Preparation method of butoconazole nitrate intermediate suitable for industrial production
A technology for butconazole nitrate and intermediates, which is applied in the field of preparation of butconazole nitrate intermediates, can solve problems such as damage to personnel and equipment, difficult control of Grignard reaction, volatilization of ether, etc., so as to reduce side reactions and increase operability. Safety and security, the effect of increasing viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
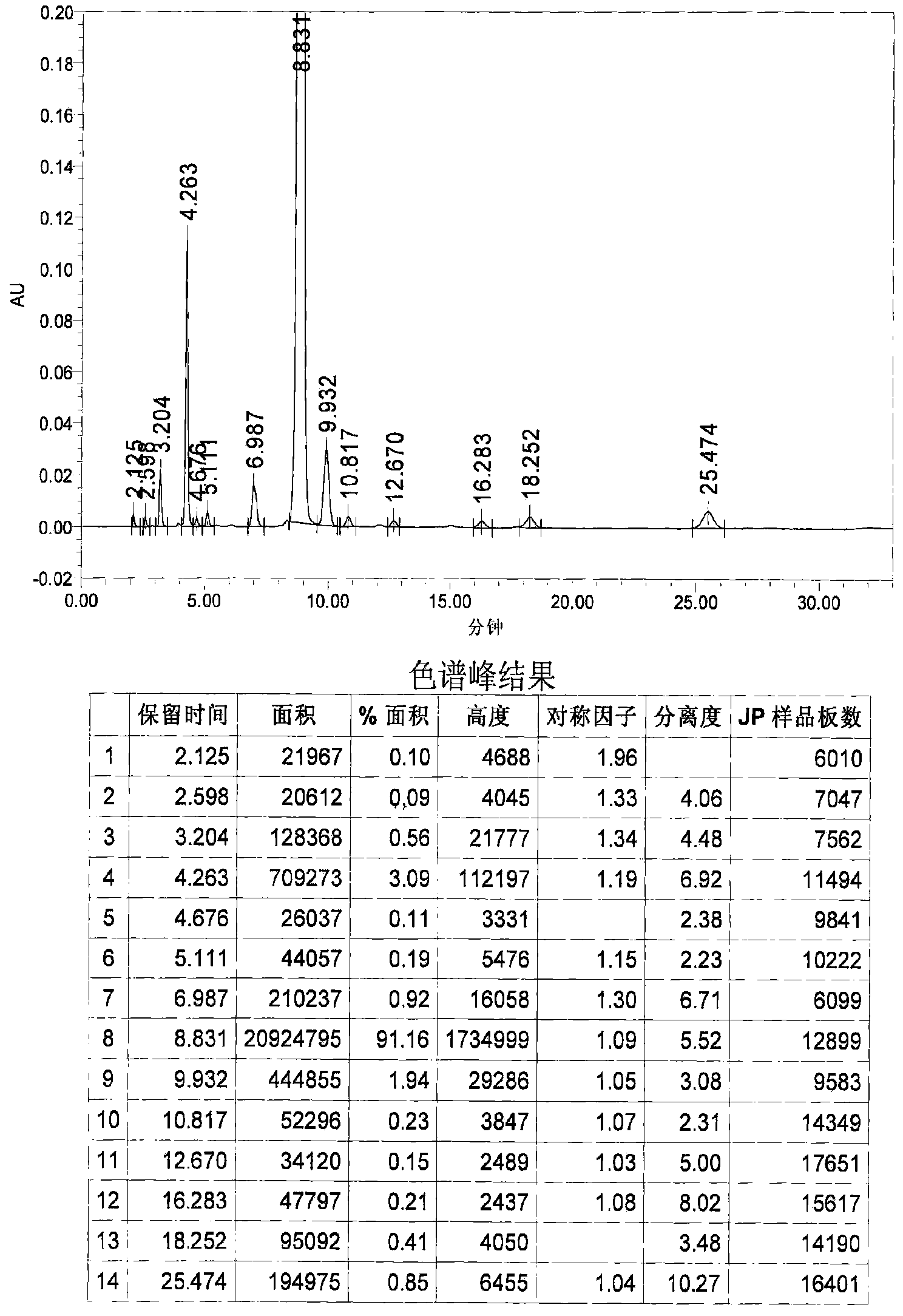
