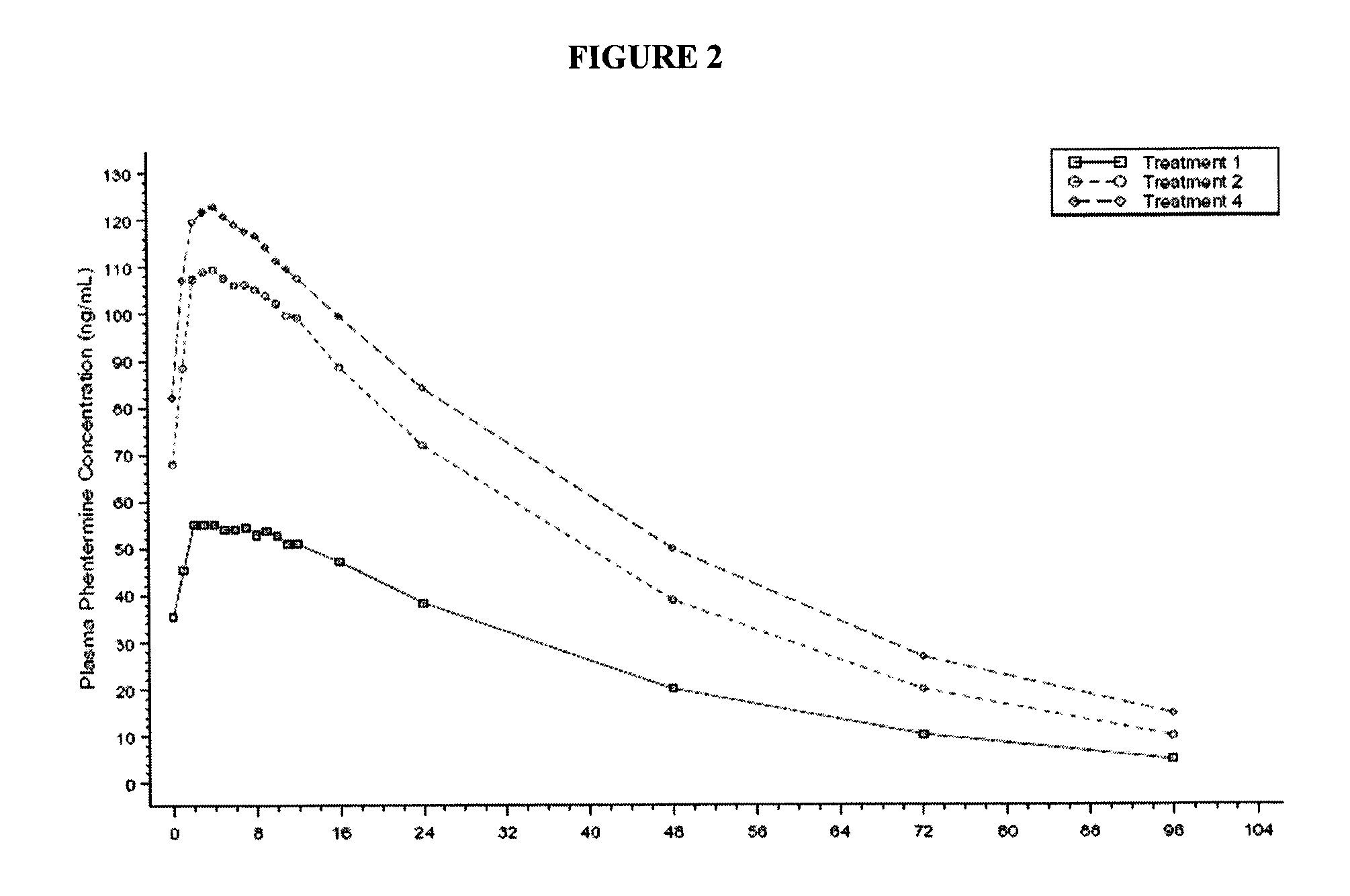
[0154]In a study comparing controlled-release formulation of topiramate according to the present invention versus immediate release topiramate (Topamax®) in combination with phentermine, the controlled release formulation of the instant invention of topiramate had a 10-15% lower effect on phentermine exposure (FIG. 2).
[0155]The mean and statistical comparisons for plasma phentermine PK parameters at steady state in multiple dose administrations are summarized in Table 2.
TABLE 2Arithmetic Mean (SD) and Statistical Comparison of Pharmacokinetic Parameters forPlasma PhentermineMean ± SDTreatment 2 Versus Treatment 4PharmacokineticTreatment 2Treatment 490% ConfidenceParameters(N = 13)(N = 12)Intervals% Mean RatioAUC0-tau (ng * hr / mL)2250 ± 563 2530 ± 644 (75.6, 105.3)89.2AUC0-96 (ng * hr / mL)4640 ± 15705550 ± 1960(67.1, 105.0)84.0AUC0-t (ng * hr / mL)4640 ± 15705550 ± 1960(67.1, 105.0)84.0Cmax,96 (ng / mL) 114 ± 23.6 127 ± 27.6(78.8, 104.5)90.7Cmin,68 (ng / mL)9.84 ± 7.2414.6 ± 11.3(42.5, 109....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Mass flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com