Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
191 results about "Pharmaceutical manufacturing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug manufacturing is the process of industrial-scale synthesis of pharmaceutical drugs by pharmaceutical companies. The process of drug manufacturing can be broken down into a series of unit operations, such as milling, granulation, coating, tablet pressing, and others.
System and method for manufacturing
ActiveUS8298054B2Increase capacityLess expensiveDomestic stoves or rangesSpace heating and ventilationFir systemOn board
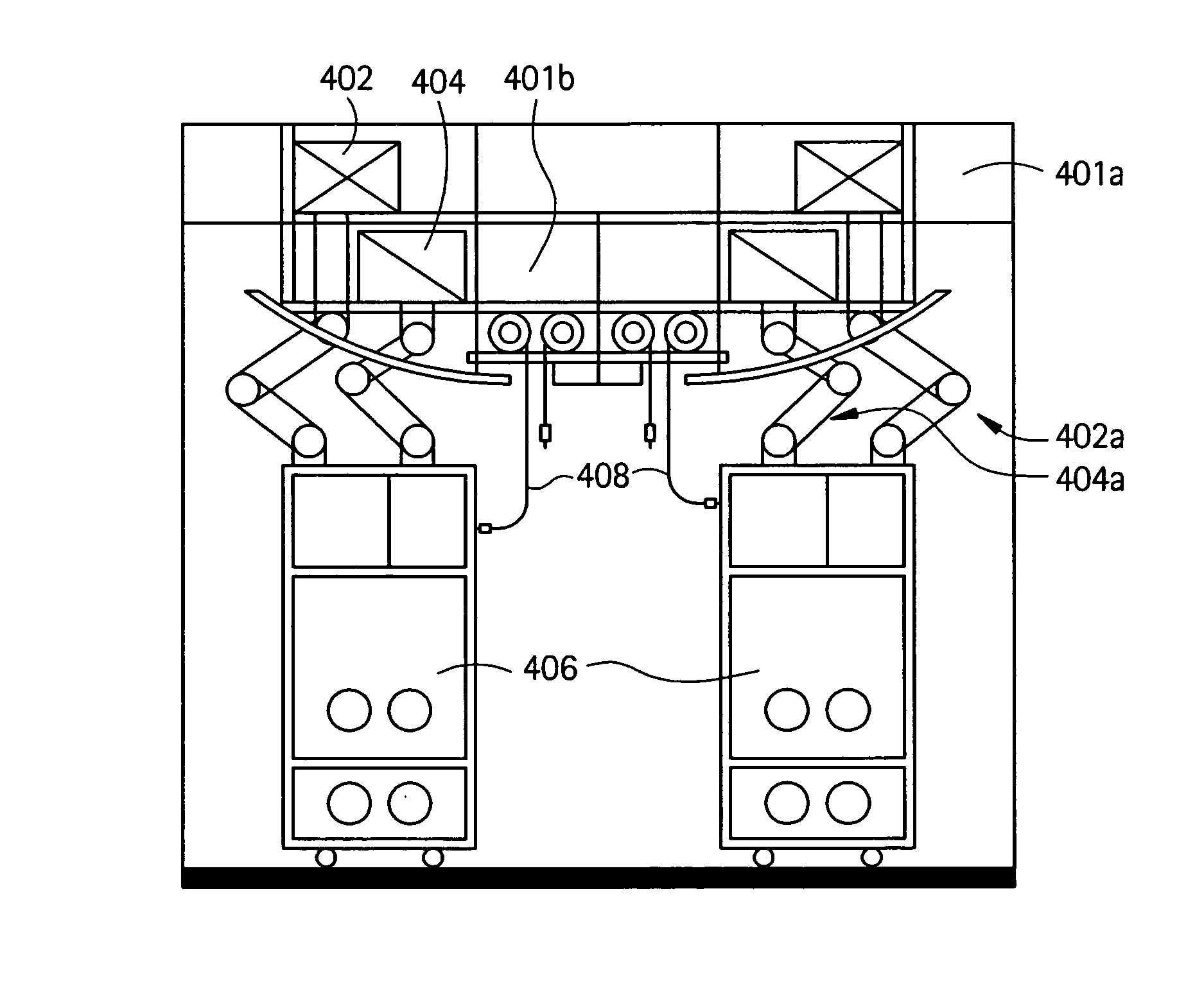
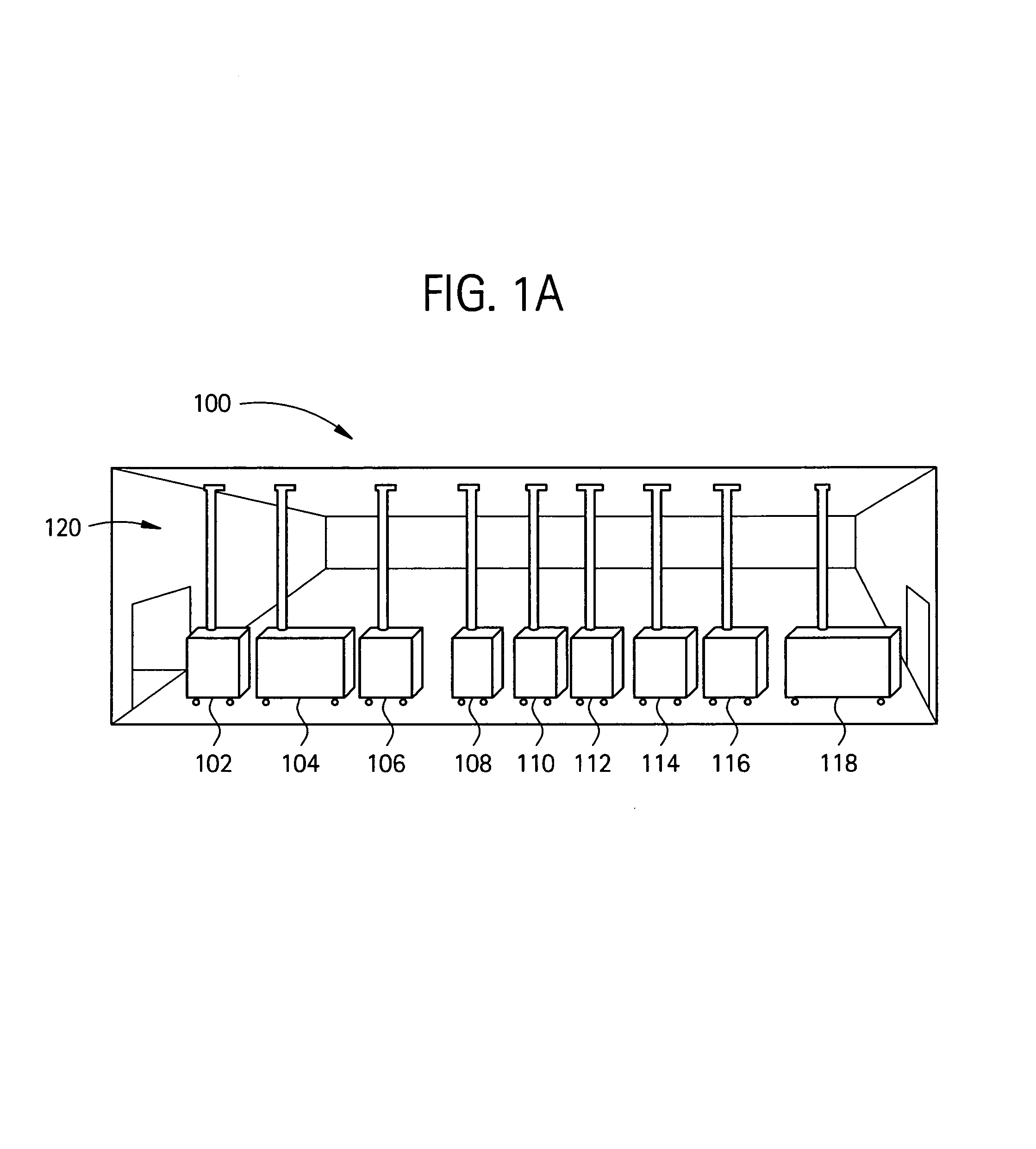
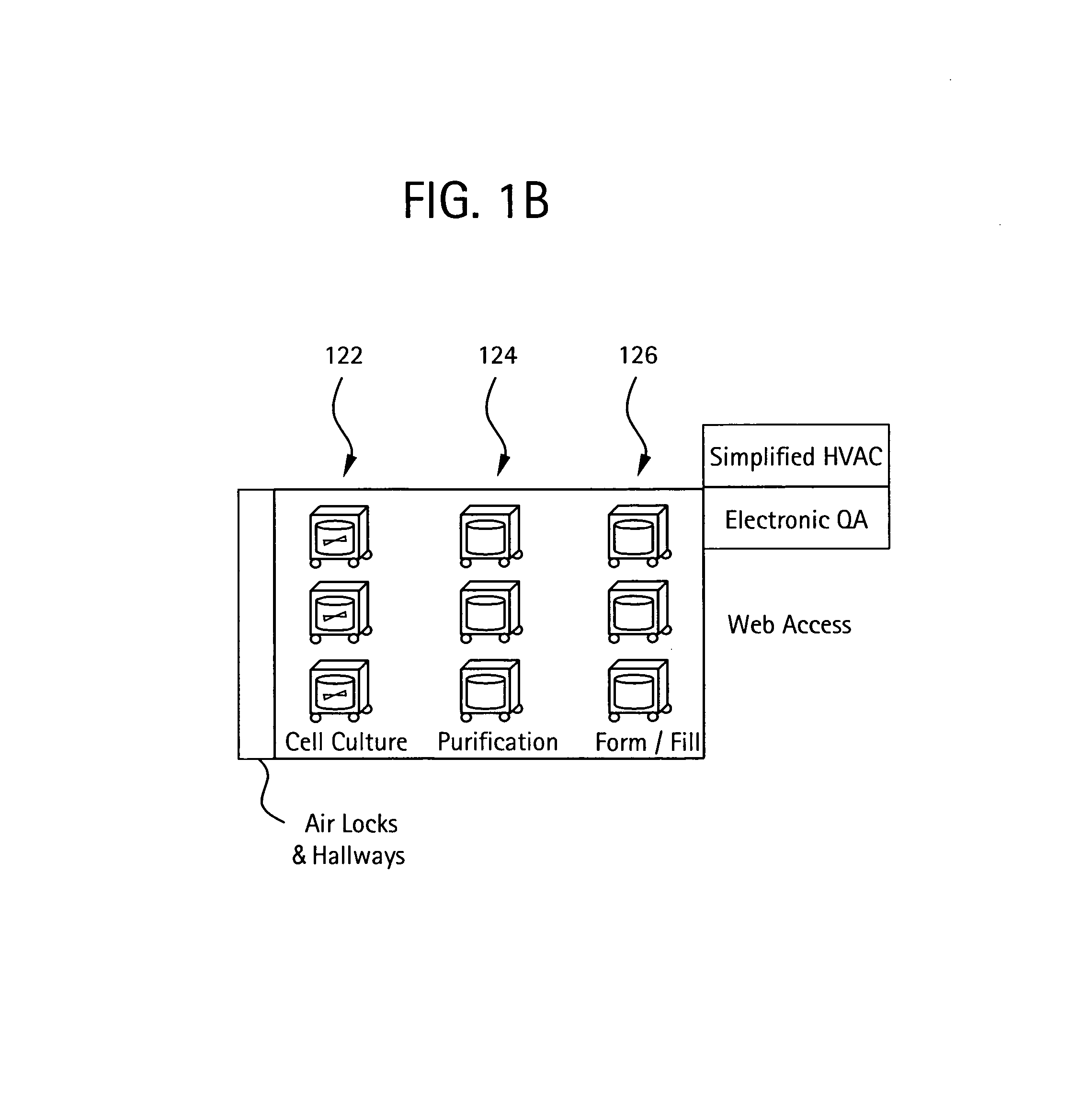
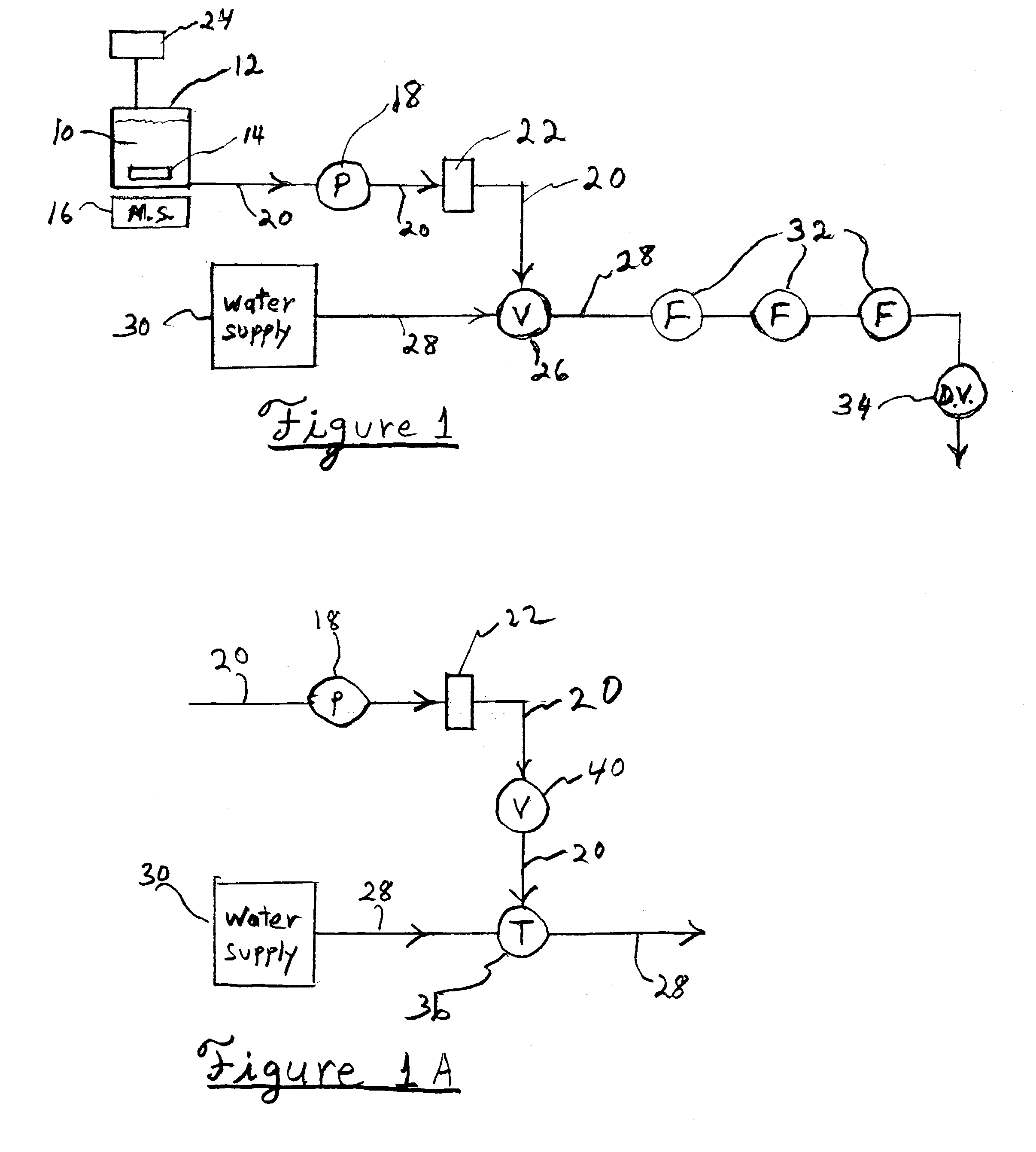
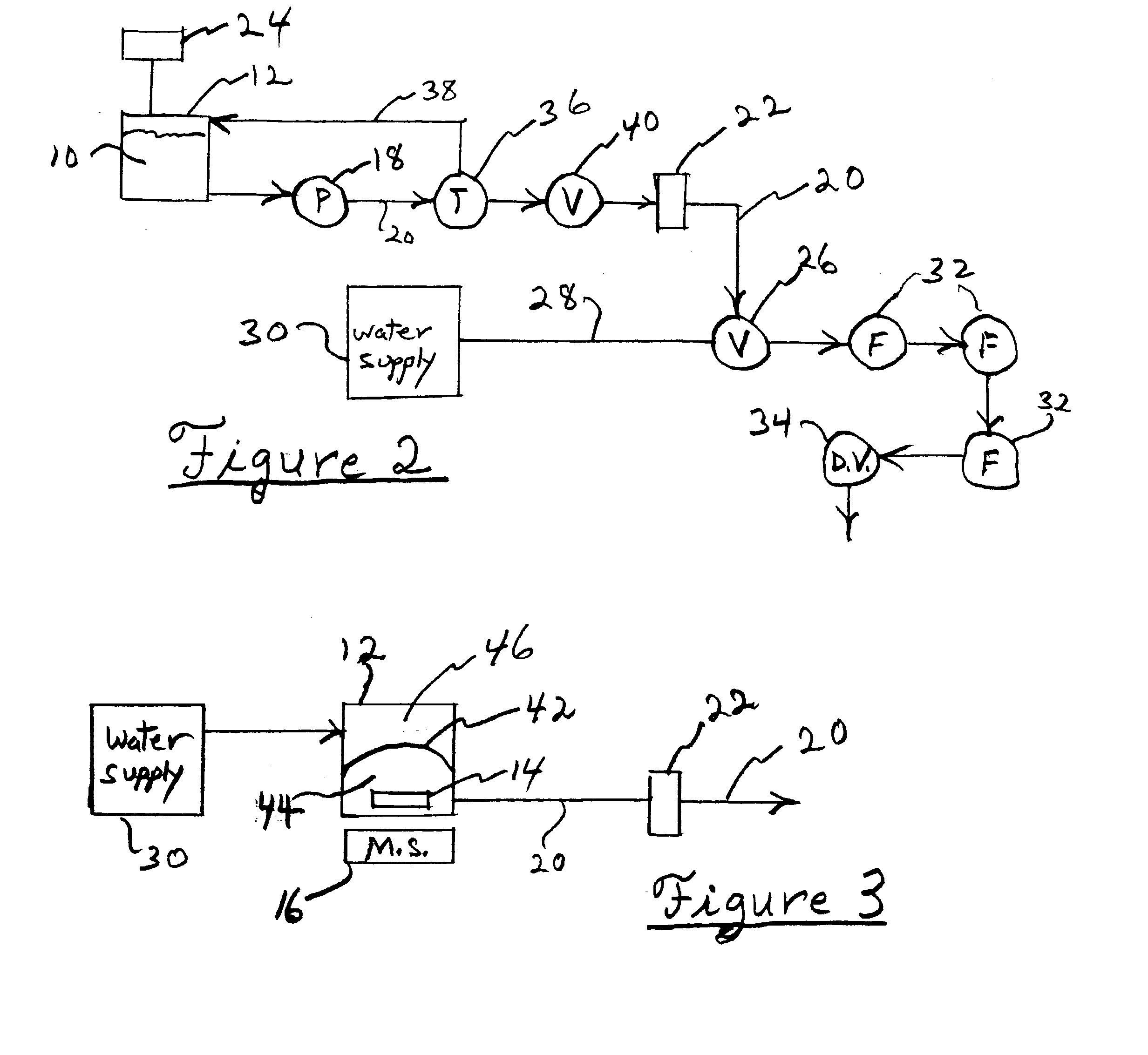
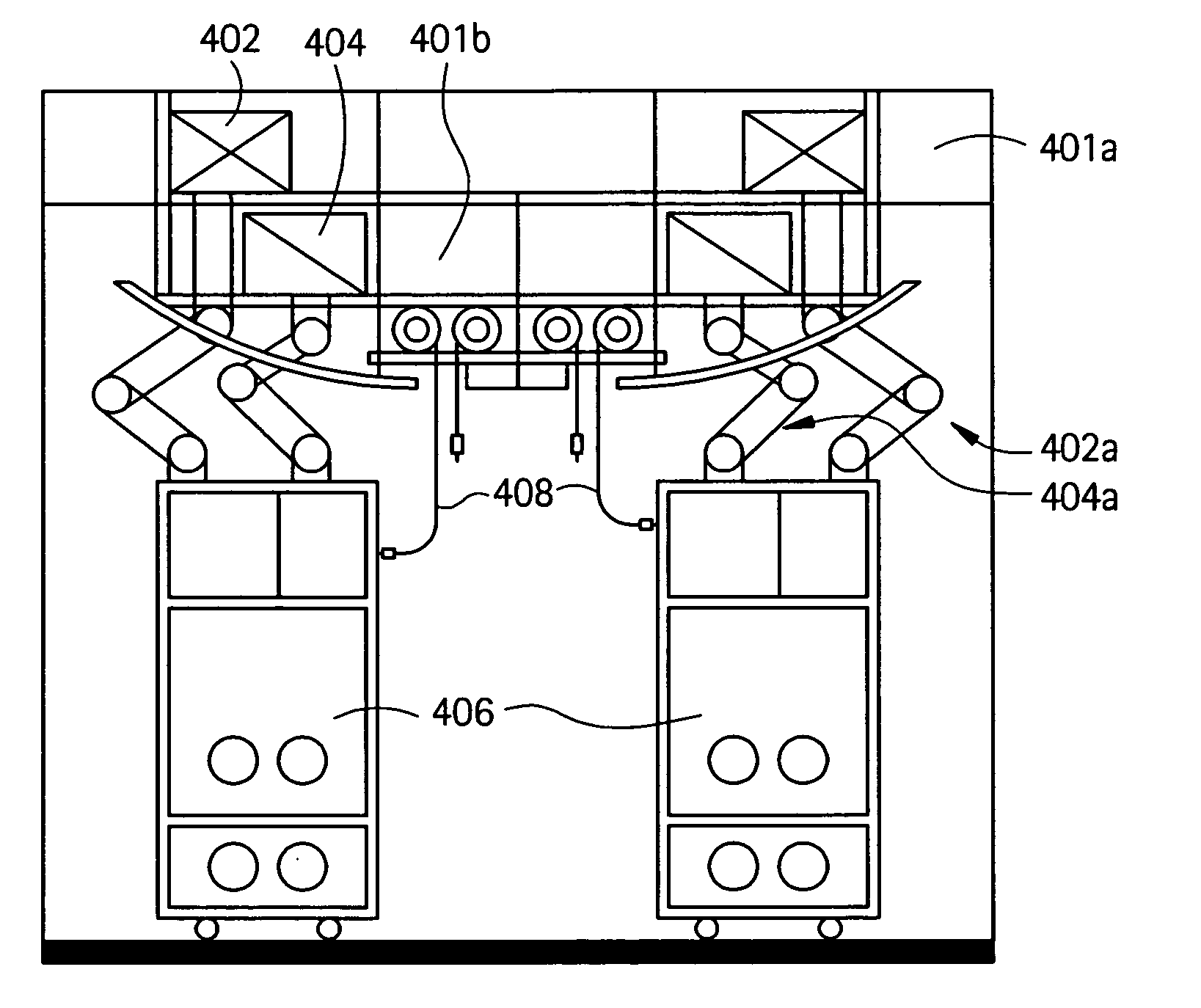
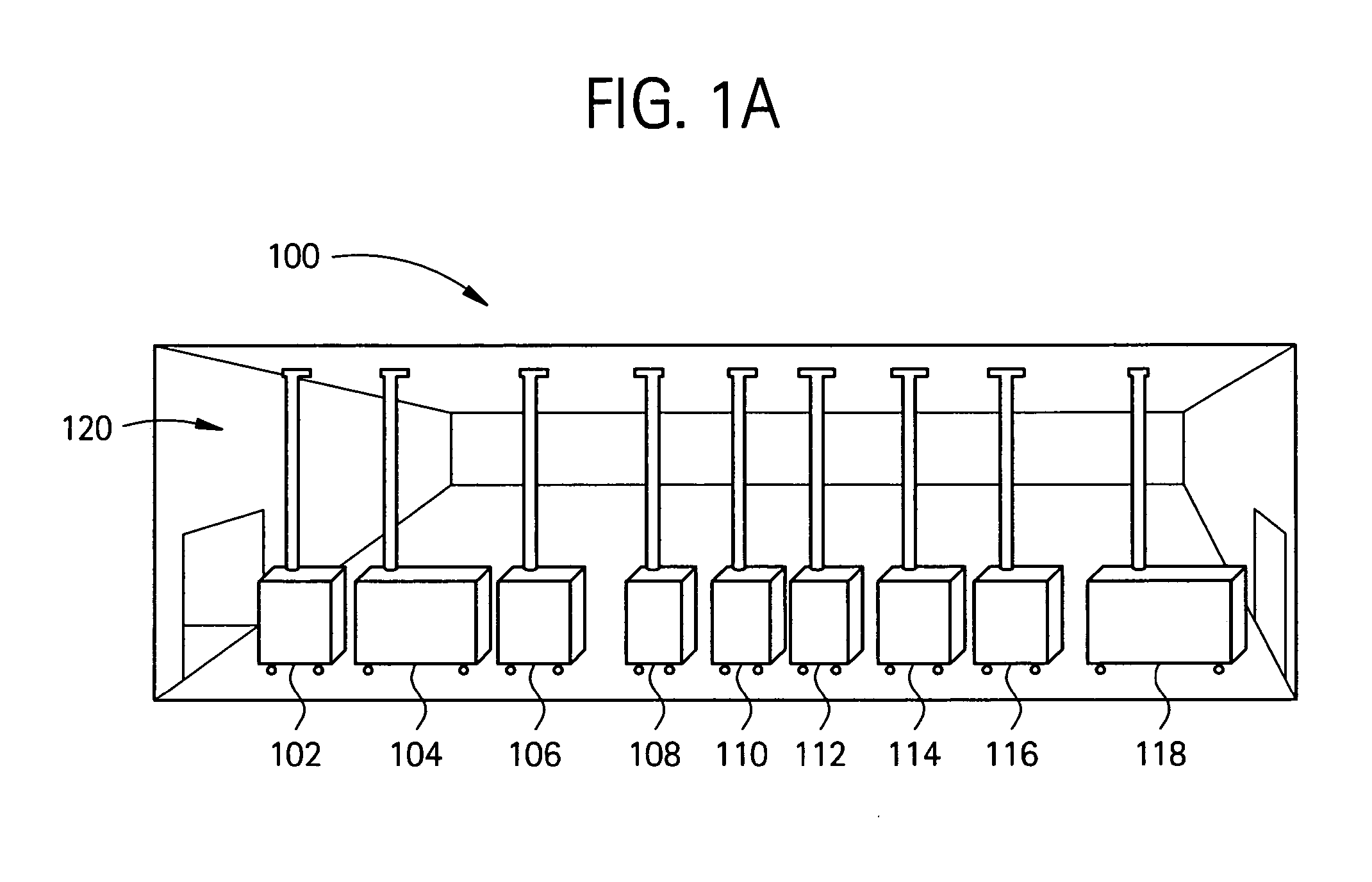
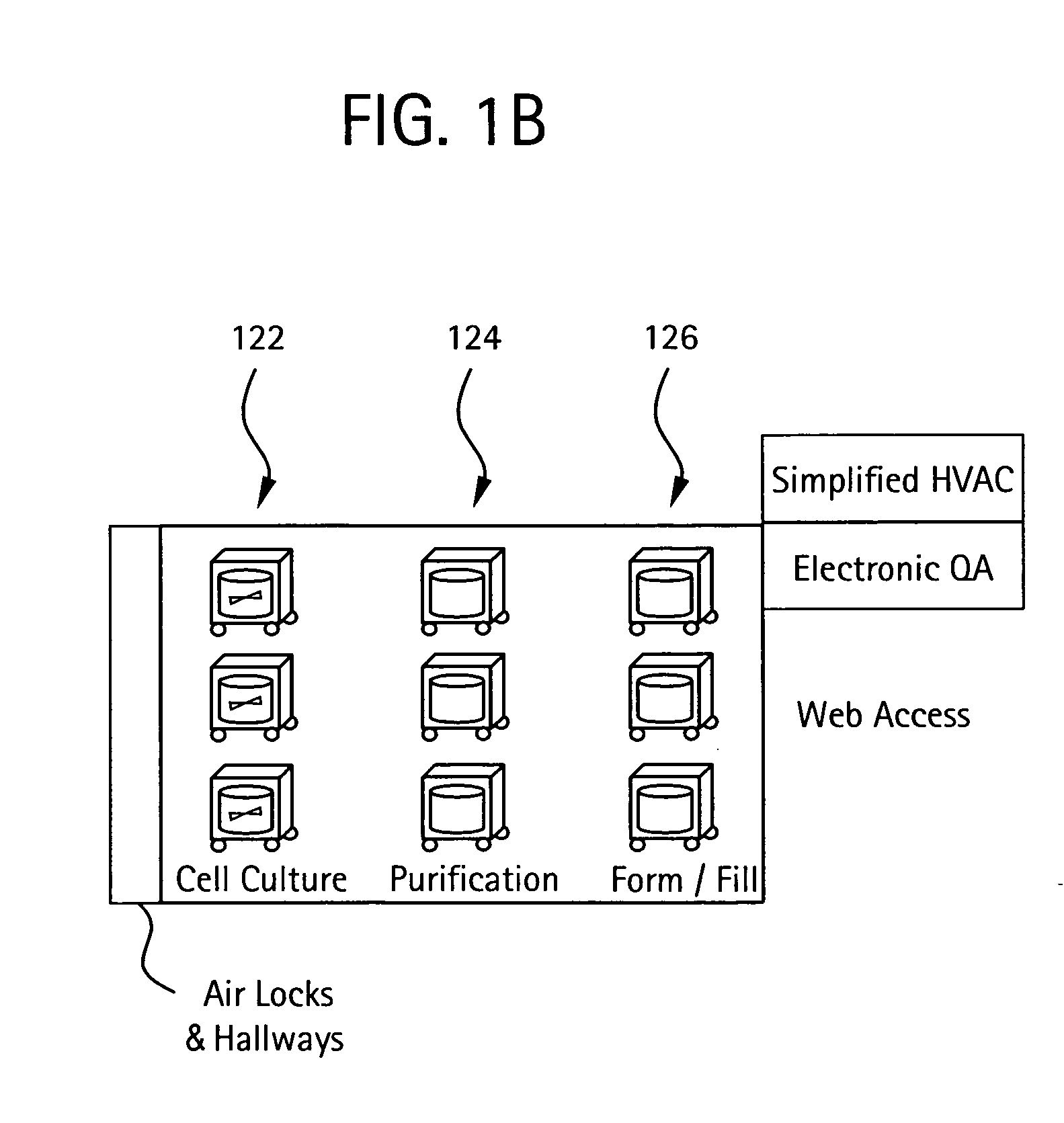
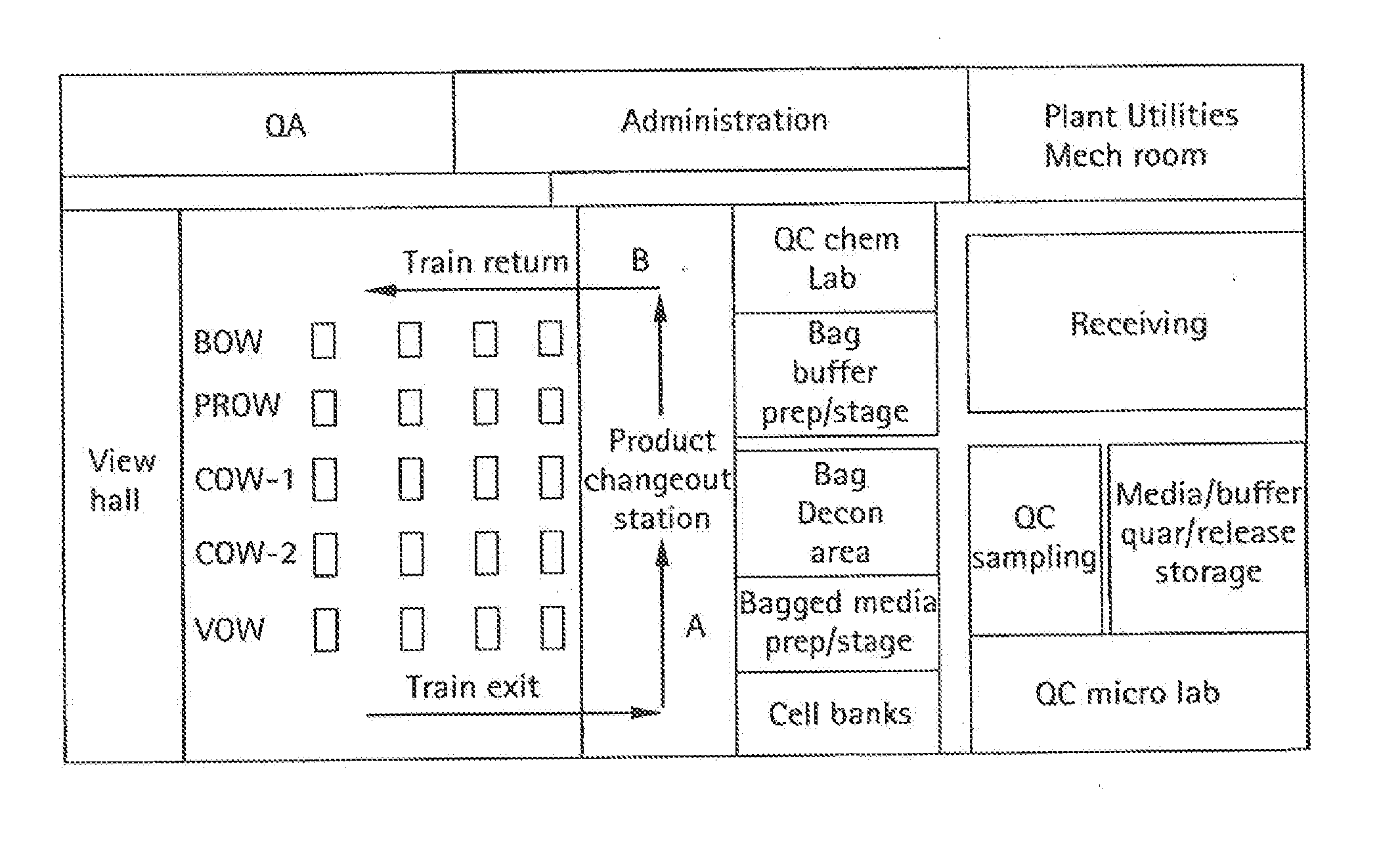
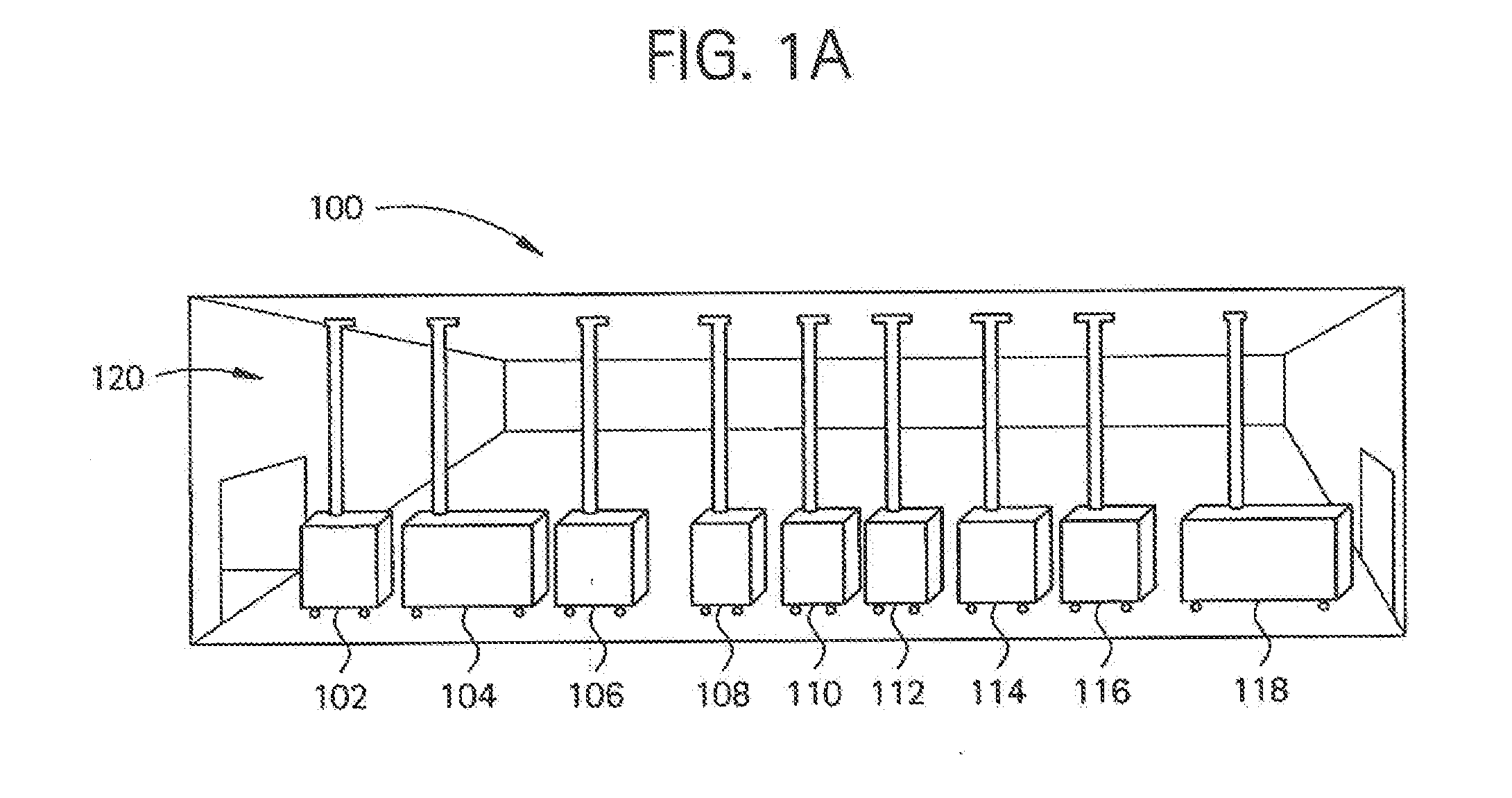
Embodiments of the present invention are directed to a customizable bio-manufacturing system which includes a manufacturing space having a first air handling system for providing supply air and a second air handling system for handling exhaust air, the supply air system being optionally provided with at least one of filtration, heating, cooling and or humidity control and a plurality of portable modules provided within the manufacturing space. At least one module having an interior capable of being interconnected with another module interior and each module's interior includes one or more components to perform at least one specific task of a biological, chemical, and / or pharmaceutical manufacturing process. At least one module includes an on-board environmental control system for controlling an environment within the module and a connection means for interconnecting the module interior with another module interior. The system also includes a central controller operating to at least perform one or more of operation and information collection for the operation of at least one of the system and one or more modules.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
Removal of biofilm from surfaces
InactiveUS20030121532A1The process is simple and effectiveLess complexInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsBiofilmPharmaceutical manufacturing
The disclosure encompasses composition, method and apparatus that provide improved and convenient removal of biofilm from surfaces. Surfaces cleaned according to the invention comprise the inner wall of conduits such as those employed in dental clinics, food and pharmaceutical manufacturing.
