Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Phenylpiperidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenylpiperidine is a chemical compound with a phenyl moiety directly attached to piperidine. There are a variety of pharmacological effects associated with phenylpiperidines including morphine-like activity or other central nervous system effects.
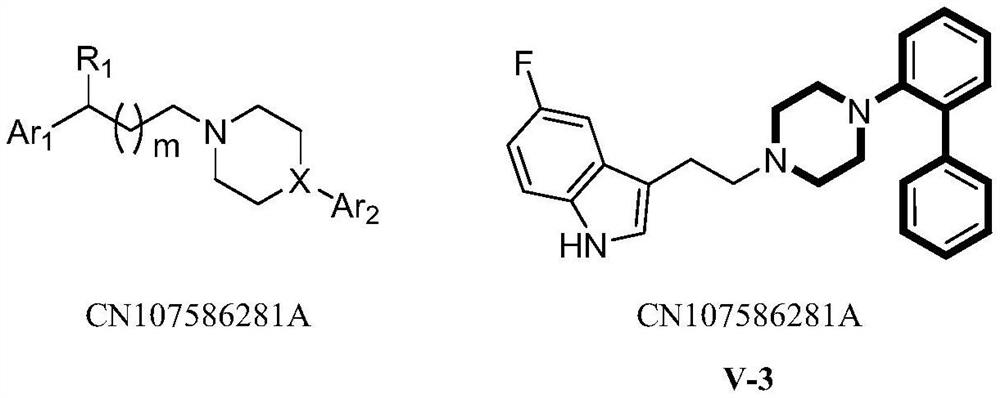
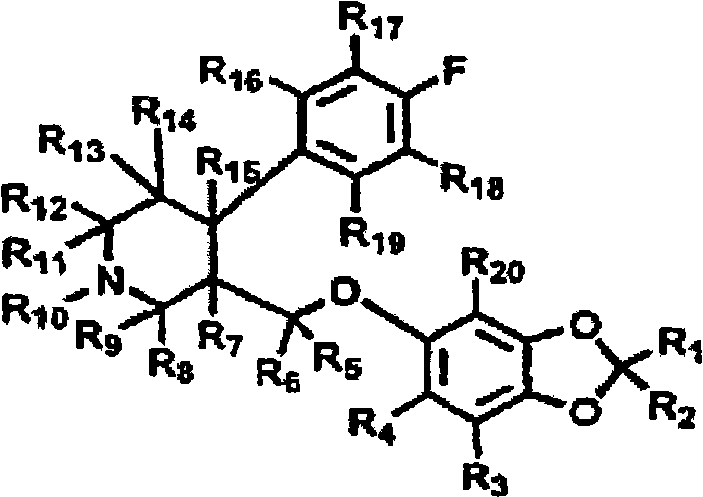
Substituted phenylpiperidines with serotoninergic activity and enhanced therapeutic properties
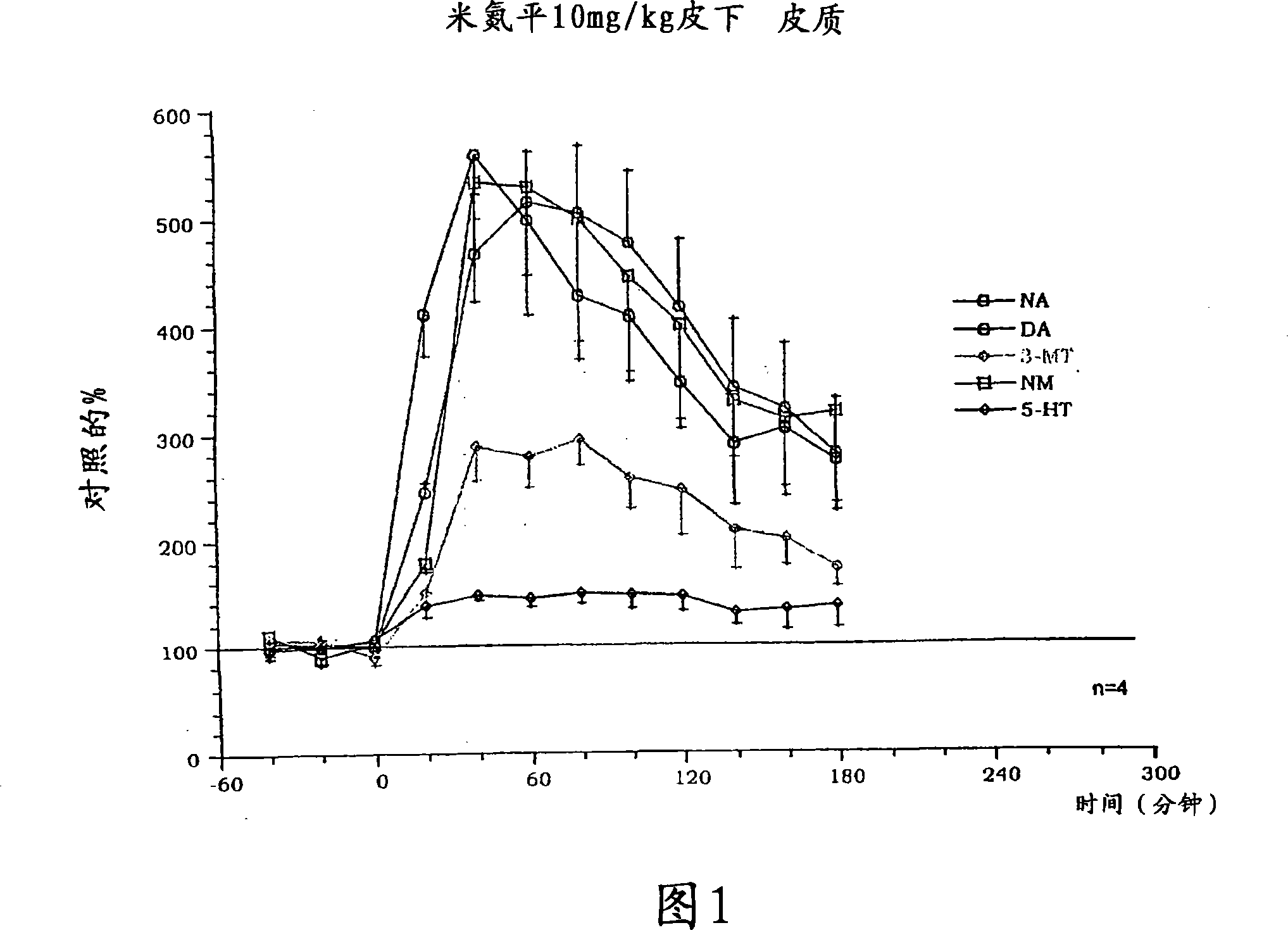
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:AUSPEX PHARMA INC
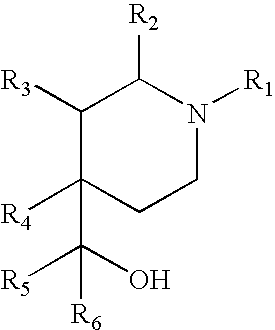
Substituted 4-phenylpiperidines, their preparation and use
The present invention provides a compound having the structure:whereinR1, R2, R3, R4, and R5 are each independently H, halogen, CF3 or C1-C4 alkyl,wherein two or