Method for catalyzing dynamic kinetic resolution of arylamine via racemization catalyst
A racemization catalyst, a technology for catalyzing arylamines, applied in organic chemistry methods, organic chemistry, chemical recovery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Add p-chlorophenol, p-chlorophenyl valerate, dicyclohexylcarbodiimide and 4-dimethylaminopyridine with a molar ratio of 1:1:1:0.03, stir for 7 hours, filter, and dry the filtrate Bathing, concentrating, and passing through the column to obtain p-chlorophenyl propionate as an acyl donor for subsequent use;
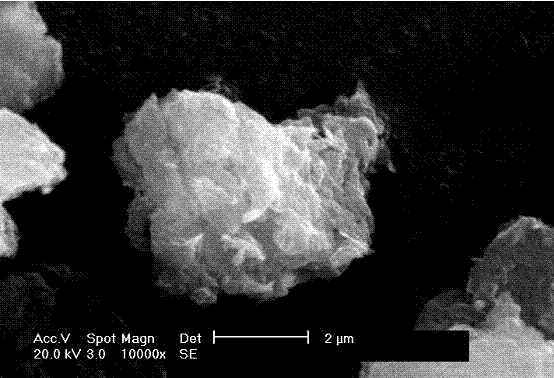
[0024] 2) Under vigorous stirring, add 10 mL of an aqueous solution of magnesium chloride and aluminum chloride with a molar percentage of 3:1 to 200 mL of 3 mol / L sodium hydroxide solution, and stir for 10 minutes under nitrogen protection. Centrifuge, wash twice with deionized water, and disperse in 40 mL of deionized water. Placed in a 60 mL stainless steel reactor, at 100 o C hydrothermal treatment for 10 hours to prepare chloride ion intercalated hydrotalcite. Add it to 100 mL of 2 mol / L sodium lauryl sulfate aqueous solution, 80 o C for 12 hours at reflux. Cooling, centrifugation, filtration, washing with water, washing with acetone, 80 o Dried for 4 ho...
Embodiment 2
[0028] 1) Add p-chlorophenol, n-valeric acid, dicyclohexylcarbodiimide, and 4-dimethylaminopyridine in a molar ratio of 1: 2: 2: 0.05, stir for 7 hours, filter, dry the filtrate, concentrate, pass column, obtain p-chlorophenyl valerate as an acyl donor for subsequent use;
[0029] 2) Under vigorous stirring, add 15 mL of an aqueous solution of magnesium chloride and aluminum chloride with a molar percentage of 4:1 to 250 mL of 3 mol / L sodium hydroxide solution, and stir for 30 minutes under nitrogen protection. Centrifuge, wash with deionized water three times, and disperse in 40 mL of deionized water. Placed in a 60 mL stainless steel reactor, at 100 o C Hydrothermal treatment for 16 hours prepared chloride ion intercalated hydrotalcite. Add it to 100 mL of 2 mol / L sodium lauryl sulfate aqueous solution, 80 oC for 12 hours at reflux. Cooling, centrifugation, filtration, washing with water, washing with acetone, 80 o Dried for 4 hours at C to obtain dodecyl sulfate interc...
Embodiment 3
[0033] 1) Add p-chlorophenol, n-valeric acid, dicyclohexylcarbodiimide and 4-dimethylaminopyridine in a molar ratio of 1:2:2:0.05, stir for 3 hours, filter, dry the filtrate, concentrate, Go through the column to obtain p-chlorophenyl valerate as an acyl donor for subsequent use;
[0034] 2) Under vigorous stirring, add 10 mL of an aqueous solution of magnesium chloride and aluminum chloride with a molar percentage of 3:1 to 200 mL of 3 mol / L sodium hydroxide solution, and stir for 10 minutes under nitrogen protection. Centrifuge, wash twice with deionized water, and disperse in 40 mL of deionized water. Placed in a 60 mL stainless steel reactor, at 100 o C hydrothermal treatment for 10 hours to prepare chloride ion intercalated hydrotalcite. Add it to 100 mL of 2 mol / L sodium lauryl sulfate aqueous solution, 80 o C for 12 hours at reflux. Cooling, centrifugation, filtration, washing with water, washing with acetone, 80 o Dried for 4 hours at C to obtain dodecyl sulfate i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com