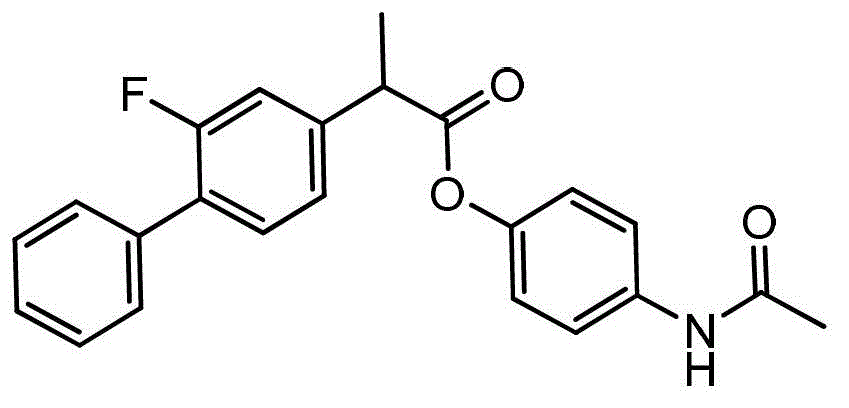
Flurbiprofen acetaminophen ester solid dispersion and preparation method thereof
A technology of acetaminophen ester and solid dispersion, which can be used in pharmaceutical formulations, drug delivery, non-central analgesics, etc., and can solve problems such as poor solubility and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 5 g of flurbiprofen acetaminophen ester, dissolve it in ethanol solution, heat 20 g of PEG4000 to 80° C. to form a molten state, slowly drop the flurbiprofen acetaminophen ester alcohol solution into the carrier material, stir, and volatilize Dry ethanol and quickly move to -20°C to solidify for 4h. The resultant was taken out, pulverized, and dried. That is, solid dispersion powder is obtained. The solid dispersion of the inventive process has an in vitro cumulative dissolution rate of 93.5±3.1% (n=6) within 45 minutes. Six samples were measured in parallel.
Embodiment 2
[0025] Take flurbiprofen acetaminophen ester 5g, dissolve in appropriate ethanol, dissolve PVP-S630 in ethanol, heat at 60°C, mix the above two solutions evenly, stir for 2 hours, evaporate the ethanol, and quickly move to Curing at -20°C for 8 hours. The resultant is taken out, pulverized, and dried to obtain a solid dispersion powder. The solid dispersion of the inventive process has an in vitro cumulative dissolution rate of 94.4±3.6% (n=6) within 45 minutes. Six samples were measured in parallel.
Embodiment 3
[0027] Take flurbiprofen acetaminophen ester 5g, kollidon VA64 20g, mix evenly, melt and extrude at 160°C, the mixture of drug and carrier is fully mixed in the extruder, dispersed, solidified, crushed and sieved to obtain Solid dispersion. The solid dispersion of the inventive process has an in vitro cumulative dissolution rate of 88.4±2.8% (n=6) within 45 minutes. Six samples were measured in parallel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com