more of R1, R2, R3, R4, or R5 are other than H;R6 is H, OH, or halogen; andB is a substituted or unsubstituted heterobicycle,wherein when R1 is CF3, R2 is H, R3 is F, R4 is H, and R5 is H, or R1 is H, R2 is CF3, R3 is H, R4 is CF3, and R5 is H, or R1 is Cl, R2 is H, R3 is H, R4 is F, and R5 is H, or R1 is CF3, R2 is H, R3 is F, R4 is H, and R5 is H, or R1 is CF3, R2 is F, R3 is H, R4 is H, and R5 is H, or R1 is Cl, R2 is F, R3 is H, R4 is H, and R5 is H, then B is other thanor a pharmaceutically acceptable salt thereof.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
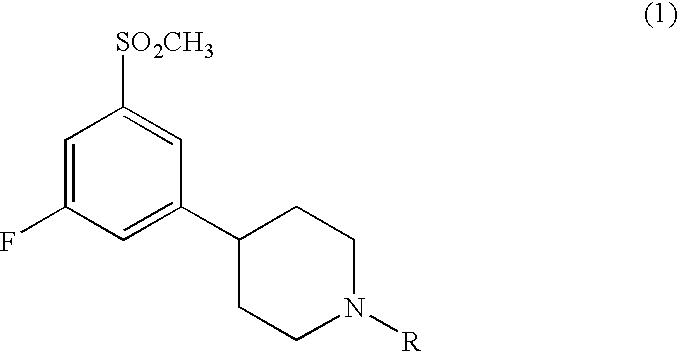
3,5-Disubstituted Phenyl-Piperidines as Modulators of Dopamine Neurotransmission
The present invention relates to compounds having therapeutic effects against disorders in the central nervous system, and in particular substituted phenylpiperidines of the formula 1:wherein R is as defined herein.