Owner:COUGHLIN ROBERT W +3
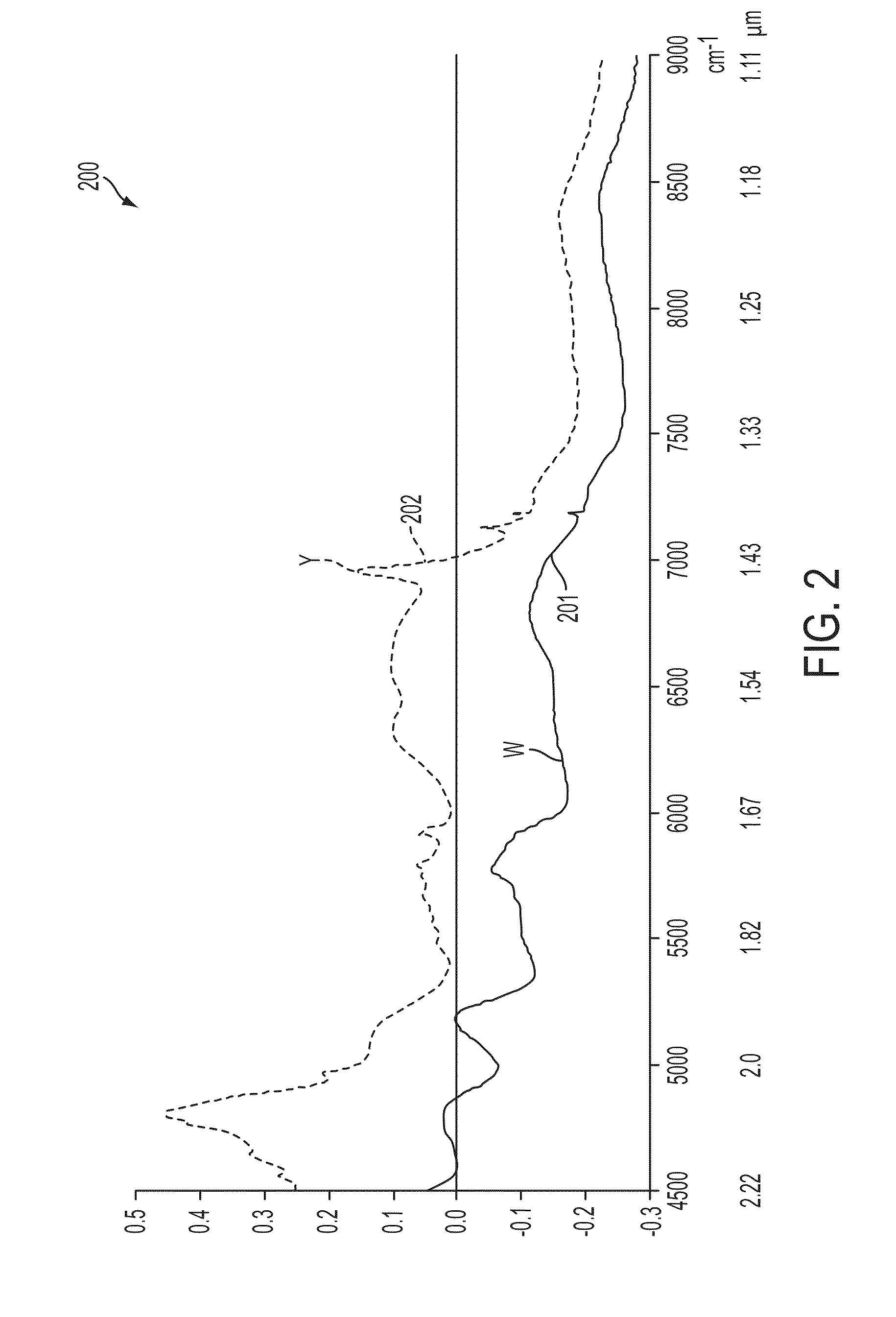
Short-wave infrared super-continuum lasers for detecting counterfeit or illicit drugs and pharmaceutical process control
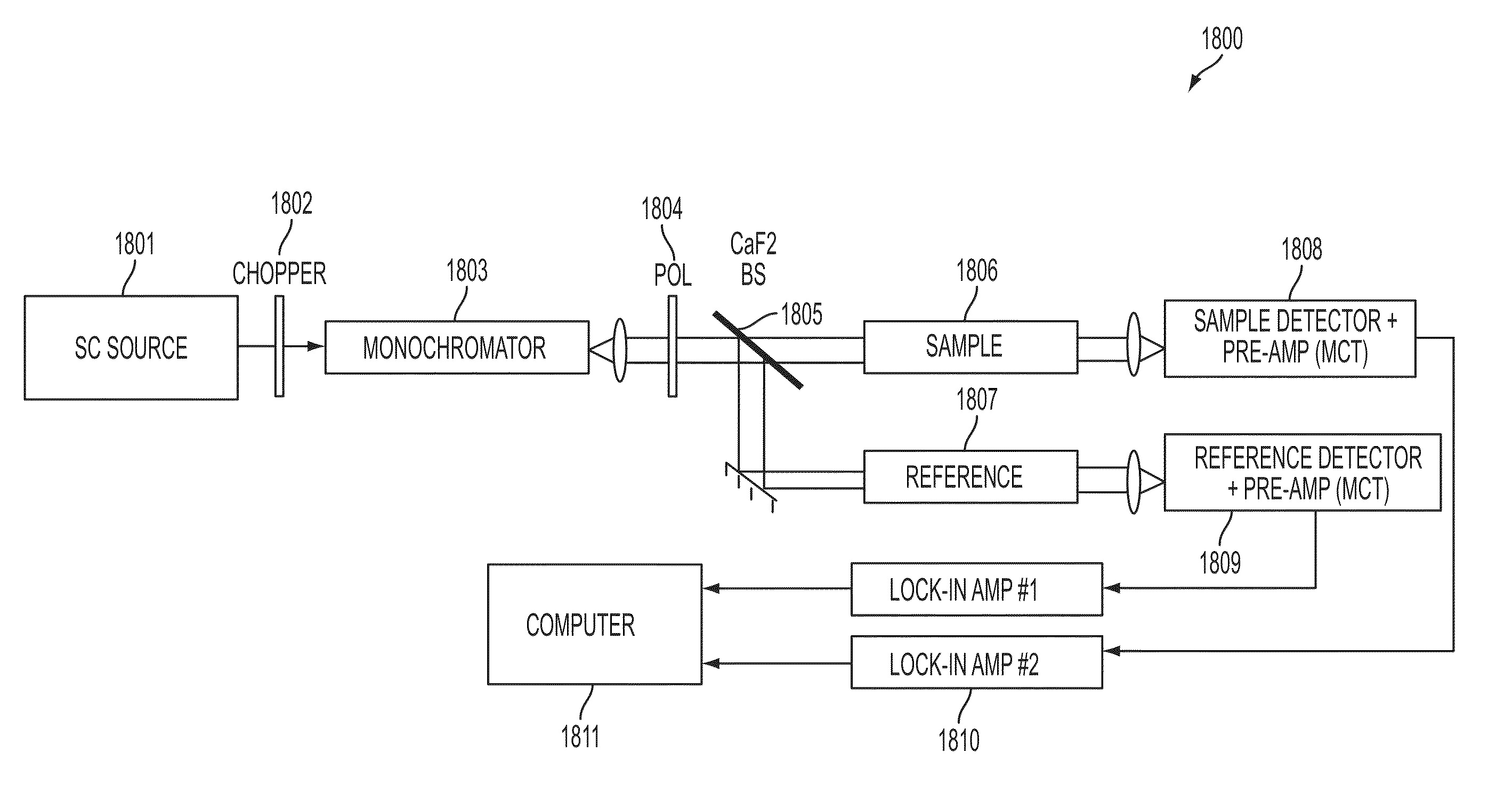
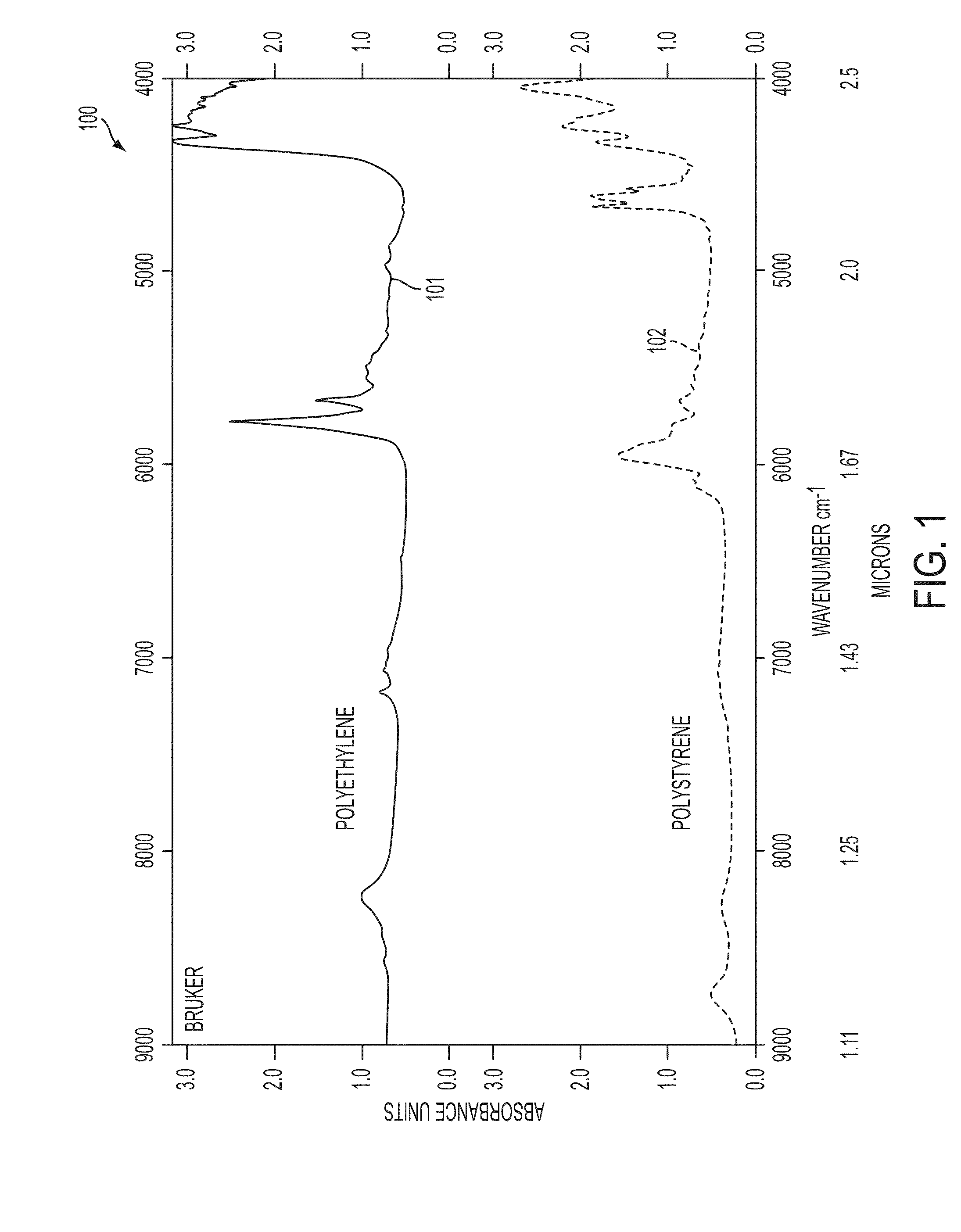
A system and method for using near-infrared or short-wave infrared (SWIR) light sources for identification of counterfeit drugs may perform spectroscopy using a super-continuum laser to provide detection in a non-contact and non-destructive manner at stand-off or remote distances with minimal sample preparation. Also, near-infrared or SWIR light may penetrate through plastic containers and packaging, permitting on-line inspection and rapid scanning. The near-infrared or SWIR spectroscopy may also be used to detect illicit drugs and their chemical composition. Moreover, the spectroscopic techniques may also be applied to quality assessment and control in pharmaceutical manufacturing, thus permitting the implementation of smart manufacturing with feedback control. Fiber super-continuum lasers may emit light in the near-infrared or SWIR between approximately 1.4-1.8 microns, 2-2.5 microns, 1.4-2.4 microns, 1-1.8 microns. In particular embodiments, the detection system may be a dispersive spectrometer, a Fourier transform infrared spectrometer, or a hyper-spectral imaging detector or camera.
