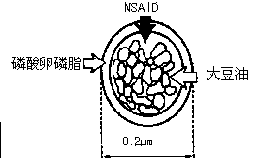
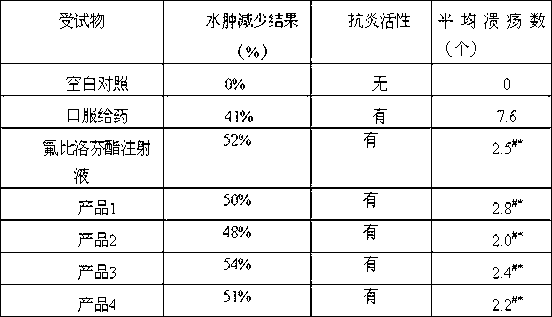
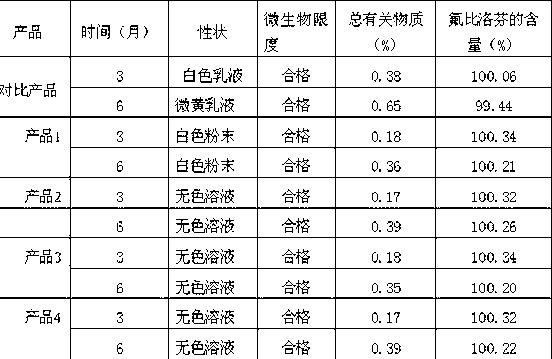
Novel 2-(2-fluorine-4biphenyl)-propionic acid pharmaceutical composition
A composition and drug technology, applied in the field of medicine, can solve problems such as adverse reactions, high cost of flurbiprofen axetil, increased difficulty in production and quality control, etc., and achieve good reconstitution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of arginine: flurbiprofen powder injection with a molar ratio of 0.6:1
[0051] prescription
[0052] Flurbiprofen 24.4g
[0053] Arginine 10.4g
[0054] Dilute to 2.44L with water for injection
[0055] A total of 1000 sticks were prepared
[0056] Arginine and flurbiprofen were both treated with activated carbon for injection according to the requirements of the powder injection workshop for material entry and exit, equipment and utensils, and the standard operating procedures for use. Add about 1.8 liters of water for injection to 10.4 g of arginine, stir at room temperature until dissolved, then add 24.4 g of flurbiprofen to the obtained arginine solution, mix at room temperature until dissolved, add water to the resulting solution to 2.44 liters, the molar ratio (arginine:flurbiprofen) is obtained as 0.6:1 Flurbiprofen solution of 10mg / ml, and then fill the qualified liquid medicine in sterile antibiotic glass bottles, then place the glass bott...
Embodiment 2-4
[0059] The same method as in Example 1 was used to prepare arginine: the lyophilized powder whose molar ratio of flurbiprofen was 0.9:1, 1:1 and 1.5:1 respectively.
Embodiment 5
[0061] Preparation of Injection with Arginine: Flurbiprofen Molar Ratio of 0.6:1
[0062] prescription
[0063] Flurbiprofen 24.4g
[0064] Arginine 10.4g
[0065] Dilute to 2.44L with water for injection
[0066] A total of 1000 sticks were prepared
[0067] The arginine of 10.4g, the flurbiprofen of 24.4g are added in 1.4 liters of water for injection, heat and stir until dissolving and then be settled to 2.44 liters with water for injection, obtain mol ratio (arginine: flurbiprofen )for 0.6:1 10mg / ml solution flurbiprofen solution, add needle carbon according to 0.15% of the total water, keep stirring at 60°C for 10-15 minutes, then filter the resulting solution through a G6 sand rod and a 0.2 micron microporous membrane , divided into receiving bottles, filled and sealed after passing the clarity inspection, and the products after filling and sealing were immediately subjected to conventional terminal sterilization, and the sterilized products were packaged after ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com