C reactive protein detection kit
A technology for detection kits and reactive proteins, applied in the field of molecular immunology, can solve problems such as short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
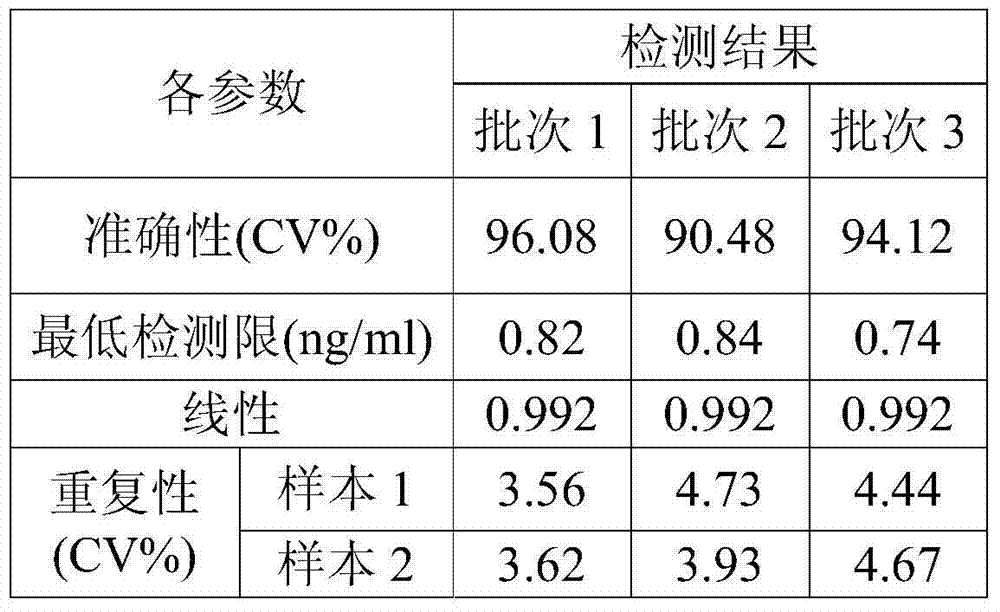
Image
Examples
Embodiment 1
[0030] Example 1: Obtaining of monoclonal antibodies
[0031] 1. Animal immunization
[0032] In order to produce CRP monoclonal antibody, 6-week-old female Balb / c mice weighing about 20 g were selected. For the first immunization, take 20-50 μg of CRP recombinant antigen (purchased from Prospec) and add complete Freund’s adjuvant for subcutaneous multi-point injection. On the 14th and 28th days, the second and third immunization doses are the same as above, plus Freund’s incomplete adjuvant Intraperitoneal injection, booster immunization 3 days before fusion, the appropriate dose is 20-50 μg, and 3 days later, spleen is taken for fusion. Generally, to prepare monoclonal antibodies of murine origin, refer to the method described in the "Antibodies" manual (Harlow and Lane, Antibodies: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor NY, pp.726, 1988) or Kohler and the technique for preparing hybridomas described by Milstein (Nature, 256:495-497, 1975). ...
Embodiment 2
[0045] Example 2. Preparation of monoclonal antibodies:
[0046] The preserved two strains of hybridoma cells were divided into 1×10 7 The concentration of cells was injected into the peritoneal cavity of BALB / c mice, and the ascites were collected 10 days later. Purify the two monoclonal antibodies separately by the following steps:
[0047] 1. The collected ascites was centrifuged at 2500 rpm, and the supernatant was taken. Add an equal volume of PBS (pH7.4) and 1 / 2 volume of saturated ammonium sulfate, and let stand at 4°C for 30 minutes;
[0048] 2. Centrifuge at 3000rpm, 4°C for 20min;
[0049] 3. Remove precipitation, add an equal volume of saturated ammonium sulfate to the supernatant, and let it stand for 30 minutes;
[0050] 4. Centrifuge at 3000rpm, 4°C for 20min;
[0051] 5. Take the precipitate, add 5ml normal saline and 5ml saturated ammonium sulfate and let stand for 30min;
[0052] 6. Centrifuge at 3000rpm, 4°C for 20min;
[0053] 7. Add 5ml of normal sal...
Embodiment 3
[0060] Embodiment 3. Preparation of detection reagent
[0061] 1. Prepare a multi-well plate coated with CRP monoclonal antibody:
[0062] a) Coating: use 0.05M carbonate buffer solution with a pH value of 9.6-9.8 to mix with an appropriate concentration of a CRP antibody (CGMCC No.8702) collected in Example 2 to prepare a coating mixture, and It was loaded on a microwell plate and coated at 4°C for 12h;
[0063] Carbonate buffer standard formula:
[0064] Anhydrous sodium carbonate 1.600g
[0065] Sodium bicarbonate 2.940g
[0066] Purified water was adjusted to 1000ml.
[0067] b) Plate washing: After diluting 20 times the concentrated washing solution (pH value 6.2-6.6) to 1 times the concentration, wash the plate twice;
[0068] 20 times concentrated lotion standard formula:
[0069]
[0070] c) Blocking: Load the blocking solution (disodium hydrogen phosphate 5.8g, sodium dihydrogen phosphate 0.59g, sodium chloride 8.0g, goat serum 100ml, 0.5ml Proclin-300, purif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com