Owner:OMNI MEDSCI INC
System and method for manufacturing
ActiveUS20050226794A1Increase capacityMinimizing fluid transfer lineDomestic stoves or rangesSpace heating and ventilationFiltrationOn board
Embodiments of the present invention are directed to a customizable bio-manufacturing system which includes a manufacturing space having a first air handling system for providing supply air and a second air handling system for handling exhaust air, the supply air system being optionally provided with at least one of filtration, heating, cooling and or humidity control and a plurality of portable modules provided within the manufacturing space. At least one module having an interior capable of being interconnected with another module interior and each module's interior includes one or more components to perform at least one specific task of a biological, chemical, and / or pharmaceutical manufacturing process. At least one module includes an on-board environmental control system for controlling an environment within the module and a connection means for interconnecting the module interior with another module interior. The system also includes a central controller operating to at least perform one or more of operation and information collection for the operation of at least one of the system and one or more modules.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
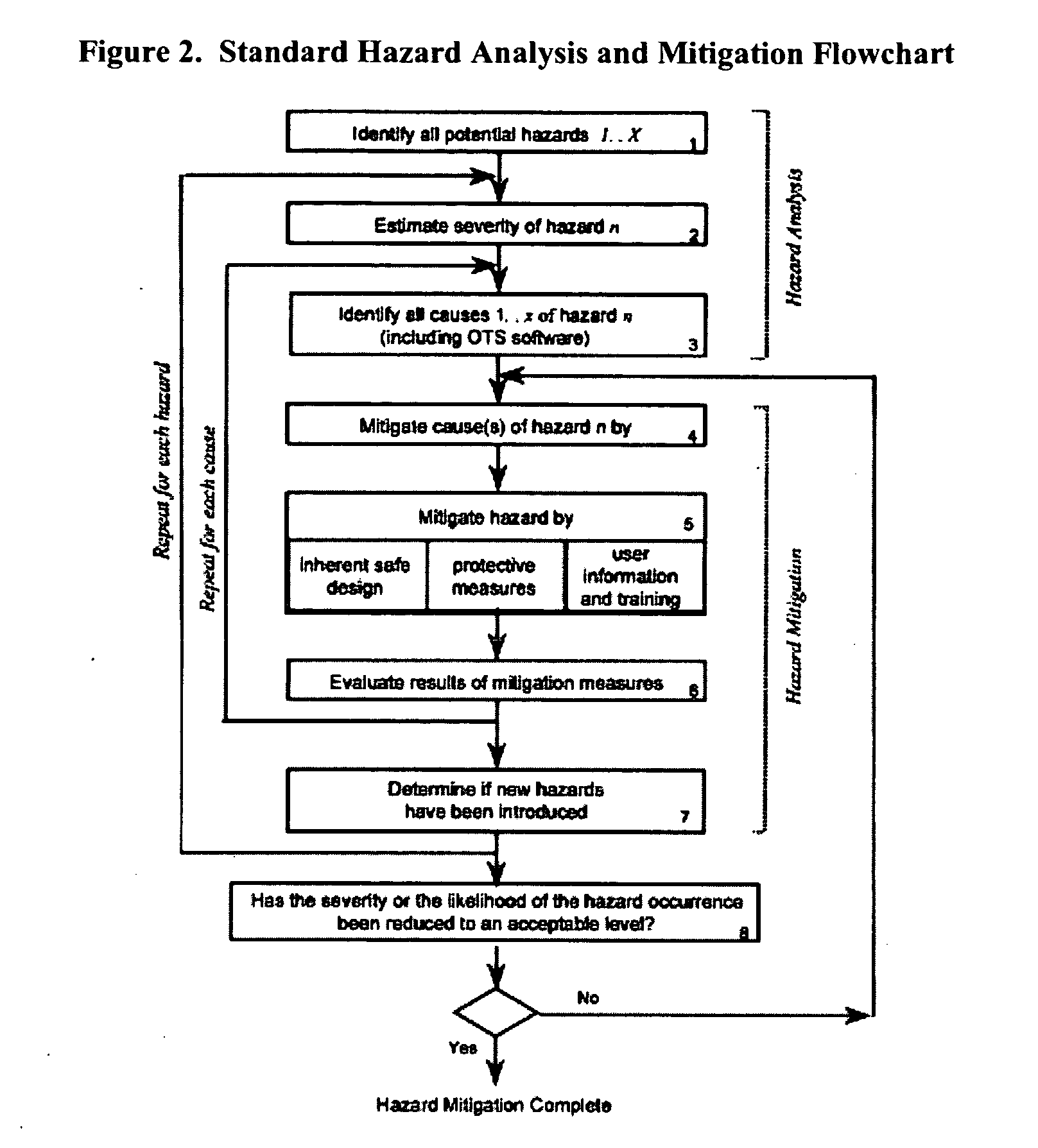
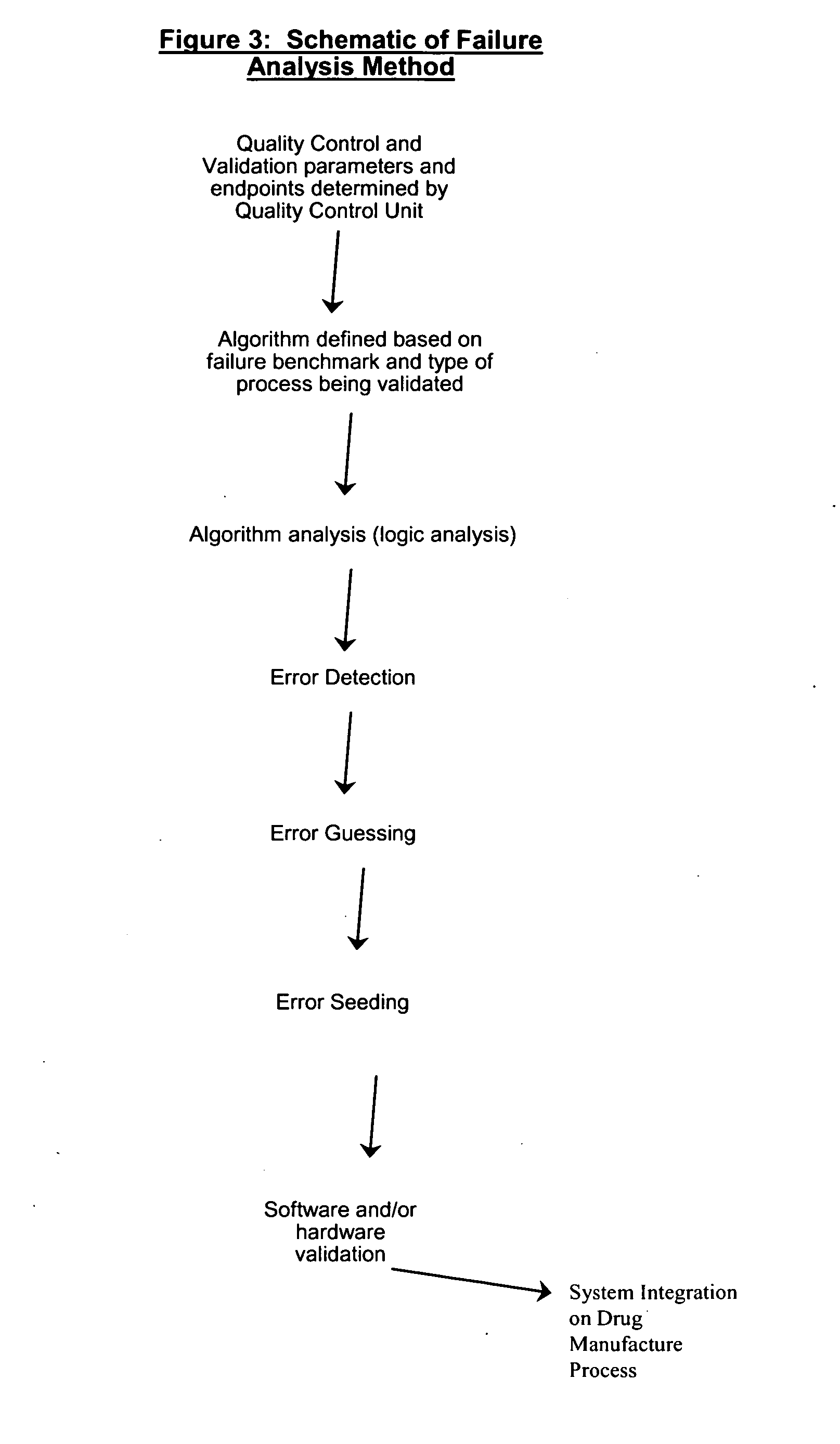
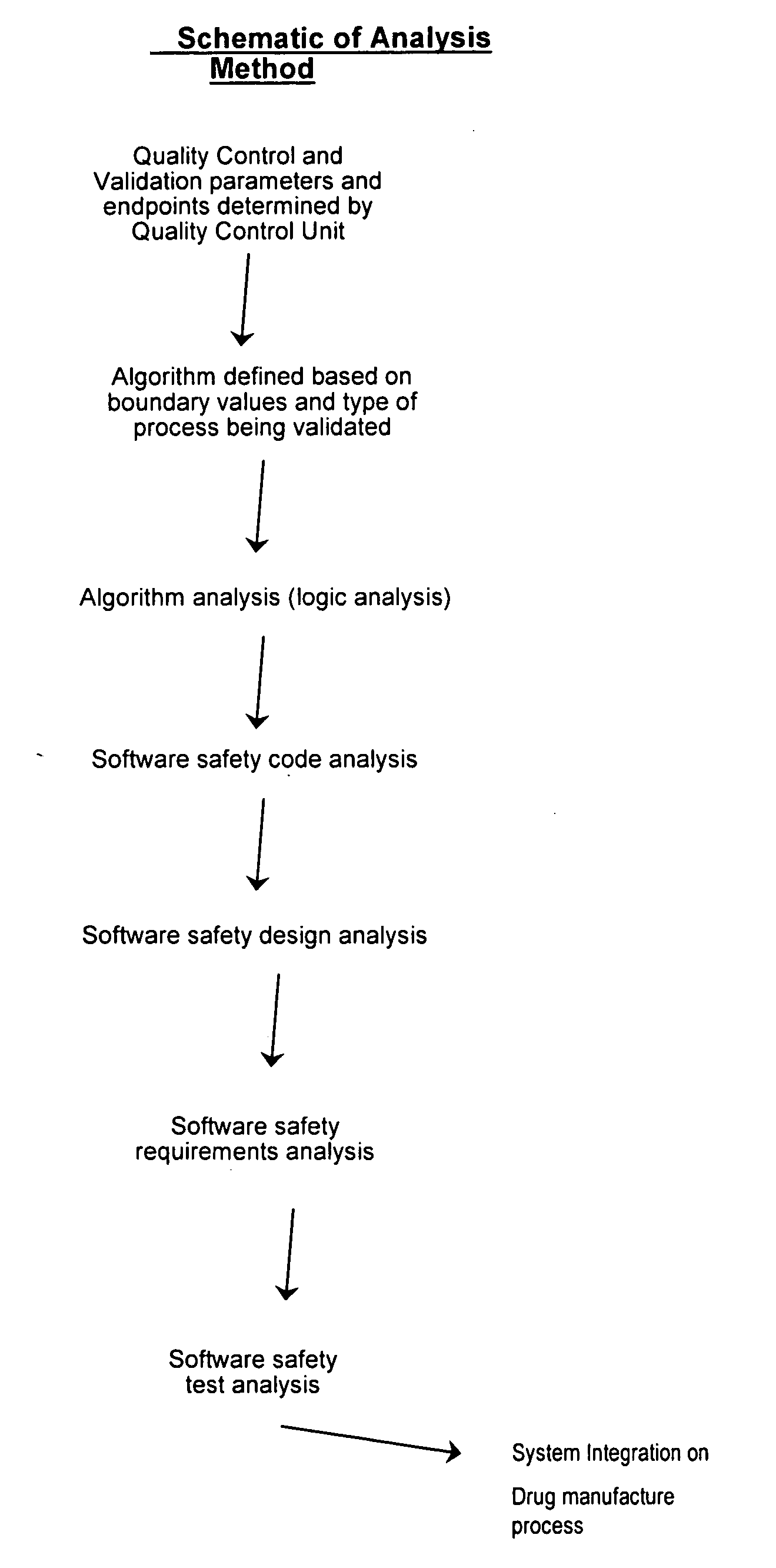
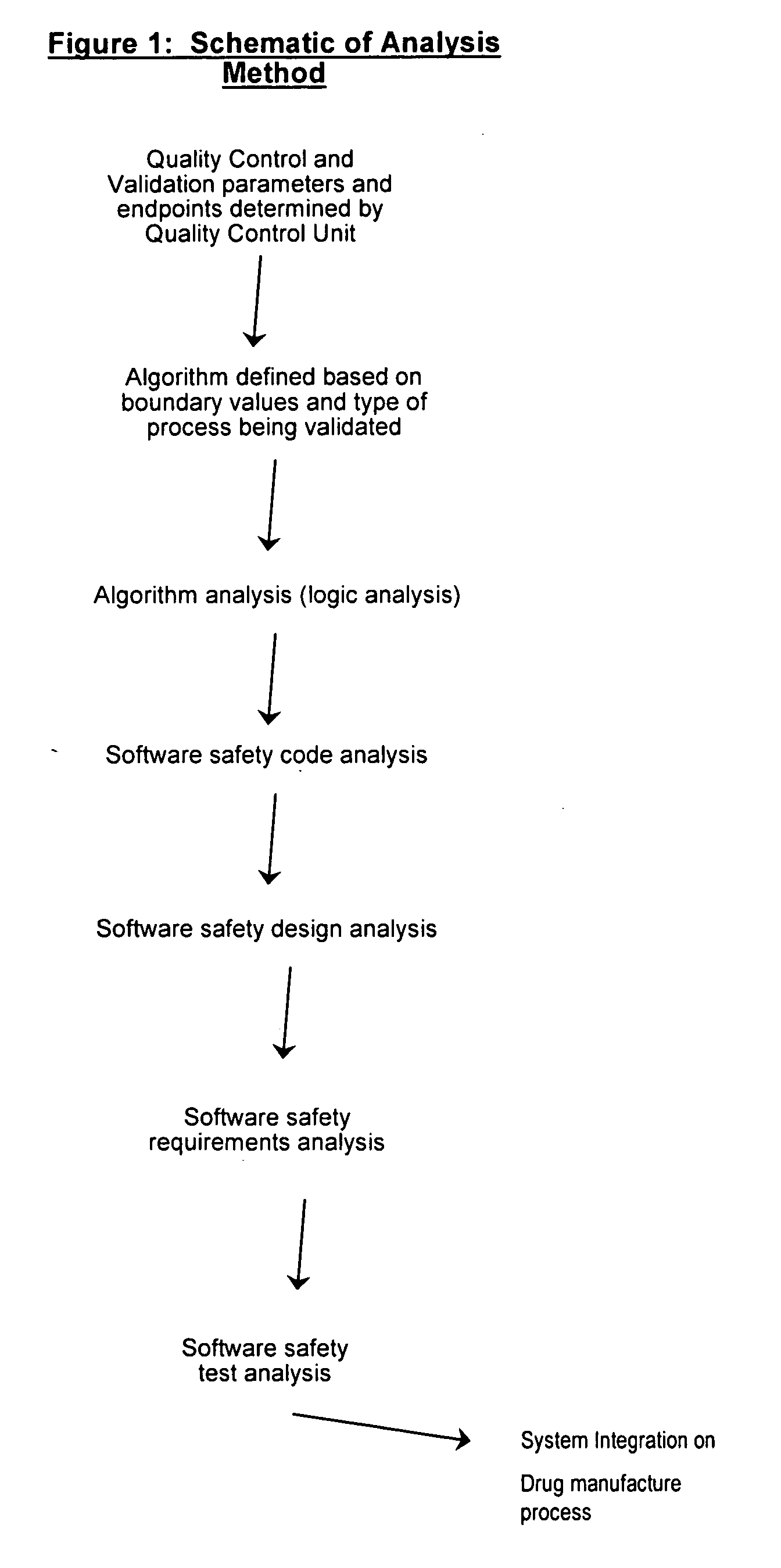
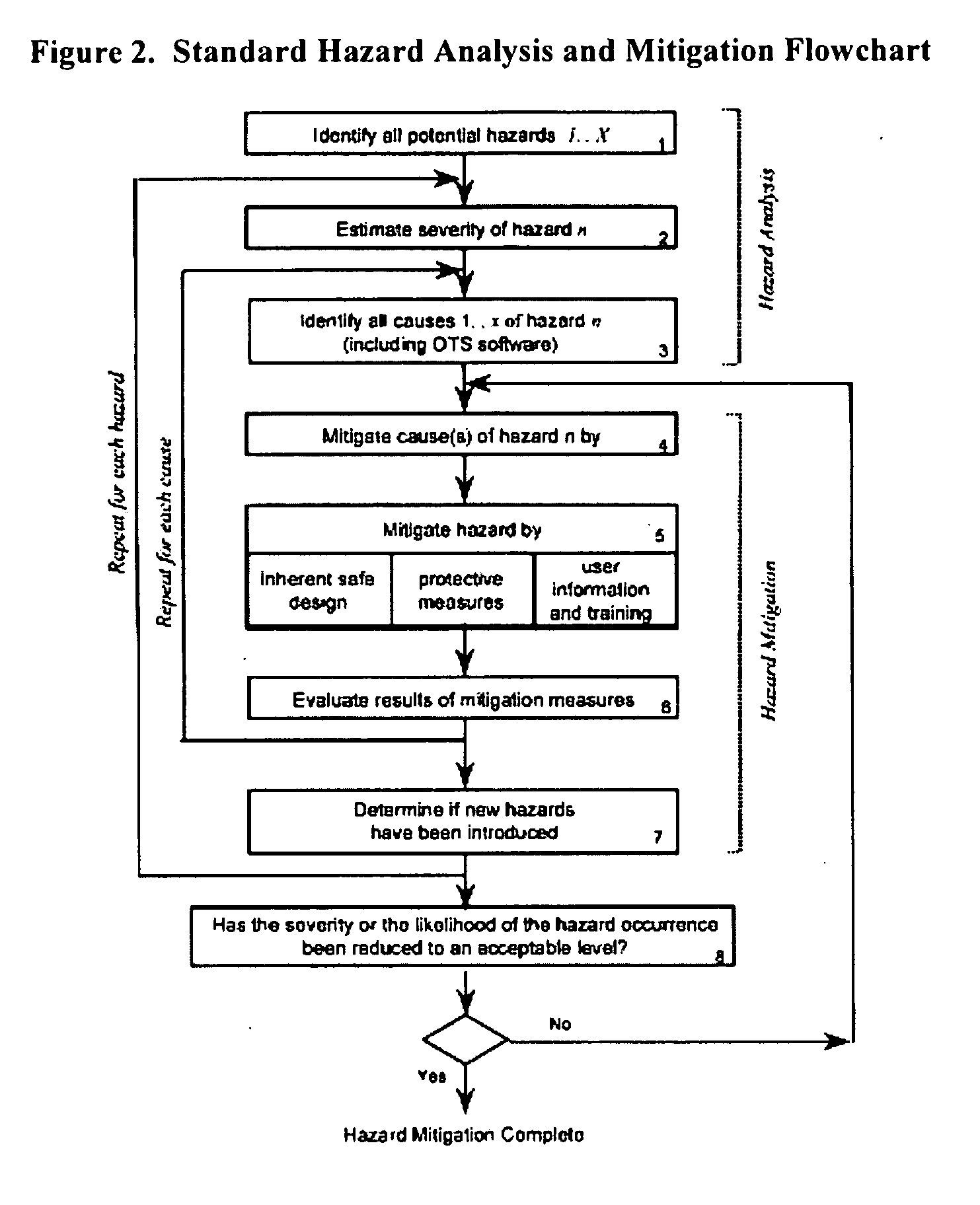
Methods, systems, and software program for validation and monitoring of pharmaceutical manufacturing processes
ActiveUS20050251278A1Testing/monitoring control systemsResourcesQuality assuranceSoftware engineering
Methods, systems, and software program for validation of pharmaceutical manufacturing processes and quality assurance process are described and disclosed herein. Consequently, the methods provide a means to perform validation on an integrated level whereby the quality control unit can ensure data and product integrity and minimize cost.