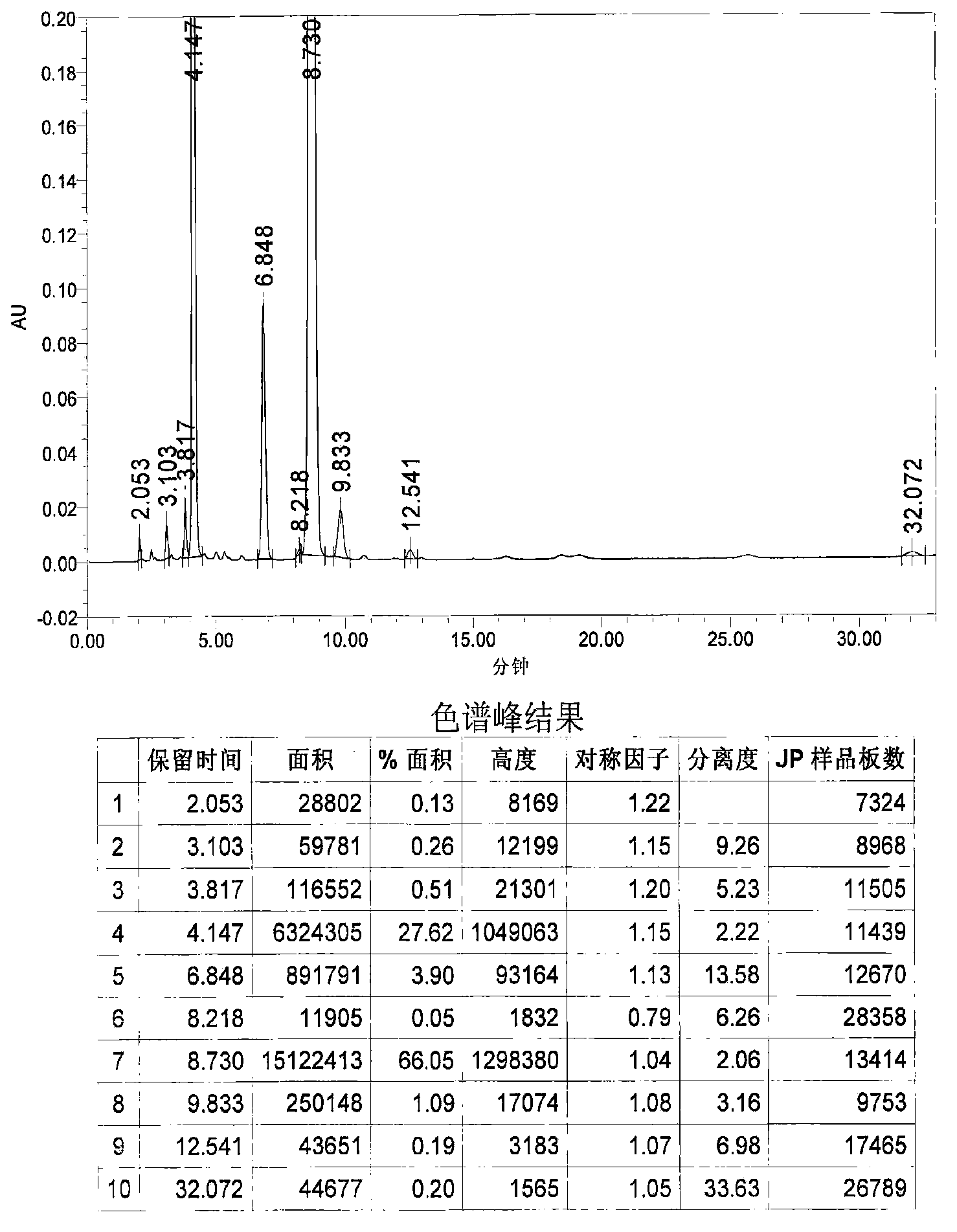
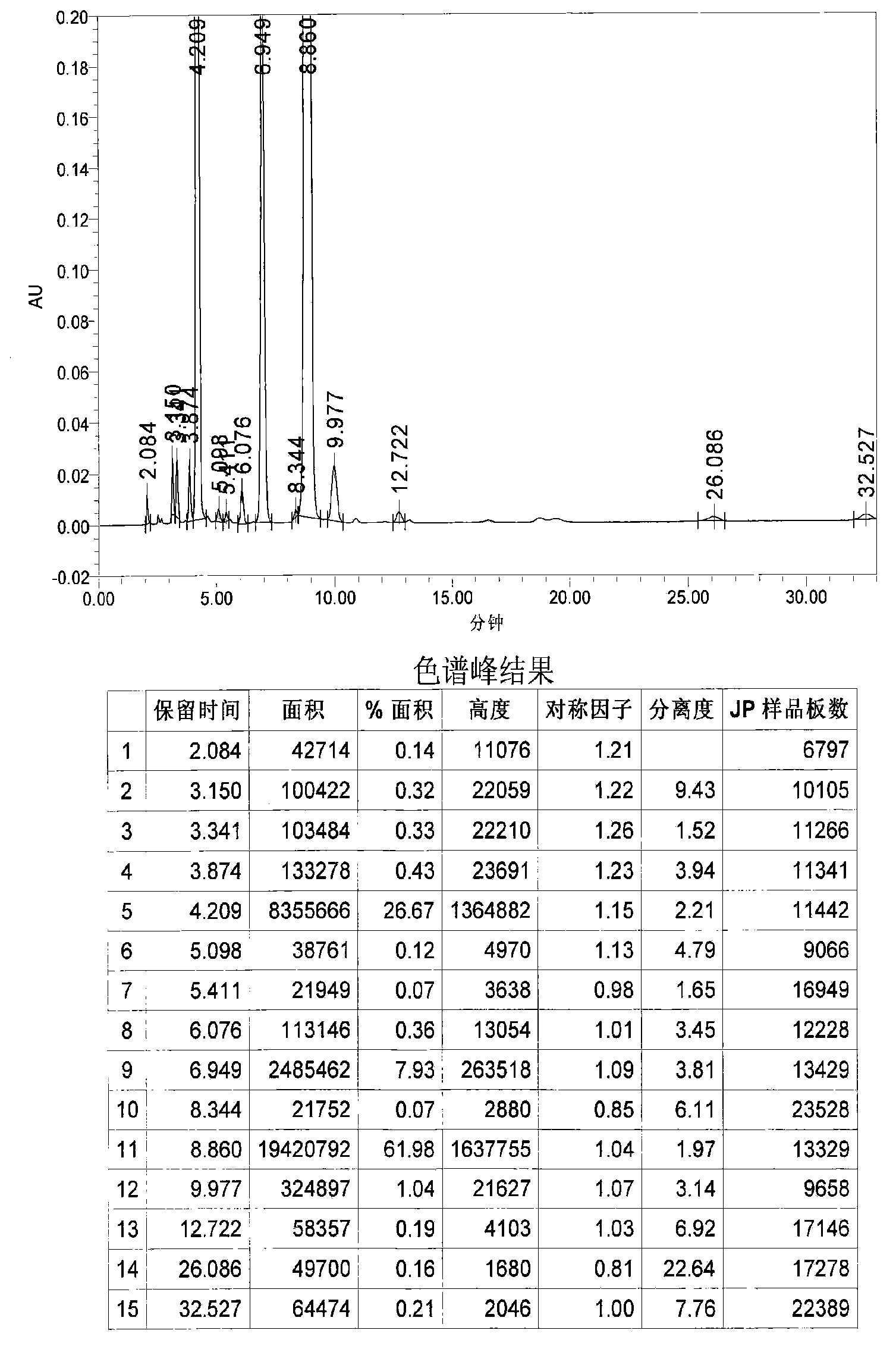
Image
Examples
Embodiment 1
[0031] 1. Grignard reaction: In a 20L dry reaction kettle, add 504g (21mol) of magnesium powder, 6.4L of anhydrous methyl tert-butyl ether and tetrahydrofuran (volume ratio 15:1) mixed solvent and a catalytic amount of iodine in sequence at room temperature , under stirring, first drop 0.6~0.8L of anhydrous methyl tert-butyl ether solution (4L in total) containing 3.22kg (20mol) of p-chlorobenzyl chloride, and stir until the reaction is initiated (the solution becomes cloudy and the temperature rises), and the temperature Raise to about 40°C, continue to add the remaining solution, keep at 40-50°C, drop for about 1-2 hours, and keep warm for 1-2 hours.
[0032] 2. Condensation reaction: the above reaction system is lowered to room temperature, and directly dropwise add a solution of 1.85kg (20mol) of epichlorohydrin 4L of anhydrous methyl tert-butyl ether to the reaction kettle under stirring, control the rate of addition, and maintain the temperature At about 40°C, drop it fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com