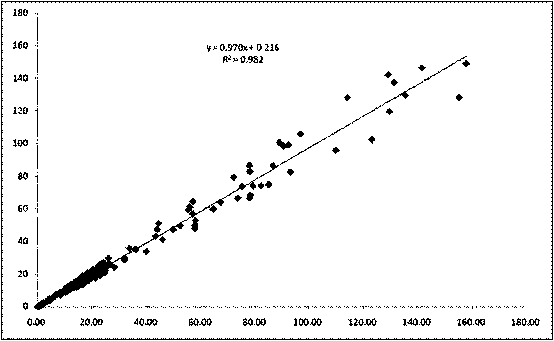
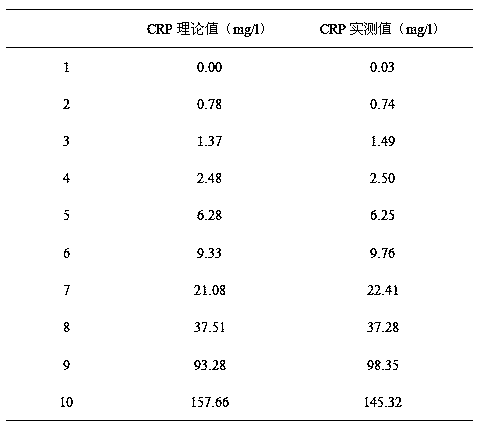
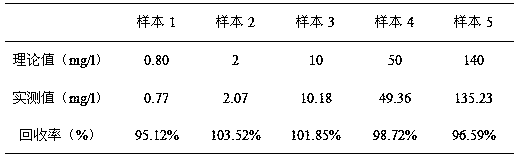
Method for improving sensitivity and linearity of latex reagent
A sensitivity and latex technology, applied in the field of medical testing, can solve the problems that the accuracy and precision cannot meet the requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1 The preparation of the C-reactive protein detection kit of mixed size latex microspheres
[0081] The main raw materials involved in the kit prepared by adopting the latex microspheres of mixed sizes and sizes of the present invention are as follows:
[0082] 1. Anti-C reactive protein antibody: mouse monoclonal antibody, the titer determined by ELISA method is 1:100000; rabbit polyclonal antibody, the titer determined by ELISA method is 1:50000.
[0083] 2. Latex microspheres: the present invention only adopts polystyrene latex microspheres with carboxyl groups to test, wherein the average particle diameter of the large-diameter latex microspheres is 300-350nm, and the small-particle-diameter latex microspheres The average particle size is 50-100nm.
[0084] The preparation of the main reagent of this embodiment is as follows:
[0085] Reagent R1: 0.2M ammonium chloride buffer solution containing 0.3% PEG8000, 3% sodium chloride, 0.5% sucrose, 0.5% Tween...
Embodiment 2
[0100] The preparation of the main reagents of this embodiment is as follows:
[0101] Reagent R1: 0.2M ammonium chloride buffer containing 0.3% PEG8000, 3% sodium chloride, 0.5% sucrose, 0.5% Tween 20, 0.2% sodium azide, this reagent is a colorless and transparent solution.
[0102] Reagent R2: 0.2M ammonium chloride buffer containing 0.25% anti-C-reactive protein antibody sensitized polystyrene latex microspheres of mixed sizes and sizes, 1.5% sucrose, 0.2% sodium azide. The reagent is a milky white solution.
[0103] The specific preparation steps are as follows:
[0104] 1) Take 1ml (100mg / ml) of latex microspheres with large particle size or small particle size, wash three times with 0.2M, pH5.0 MES solution (2-morpholine ethanesulfonic acid buffer), and disperse;
[0105] 2) Add 0.1ml of 10mg / ml ethyldimethylaminopropylcarbodiimide EDAC solution freshly prepared with 0.2M, pH5.0 MES solution, and react at room temperature for 1 hour;
[0106] 3) Add 0.1ml of 100mg / ml ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com