Owner:SMP LOGIC SYST
Single-use controlled environment module
ActiveUS20130017131A1Less laborLess materialBacterial antigen ingredientsSamplingBiochemical engineeringComputer module
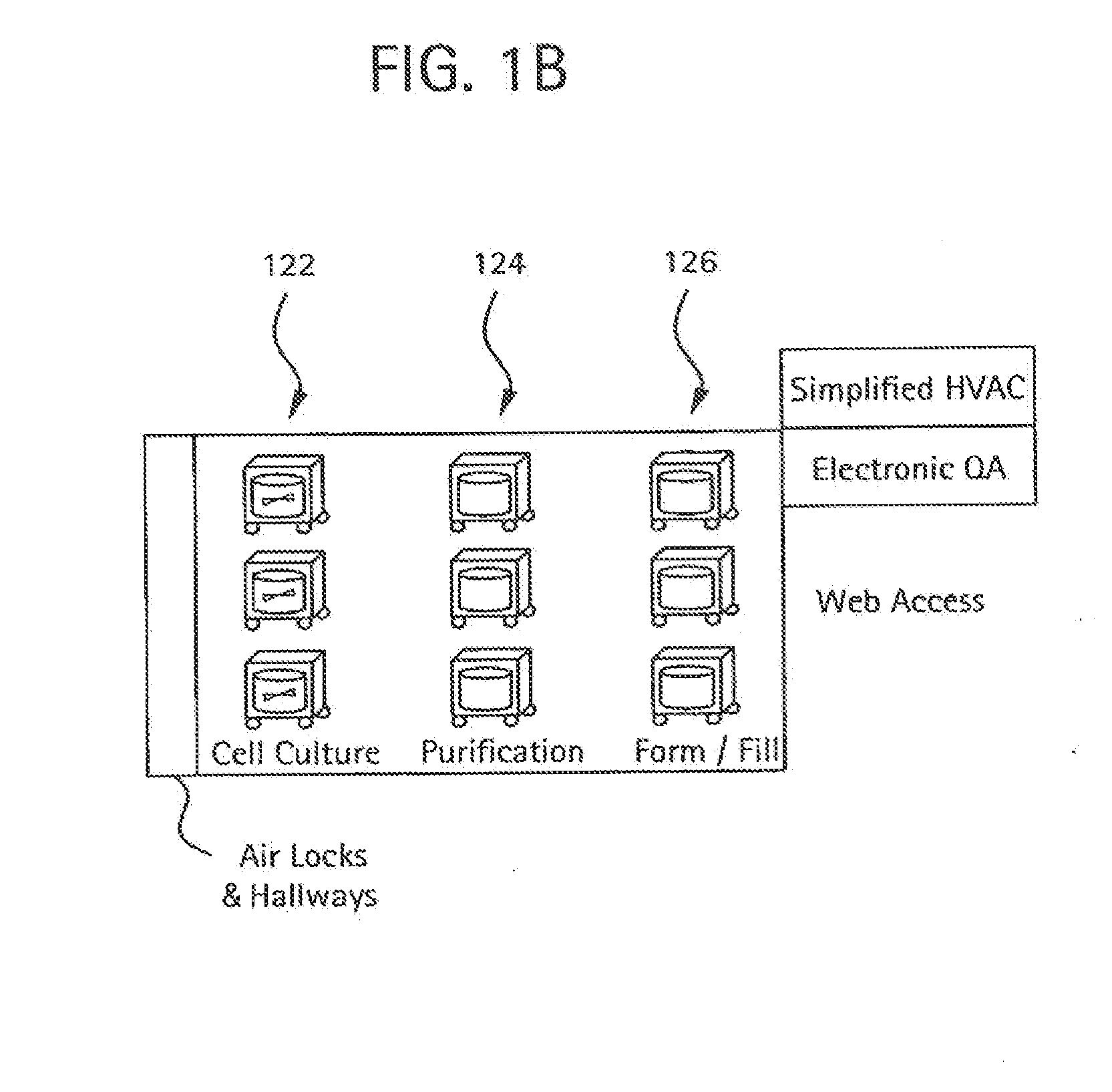
Disclosed herein is a single use, controlled environment manufacturing module in the form of a sterile sealed bag formed of a substantially flexible material, such that the bag can be inflated and deflated for transport and / or disposal. The flexible bag has one or more access ports and connectors to accommodate a variety of biochemical or pharmaceutical manufacturing processes to be carried out within the flexible bag. The interiors of one or more disclosed modules can be connected, forming a module train.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
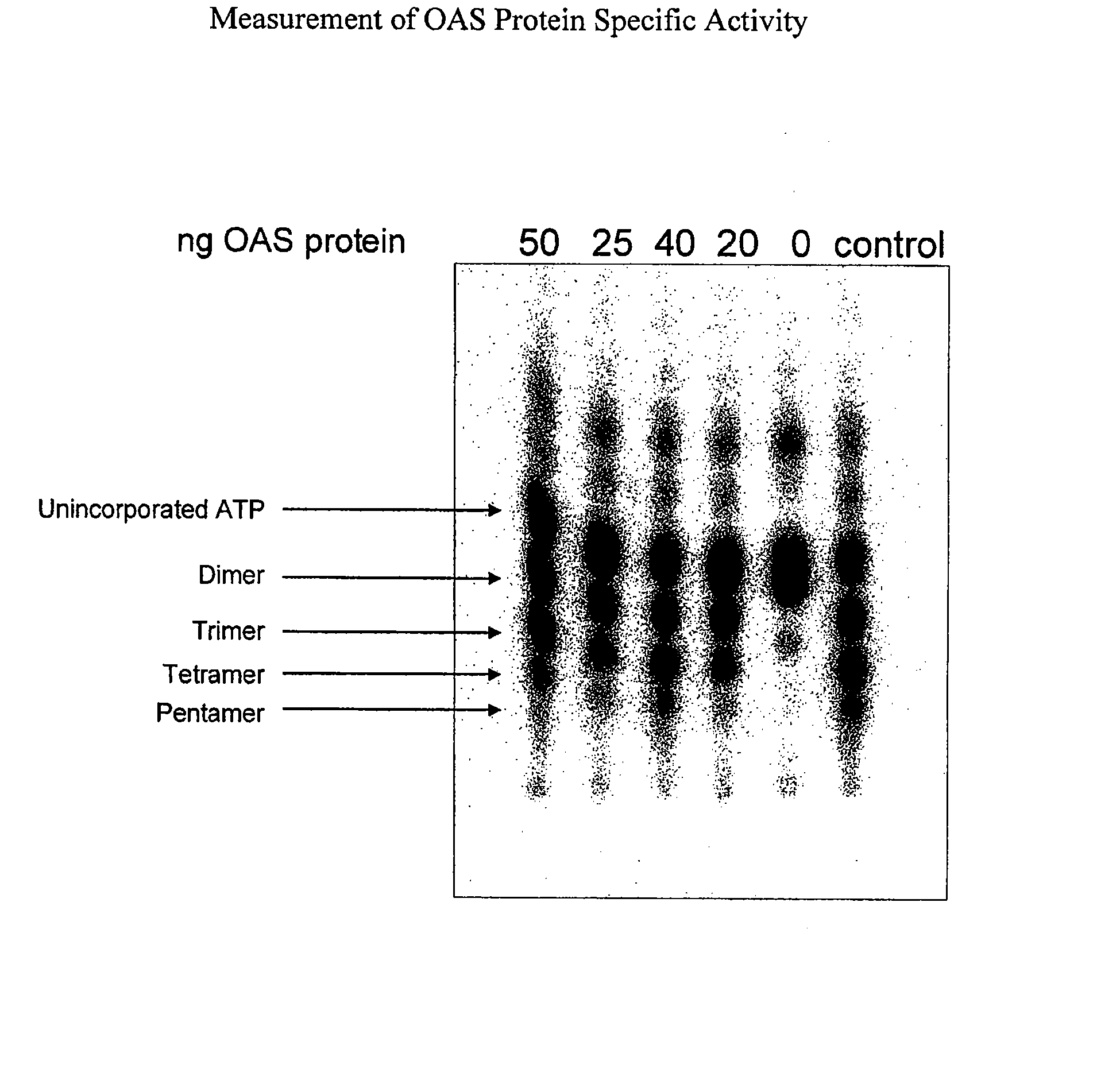
Pharmaceutical manufacturing methods
The invention describes methods for manufacturing oligoadenylate synthetase (OAS) proteins for use as active pharmaceutical ingredients in pharmaceutical compositions. A manufacturing method is described that produces large quantities of concentrated, highly active OAS protein for use in pharmaceutical compositions for the treatment of a variety of diseases including viral infection. Methods for monitoring and validating the manufacturing process are also described.
Owner:KINETA TWO LLC
Short-wave infrared super-continuum lasers for detecting counterfeit or illicit drugs and pharmaceutical process control
A system and method for using near-infrared or short-wave infrared (SWIR) light sources for identification of counterfeit drugs may perform spectroscopy using a super-continuum laser to provide detection in a non-contact and non-destructive manner at stand-off or remote distances with minimal sample preparation. Also, near-infrared or SWIR light may penetrate through plastic containers and packaging, permitting on-line inspection and rapid scanning. The near-infrared or SWIR spectroscopy may also be used to detect illicit drugs and their chemical composition. Moreover, the spectroscopic techniques may also be applied to quality assessment and control in pharmaceutical manufacturing, thus permitting the implementation of smart manufacturing with feedback control. Fiber super-continuum lasers may emit light in the near-infrared or SWIR between approximately 1.4-1.8 microns, 2-2.5 microns, 1.4-2.4 microns, 1-1.8 microns. In particular embodiments, the detection system may be a dispersive spectrometer, a Fourier transform infrared spectrometer, or a hyper-spectral imaging detector or camera.
Owner:OMNI MEDSCI INC
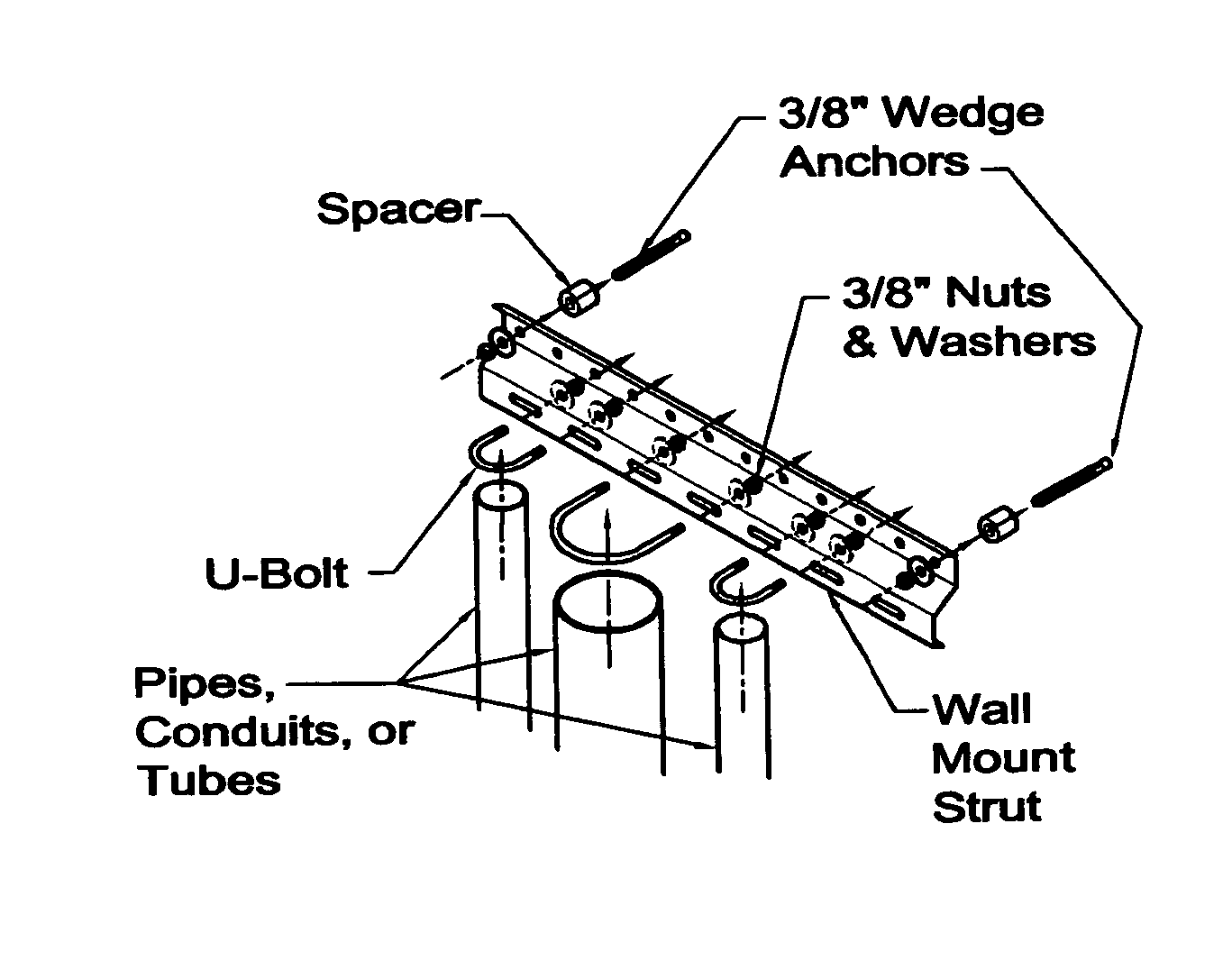
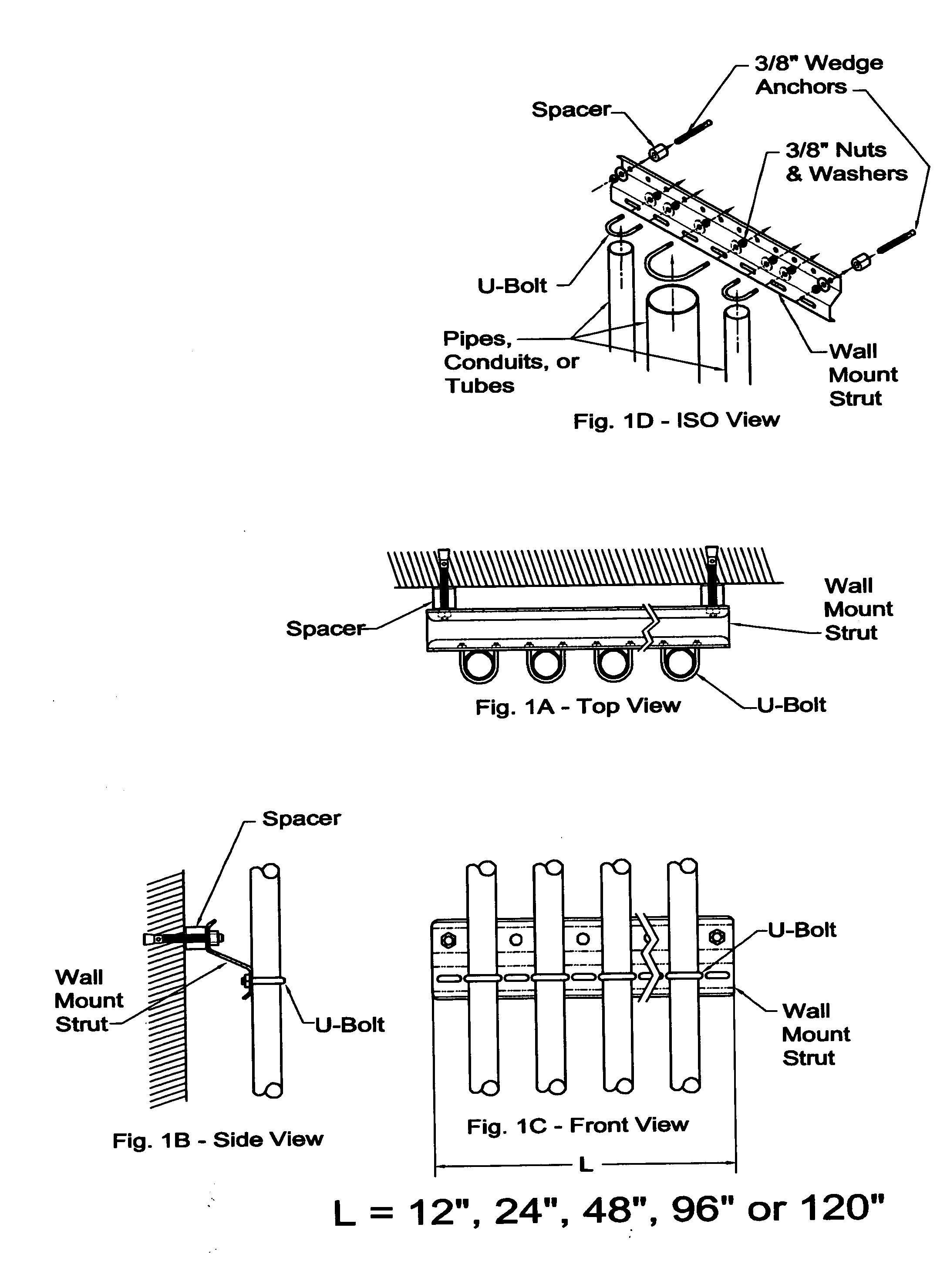
Sanitary pipe mounting system
InactiveUS20060186278A1Resists contaminationEasy to checkPipe supportsStands/trestlesSupporting systemSystems design
Have invented a new strut system designed for the attachment of pipes, conduit, and tubes to vertical and horizontal surfaces in a sanitary manner. The mounting of pipes, conduits or tubes in an environment that demands a high level of sanitation, (i.e.; food processing and pharmaceutical manufacturing) is best accomplished through the use of my invention. Unlike strut systems currently available, my strut system repels contaminants, is easy to inspect, and easy to clean.
Owner:TJERRILD JAMES WILLIAM
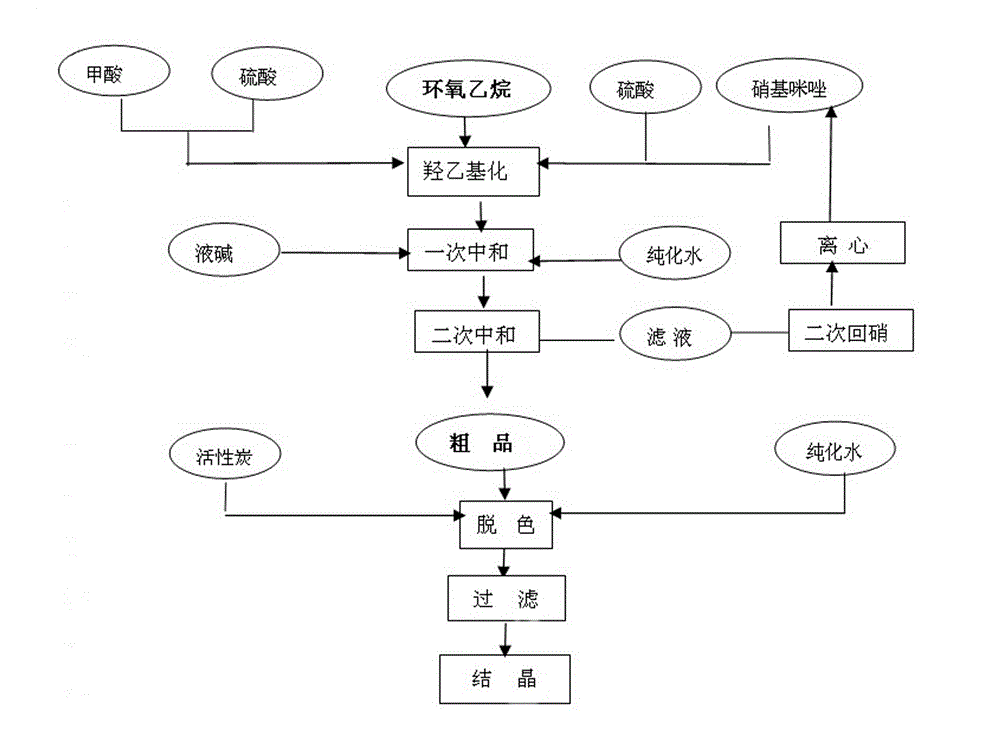
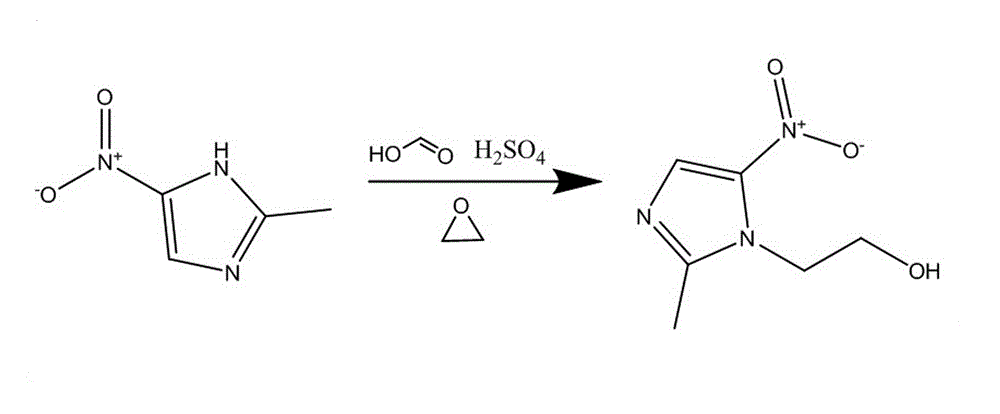
Metronidazole preparation method
The invention relates to the field of medicine manufacturing techniques, in particular to a metronidazole API preparation method, and solves the problems that a current metronidazole preparation method is high in cost and low in yield. The method comprises the following steps: (1), acid mixing, adding 100 parts (by weight) of formic acid of concentration of 95-99 percent in a reaction kettle for stirring to a temperature of 20 DEG C, then adding 25-35 parts (by weight) of concentrated sulphuric acid, so as to prepare the mixed acid for further use; (2), synthesis reaction, adding 100-120 parts (by weight) of reaction raw materials 2-methyl-5-nitroimidazole into a reaction tank, and adding the mixed acid prepared in the step 1, stirring to a temperature of 75-80 DEG C, completely dissolving the 2-methyl-5-nitroimidazole, and maintaining 10-20 minutes. According to the invention, the design is reasonable; and the mixed acid is adopted to provide an acidic condition, an applicable reaction raw material ratio and an appropriate control reaction parameter, so as to allow the yield of the nitroimidazole to reach 65-70 percent.
Owner:SHANXI TONGJI PHARMA
System and method for manufacturing
ActiveUS20130274929A1Increase capacityLess expensiveSampled-variable control systemsComputer controlOn boardFiltration
Embodiments of the present invention are directed to a customizable bio-manufacturing system which includes a manufacturing space having a first air handling system for providing supply air and a second air handling system for handling exhaust air, the supply air system being optionally provided with at least one of filtration, heating, cooling and or humidity control and a plurality of portable modules provided within the manufacturing space. At least one module having an interior capable of being interconnected with another module interior and each module's interior includes one or more components to perform at least one specific task of a biological, chemical, and / or pharmaceutical manufacturing process. At least one module includes an on-board environmental control system for controlling an environment within the module and a connection means for interconnecting the module interior with another module interior. The system also includes a central controller operating to at least perform one or more of operation and information collection for the operation of at least one of the system and one or more modules.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
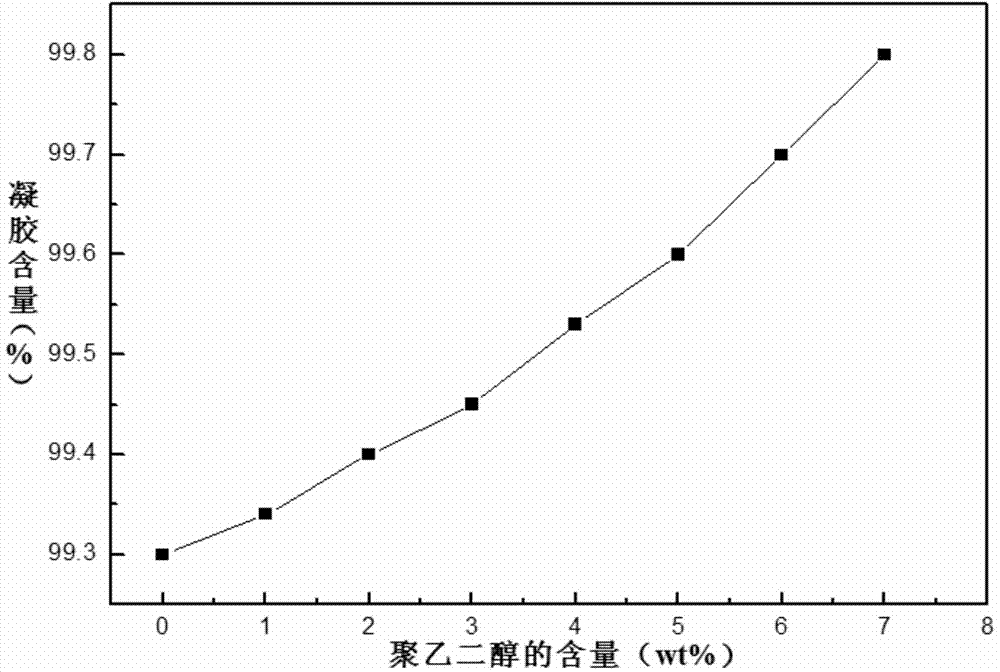
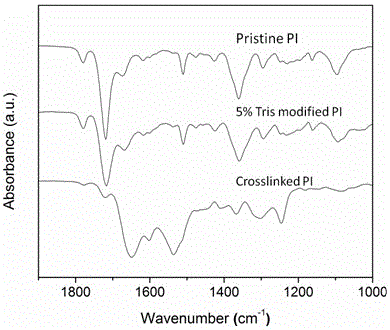
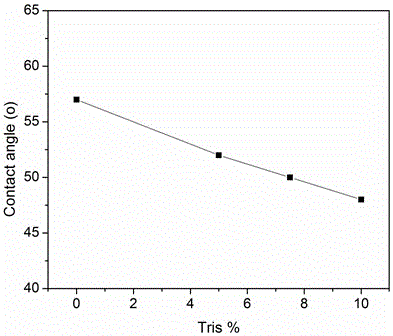
Preparation method for diamine cross-linked modified polyimide nano-filtration membrane added with polyethylene glycol
InactiveCN103768964AGood chemical stabilityHigh retention rateSemi-permeable membranesCross-linkPhase conversion
The invention provides a preparation method for a diamine cross-linked modified polyimide nano-filtration membrane added with polyethylene glycol and aims to solve the problems that the permeation flux of the prepared polyimide nano-filtration membrane is low, the rejection rate is low and the preparation cost is high in an existing method. The preparation method provided by the invention comprises the following steps: 1, preparing polyimide membrane casting liquid with the mass percent of 15%-25%; 2, preparing polyethylene glycol-polyimide membrane casting liquid, wherein the mass percent of the polyethylene glycol in the polyethylene glycol-polyimide membrane casting liquid is 1-20wt%; 3, carrying out membrane preparation by using an immersing precipitated phase conversion method to obtain the polyimide nano-filtration membrane; 4, processing the obtained polyimide nano-filtration membrane by using diamine to obtain a modified polyimide nano-filtration membrane; and 5, immersing and washing 2-4 times to obtain the diamine cross-linked modified polyimide nano-filtration membrane added with the polyethylene glycol. The preparation method for the diamine cross-linked modified polyimide nano-filtration membrane added with the polyethylene glycol is applied to fields including pharmaceutical manufacturing, biology, catalyst recycling and the like.
Owner:HARBIN INST OF TECH
Manufacturing execution system for validation, quality and risk assessment and monitoring of pharmaceutical manufacturing processes
ActiveUS20080038833A1Quality improvementImprove productivityComponent separationDrug and medicationsManufacture execution systemQuality assurance
Manufacturing execution systems relating to methods, systems, and software program for validation of pharmaceutical manufacturing processes and quality assurance process are described and disclosed herein. Consequently, the methods provide a means to perform validation on an integrated level whereby the quality control unit can ensure data and product integrity and minimize cost.
Owner:SMP LOGIC SYST
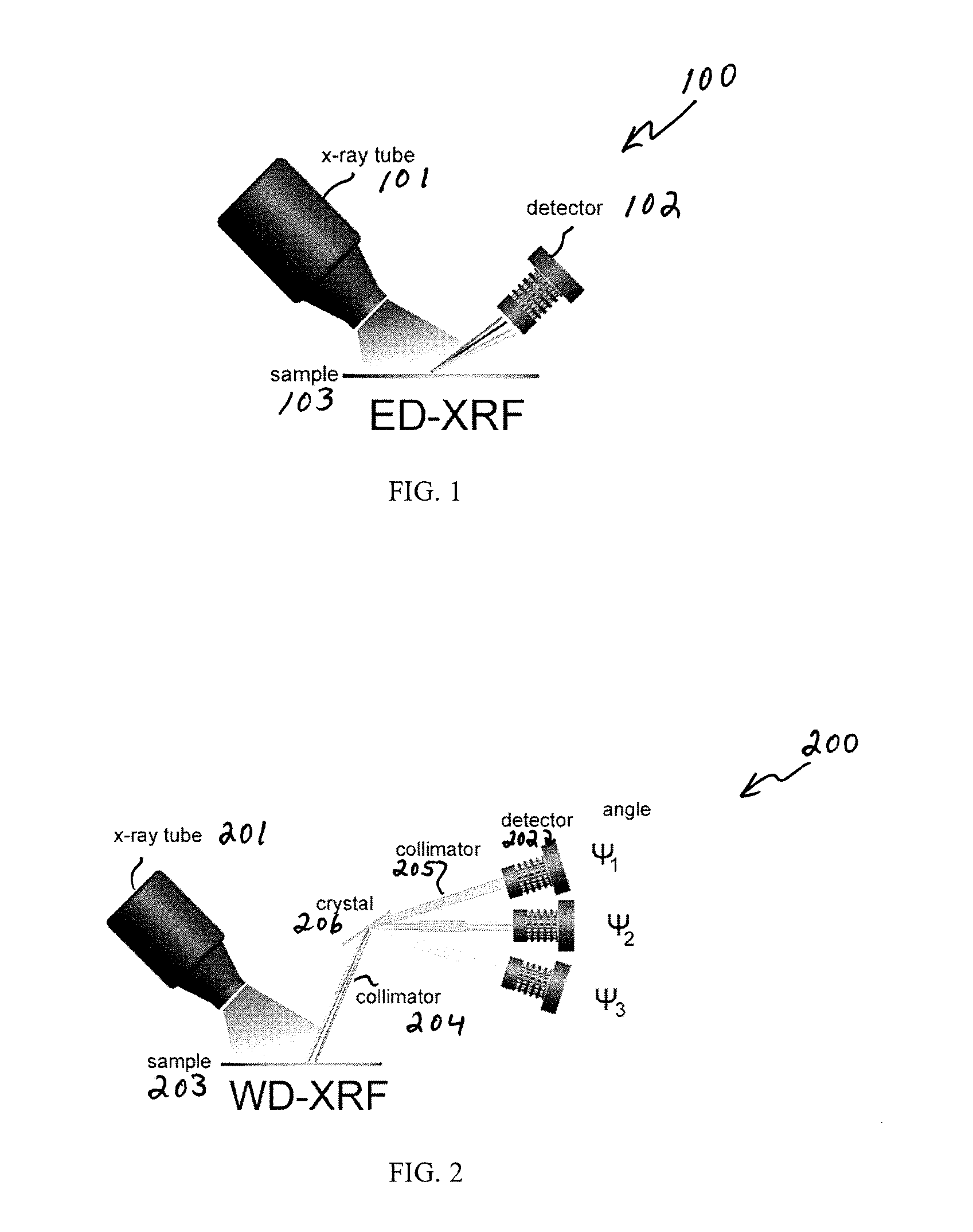
Metal analysis during pharmaceutical manufacturing
ActiveUS20170038319A1Material analysis using wave/particle radiationTesting medicinal preparationsMetal impuritiesPharmaceutical drug
A system and method for detecting, measuring, and analyzing for metallic impurities in pharmaceutical drugs and compounds utilizes an x-ray fluorescence system. The system and method may be co-located with a pharmaceutical manufacturing process for in-line continuous monitoring of metal impurities. The pharmaceutical products may be in a form selected from a powder, slurry, pill, tablet, and gel.
Owner:UHV TECH INC
Preparation method for monoamine-grafted-and-modified crosslinked polyimide solvent-resistant nanofiltration membrane
ActiveCN104959047AHydrophilic regulationImprove stabilitySemi-permeable membranesPolymer scienceAlcohol
The invention provides a preparation method for a monoamine-grafted-and-modified crosslinked polyimide solvent-resistant nanofiltration membrane. The preparation method comprises the following steps: a, preparing a mixed solution from a solvent and a cosolvent according to a mass ratio of 1: 0.1-5; b, adding 15 to 28% of a polyimide solution into the mixed solution so as to prepare a membrane casting liquid; c, adding 2.5 to 20% of monoamine into the membrane casting liquid and carrying out mixing at 25 to 80 DEG C for 3 to 24 h under stirring so as to guarantee complete grafting; d, preparing a membrane by using a immersion-precipitation phases inversion process, washing the prepared membrane with deionized water a plurality of times and standing the washed membrane for subsequent usage; e, preparing an alcoholic solution of diamine with a concentration of 0.5 to 20%; f, putting the prepared membrane in the alcoholic solution of diamine prepared in the previous step for crosslinking modification for 0.25 to 48 h; and g, after completion of the reaction, soaking and cleaning the membrane in an alcohol solvent so as to obtain the polyamide nanofiltration membrane with excellent performance. The polyimide solvent-resistant nanofiltration membrane prepared in the invention has the advantages of simple preparation, adjustable hydrophilicity, outstanding solvent resistance, great permeation flux, a high retention rate, etc. and is applicable to separation of substances in all the organic solvents and to the fields of pharmaceutical manufacturing, biology, catalyst recovery, etc.
Owner:宜兴环保产业有限公司
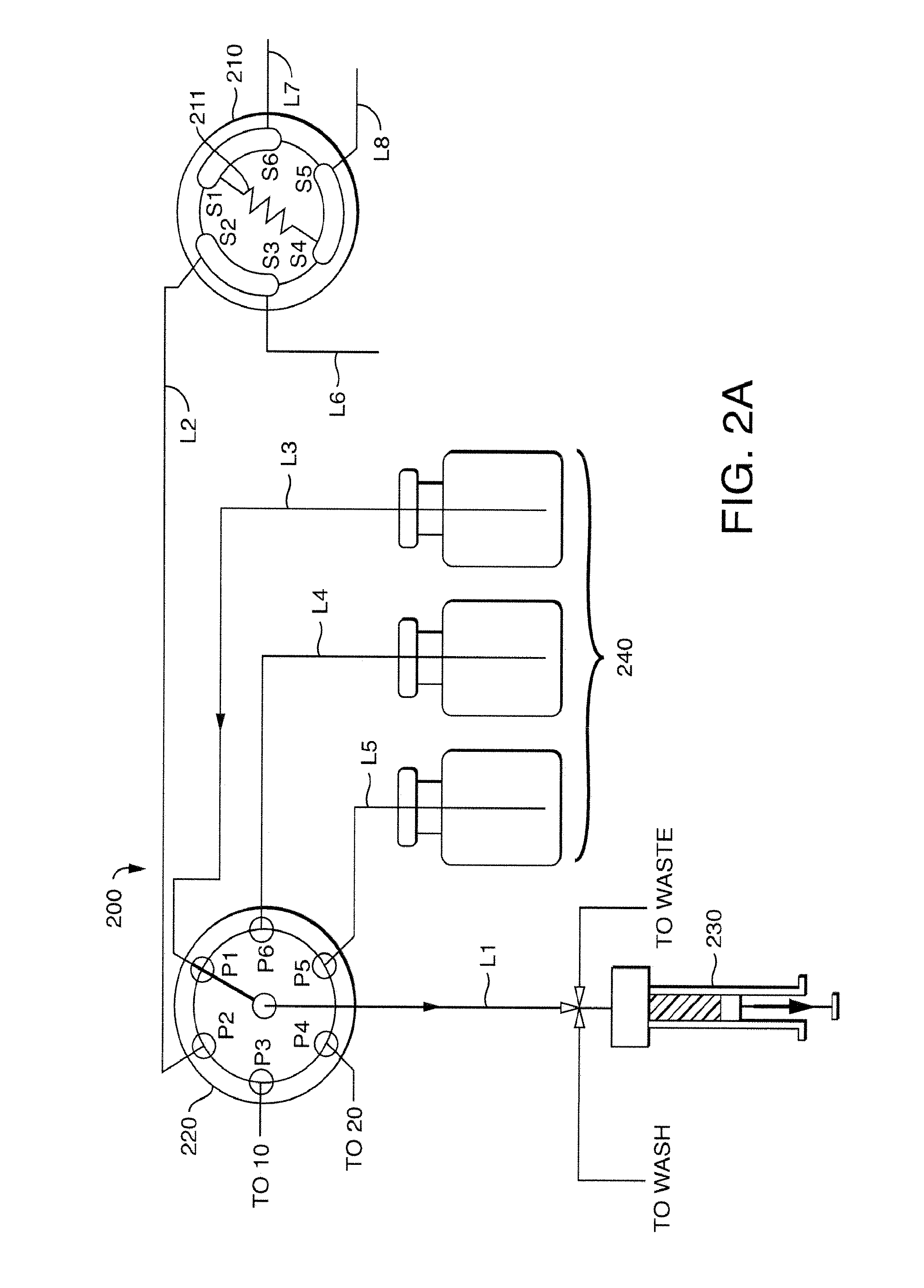
Chromatography-based monitoring and control of multiple process streams
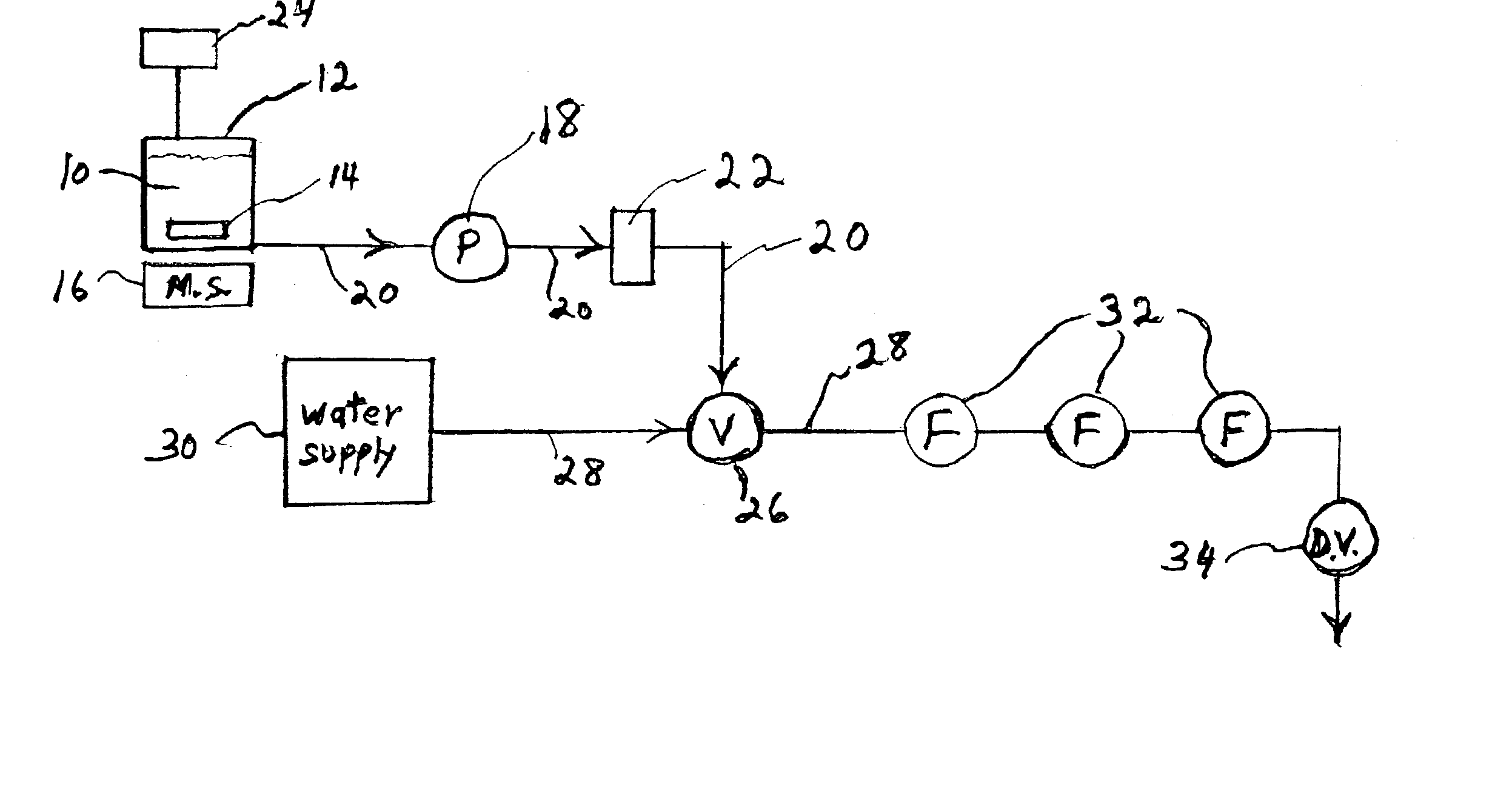
ActiveUS8343774B2High frequencyReduce time lagComponent separationIon-exchanger regenerationLine tubingDrug compound
An analytical apparatus includes a sample-injection valve, a sample pump, at least two sources of standards, and a selection valve. The sample-injection valve has an output port in fluid communication with a LC column, and an input port in fluid communication with a mobile-phase supply line. The at least two sources of standards are associated with at least two pharmaceutical compounds. The selection valve fluidically and selectably connects the sample pump to the at least two sources of standards, to the sample-injection valve, and to at least two pharmaceutical-manufacturing process lines associated with the at least two pharmaceutical compounds. A method for controlling a pharmaceutical manufacturing process includes switching the selection valve to alternately and repeatedly sample the at least two sources of standards and material flowing through the at least two pharmaceutical-manufacturing process lines.
Owner:WATERS TECH CORP
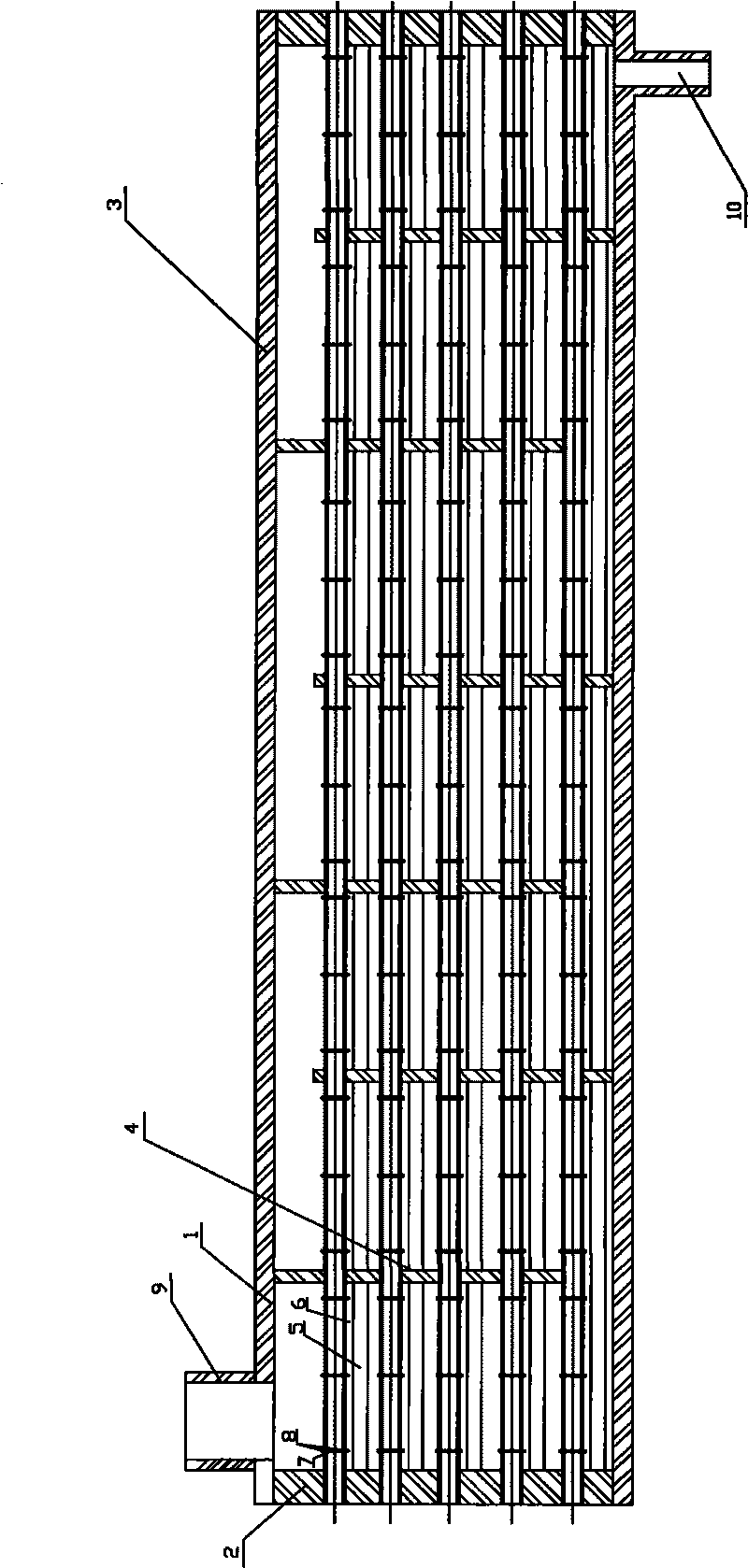
Horizontal shell and tube type condenser with liquid guide devices
InactiveCN101709923AAvoid drippingEnhanced condensation heat transfer capacitySteam/vapor condensersHeat exchanger casingsChemical industryPharmaceutical manufacturing
The invention relates to a horizontal shell and tube type condenser with liquid guide devices. At present, when designing the horizontal shell and tube type condenser, people compensate the negative influence of tube bundle effect on the condensation heat exchange only by increasing the amount of heat exchange tubes so as to cause increase of the volume of heat-exchange equipment and the improvement of the production cost. The horizontal shell and tube type condenser with the liquid guide devices comprises a shell (1). Both sides of the shell are connected with a tube plate (2). A space inside the shell surrounded by the shell and the tube plate is provided with a set of heat exchange tubes (3). The heat exchange tubes are provided with support plates (4). Continuous liquid guide devices (5) are installed below the heat exchange tube and are collection liquid guide device or separation liquid guide devices. The shell is provided with an air inlet (9) and a liquid outlet (10). The invention can be widely applied in the fields of petroleum, chemical industry, metallurgy, pharmaceutical manufacturing, air conditioning, refrigeration, heat supply and the like.
Owner:张吉礼
Manufacturing execution system for validation, quality and risk assessment and monitoring of pharamaceutical manufacturing processes
ActiveUS20070032897A1Quality improvementImprove productivityDrug and medicationsTechnology managementManufacture execution systemQuality assurance
Manufacturing execution systems relating to methods, systems, and software program for validation of pharmaceutical manufacturing processes and quality assurance process are described and disclosed herein. Consequently, the methods provide a means to perform validation on an integrated level whereby the quality control unit can ensure data and product integrity and minimize cost.
Owner:SMP LOGIC SYST
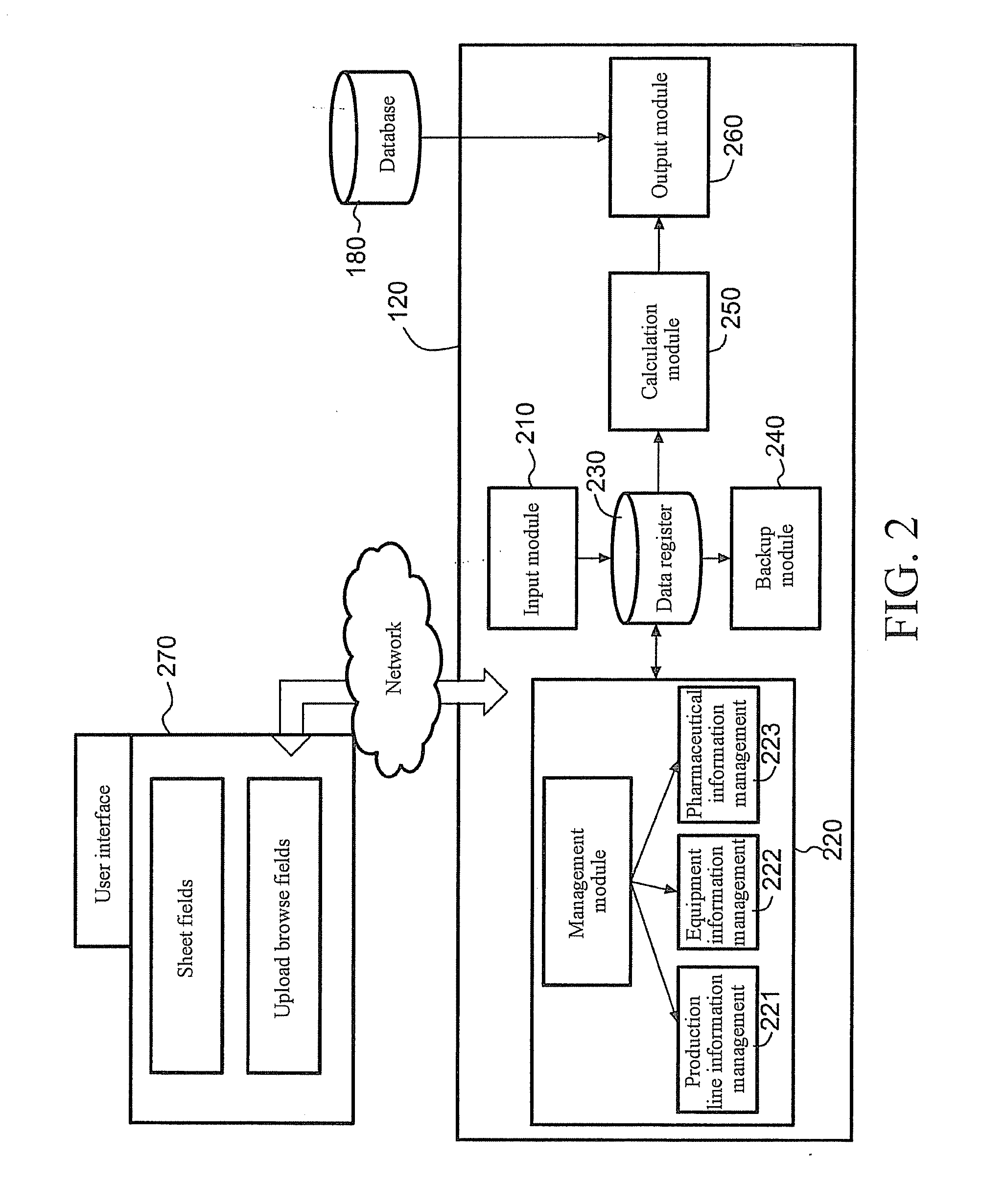
Server for Integrated Pharmaceutical Analysis and Report Generation Service, Method of Integrated Pharmaceutical Manufacturing and Research and Development Numerical Analysis, and Computer Readable Recording Medium
ActiveUS20110276161A1Easy to operateImprove accuracyDrug and medicationsOffice automationPharmaceutical manufacturingComputer science
A web-based tool (as a server) for integrated pharmaceutical analysis and report generation service is provided in the present invention. The server can be used for numerical analysis and report generation for pharmaceutical manufacturing, research and development, and has advantages such as simple operation, complicated but fast calculation and professional report generation, and high accuracy. The server includes at least one pharmaceutical manufacturing and research and development numerical analysis system configured to perform different pharmaceutical manufacturing and research and development numerical analyses and generate different reports. Each of the at least one pharmaceutical manufacturing and research and development numerical analysis system includes an input module configured to receive, via a user interface, at least one of a template file and a backup file previously output by the server as at least one input file, wherein the at least one input file includes a plurality of data fields to provide corresponding data; at least one calculation module, each configured to execute a built-in pharmaceutical manufacturing and research and development numerical analysis calculation program, thereby automatically performing a pharmaceutical manufacturing and research and development numerical analysis calculation on at least one of the data of the at least one input file and on-line filled data; and an output module configured to generate at least one of a backup file and a report file as at least one output file based on the result of the pharmaceutical manufacturing and research and development numerical analysis calculation performed by the at least one calculation module and provide the at least one file via the user interface.
Owner:TAIWAN BIOTECH
Transfer faction solution and preparation method thereof
ActiveCN104095879AIncrease contentImprove stabilityUnknown materialsImmunological disordersFreeze thawingPharmaceutical manufacturing
The invention belongs to the technical field of biological pharmaceutical manufacturing, and particularly relates to a transfer faction solution and a preparation method thereof. The preparation method comprises the following steps: mixing animal spleen and NaCl solution according to a weight ratio of 1:(1.3-1.4), performing repeated freeze-thaw, adjusting the pH value to 6.2-7.2, and filtering to obtain the transfer faction solution. By the adoption of the method provided by the invention, the content of effective components in the transfer faction solution can be increased advantageously, the product yield is improved, and the stability and the safety of a medicine are improved.
Owner:卫材(辽宁)制药有限公司
Medicinal composition of paclitaxel
InactiveCN104511022AMeet treatment requirementsHigh stability in vitroPowder deliveryOrganic active ingredientsBenproperineFreeze-drying
The invention belongs to the technical field of medicinal preparations, and concretely relates to a medicinal composition of paclitaxel. The medicinal composition comprises, by weight, 2.87-27.66 parts of paclitaxel and 100 parts of mPEG-PLA-phenylalanine. The medicinal composition has the following advantages: 1, the in vitro stability is high, and a re-dissolved paclitaxel solution keeps stable at room temperature for above 12h to meet medicinal treatment requirements; 2, the medicinal composition has high stability in blood and small particle size, and can easily perform the ERP effect; 3, a novel micelle carrier is adopted, so the toxicity is low, and the safety is high; and 4, a pharmacy common freeze-drying technology is directly adopted to prepare freeze-dried powder, so the medicinal composition is simple to prepare, and is convenient to transport and store, and untoward effects brought by the addition of an excipient are avoided The medicinal composition can be instantly re-dissolved by using common normal saline or glucose injection, so the administration process is greatly simplified.
Owner:POLYMERCHEM
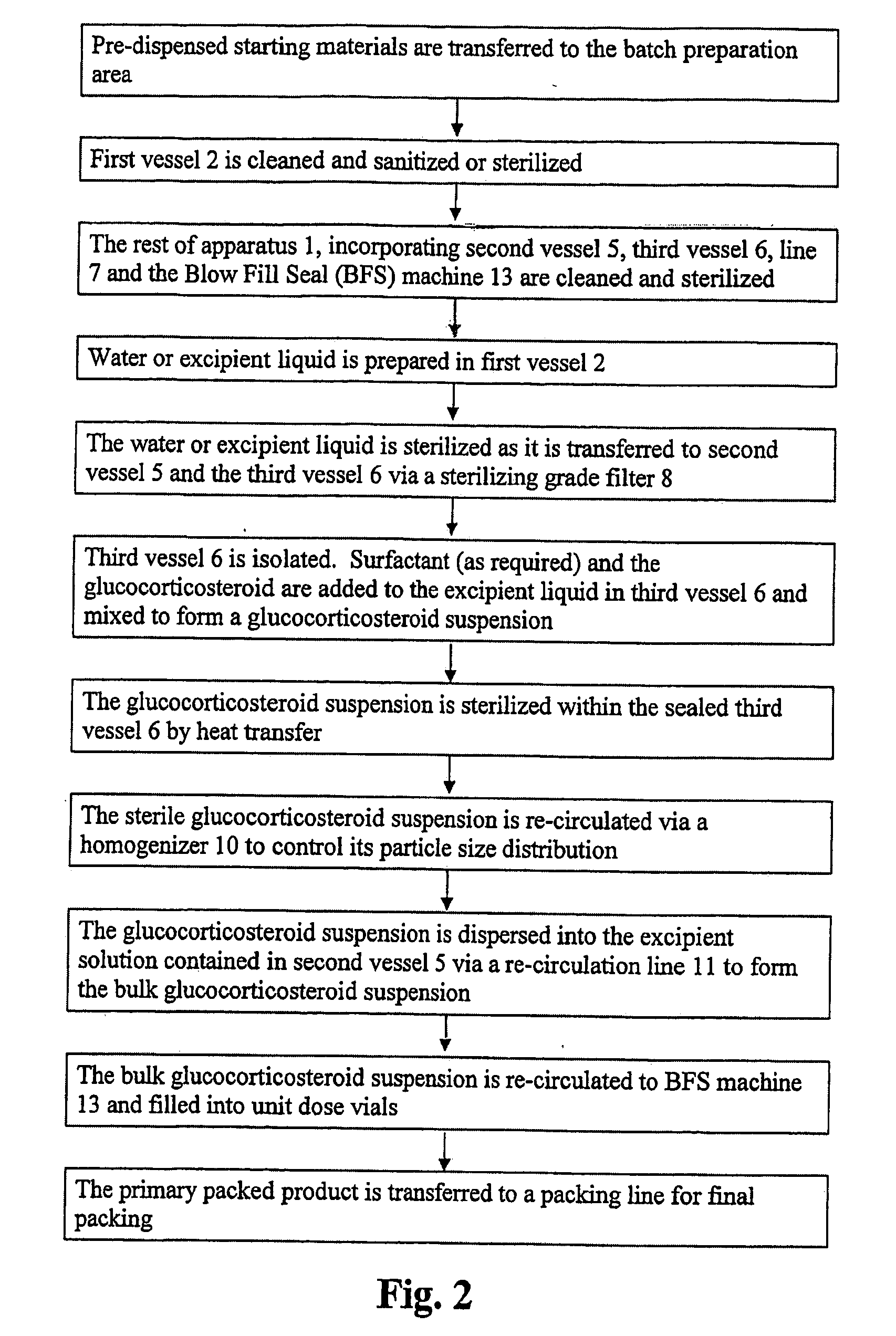
Pharmaceutical Manufacturing Process For Heat Sterilized Glucocorticoid Suspensions
ActiveUS20080269178A1Powder deliveryOrganic active ingredientsBiochemical engineeringPharmaceutical manufacturing
The present invention provides a method for preparing a sterile suspension of a glucocorticosteroid. The glucocorticosteroids used in the invention are preferably antiinflammatory glucocorticosteroids. By making the last stage of product preparation be the sterilization process, the potential for contamination during manufacture and heat degradation of products is greatly reduced.
Owner:NORTON HEALTHCARE
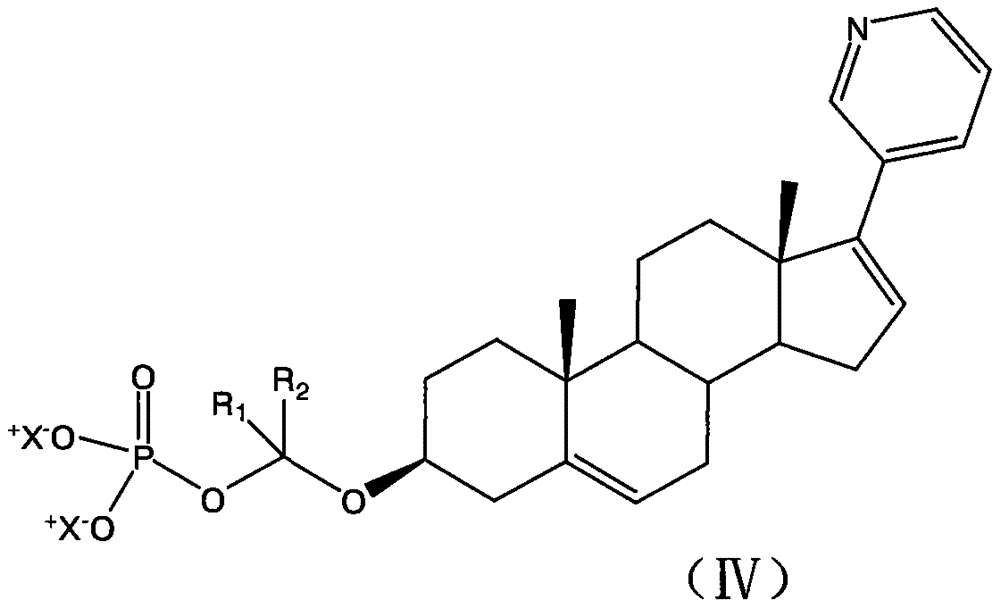
Abiraterone derivative with anti-cancer effect
InactiveCN104974212AImprove solubilityImprove bioavailabilitySteroidsAntineoplastic agentsSolubilityAbiraterone
The invention provides an abiraterone derivative with an anti-cancer effect. The general structural formula of the abiraterone derivative is as shown in the description. The abiraterone derivative can ensure improvement of biophysics properties such as abiraterone solubility and bioavailability; the stability of the abiraterone derivative is maintained very well during pharmaceutical manufacturing and drug administration; and after drug administration, the abiraterone derivative can be degraded and release active compounds; and the abiraterone derivative has the characteristics of low toxicity and the like.
Owner:BEIJING LANG REBONE TECH CO LTD
Method for extracting and further processing ainsliaea fragrans total flavonoid
InactiveCN105287677APromote dissolutionFacilitated DiffusionPlant ingredientsMicropore FilterMedicinal herbs
The invention relates to the technical field of Chinese pharmaceutical manufacturing, particularly to a method for extracting and further processing ainsliaea fragrans total flavonoid. With ainsliaea fragrans adopted as the raw medicinal material, the method comprises the following steps: firstly, smashing ainsliaea fragrans; carrying out ultrasonic immersion extraction on the smashed ainsliaea fragrans in ethanol water for a plurality of times; then, combining extracting solutions; decompressing and concentrating the extracting solutions; adding ethyl alcohol into the concentrated solution, and carrying out low-temperature standing for alcohol precipitation; filtering with a micropore filter membrane; decompressing and concentrating the filtrate; carrying out vacuum drying on the concentrated solution to obtain refined ainsliaea fragrans total flavonoid extract; finally, adding pharmaceutical excipients for further preparing the ainsliaea fragrans total flavonoid extract into various pharmaceutical preparations. According to the method, ultrasonic wave and continuous countercurrent extraction technologies are combined, so that the dissolution and spreading of the effective components are quickened, the extraction time is greatly shortened, the extraction efficiency is improved, the degradation of heat sensitive components is avoided, the energy consumption and production cost are further reduced, the extraction preparation technology is relatively simple and convenient, and the method is applicable to industrial large-scale production.
Owner:JIANGXI KINGSTONE UNITE PHARMA CO LTD
Methods, systems, and software program for validation and monitoring of pharmaceutical manufacturing processes
ActiveUS20060271227A1Testing/monitoring control systemsResourcesQuality assuranceSoftware engineering
Methods, systems, and software program for validation of pharmaceutical manufacturing processes and quality assurance process are described and disclosed herein. Consequently, the methods provide a means to perform validation on an integrated level whereby the quality control unit can ensure data and product integrity and minimize cost.
Owner:SMP LOGIC SYST
Novel gear pump
ActiveCN104214086AImprove the accuracy of useImprove the level of measurementRotary piston pumpsRotary/oscillating piston combinationsElastomerNon compliance
The invention relates to a novel gear pump, belongs to the technical field of pumps, and particularly relates to a novel gear pump which can be used for conveying various chemical mediums, oil, water, or poisonous and harmful or inflammable and explosive liquids, and is corrosion resistant, acid and alkali resistant, heat resistant, high pressure resistant and wearproof. The gear pump provided by the invention overcomes the defects in the prior art through an innovatively designed structure, improved processing technique and reasonable selection of metal and non-metal combined materials, thereby ensuring that the novel gear pump can meet working conditions of high temperature and high pressure as well as various viscosities, and can be used for efficiently conveying chemical mediums which are poisonous, harmful, volatile, corrosive, or easy-to-crystallize or relatively oppressive. The novel gear pump is connected with a motor for driving through a designed coupler with a double flange elastic body self-alignment structure; self alignment connection between the pump and the motor is really ensured; radial load caused by misalignment is eliminated; troubles in manual adjustment and non-compliance can be solved; and the novel gear pump can be extensively applied to various industries of tobaccos, petroleum, chemical engineering, papermaking, foods, beverages, pharmaceutical manufacturing, laboratories and the like.
Owner:何祥军
Special fungicide for livestock and poultry, its production method and application
InactiveCN102266314AAnti-inflammatoryWith convergenceAntibacterial agentsBiocideDiseaseSodium bicarbonate
The invention relates to a fungicide specially used for livestock and poultry, its production method and application. Take 0.01-5 parts of chlorhexidine acetate, 0.002-10 parts of polyethylene glycol octylphenyl ether, and 63-99.958 parts of tap water, put them into a container, mix and stir evenly, heat to 65-70°C, and then add benzene 0.02-12 parts of ammonium chloride, stir and heat to 92-94°C, then cool to 18-26°C, add 0.01-10 parts of glutaraldehyde and stir evenly, then adjust the pH value with sodium hydroxide or sodium bicarbonate , so that the pH value is 6-10, which is obtained. The invention is specially used for the sterilization and disinfection of livestock and poultry fur, oral cavity, endometrium, inner and outer vulva, breast, barn, air and object surface, as well as the treatment of fur and mucous membrane diseases. Compared with the existing sterilizing and disinfecting liquid, the present invention has the characteristics of many types of sterilizing, high sterilizing rate, simple manufacturing process, low cost, clear curative effect when applied to livestock and poultry, and safe and reliable use.
Owner:崔秀梅
Peach seed-safflower Siwu granule originated from peach seed-safflower Siwu decoction, and preparation method thereof
The invention relates to a peach seed-safflower Siwu granule containing medicinal materials like peach seed, safflower, Chinese angelica, common peony root, prepared rehmannia root and Ligusticum wallichii, and a preparation method thereof, belonging to the technical field of Chinese pharmaceutical manufacturing. The preparation method comprises the following steps: crushing peach seed, safflower, Chinese angelica, common peony root, prepared rehmannia root and Ligusticum wallichii, then adding water for decoction, combining two obtained decoctions, and then successively carrying out filtering, pressure-reduced concentration and spray drying so as to obtain dry powder; and then adding sugar and dextrin and then successively carrying out uniform mixing, granulation, drying and size finishing so as to obtain the peach seed-safflower Siwu granule. According to the invention, a spray drying manner is employed; product quality can be improved; quality indexes like the particle size distribution, moisture content, biological activity and dissolvability of drugs are controlled; and product purity and production efficiency are enhanced.
Owner:ANHUI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Cold and hot air alternative drying system
InactiveCN103185452AGuaranteed drynessSolve the problem of sterilizationDrying gas arrangementsLavatory sanitoryCold airPulp and paper industry
The invention relates to the field of pharmaceutical manufacturing equipment, in particular to a drying system. A cold and hot air alternative drying system comprises a drying cylinder and a heat pump connected with the drying cylinder; an air inlet pipeline is arranged between an air outlet of the heat pump and the drying cylinder; the cold and hot air alternative drying system further comprises a cold air blower; and an air outlet of the cold air blower is communicated with the drying cylinder through the air inlet pipeline. A photocatalyst device is arranged in the air inlet pipeline. Due to the adoption of the technical scheme, herbs are dehydrated at first, and then are dried through alternation of cold air and hot air to fully ensure the drying property of herbs; and the arrangement of the photocatalyst device solves the problem of sterilization of the cold air and hot air in the drying process.
Owner:SHANGHAI JINPIBAO PHARMA
Medicament for treating carbuncle, gangrene, turgescence toxin and skin ulcer, and method of producing the same
InactiveCN101172142ALow costLess drug componentsHeavy metal active ingredientsDermatological disorderTreatment effectMyrrh
A kind of medicine for treating carbuncle, gangrene, swollen poison and skin ulcer. It is made from Aconitum aconitum, Aconitum aconitum, Forsythia, Rehmannia glutinosa, frankincense, myrrh, wood turtle seed, cinnamon, dried blood, mercury powder, yellow dan, and sesame oil When making this drug, the weighed medicinal material should be immersed in the weighed sesame oil, soaked for 2 hours to 5 days, then heated, fried until the drug is browned, and then the fried drug is filtered out, and Huangdan is added and dried. Stir continuously, after cooling, it is the finished product; since there are fewer drug components in the drug of the present invention, and the price of each component is cheaper, the cost of this drug is lower; these medicinal materials are produced through proper compatibility Good therapeutic effect is obtained; the medicine in the present invention also has the advantage of simpler manufacturing process.
Owner:黄朵
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com