Owner:SANIONA AB
Substituted Phenylpiperidine Derivatives As Melanocortin-4 Receptor Modulators
InactiveUS20110086836A1Valid choiceUseful in treatmentBiocideNervous disorderDiseasePhenylpiperidine
The present invention relates to substituted phenylpiperidine derivatives as melanocortin-4 receptor modulators. Depending on the structure and the stereochemistry the compounds of the invention are either selective agonists or selective antagonists of the human melanocortin-4 receptor (MC-4R). The agonists can be used for the treatment of disorders and diseases such as obesity, diabetes and sexual dysfunction, whereas the antagonists are useful for the treatment of disorders and diseases such as cancer cachexia, muscle wasting, anorexia, anxiety and depression. Generally all diseases and disorders where the regulation of the MC-4R is involved can be treated with the compounds of the invention.
Owner:SANTHERA PHARMA SCHWEIZ
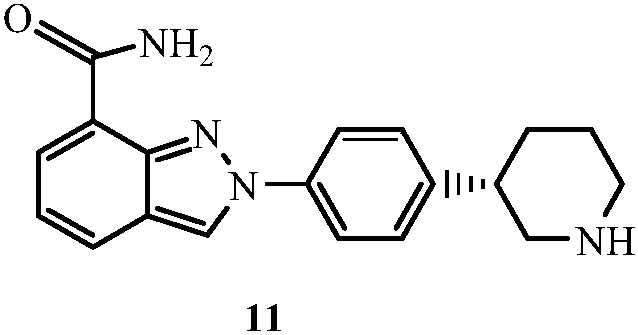
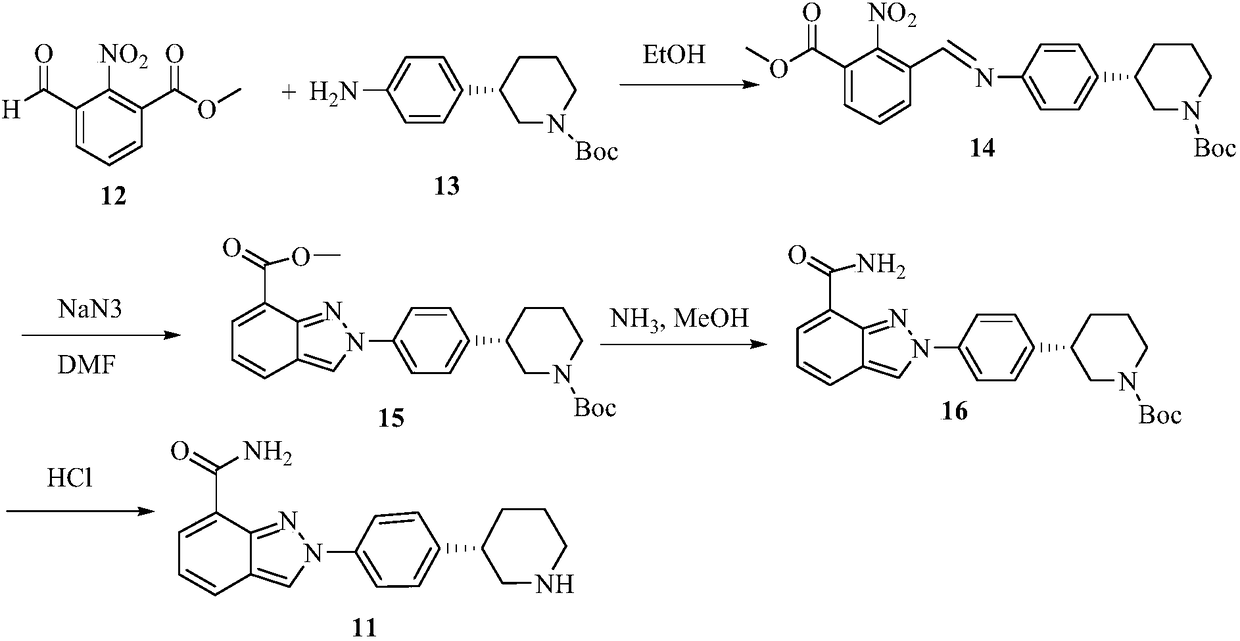
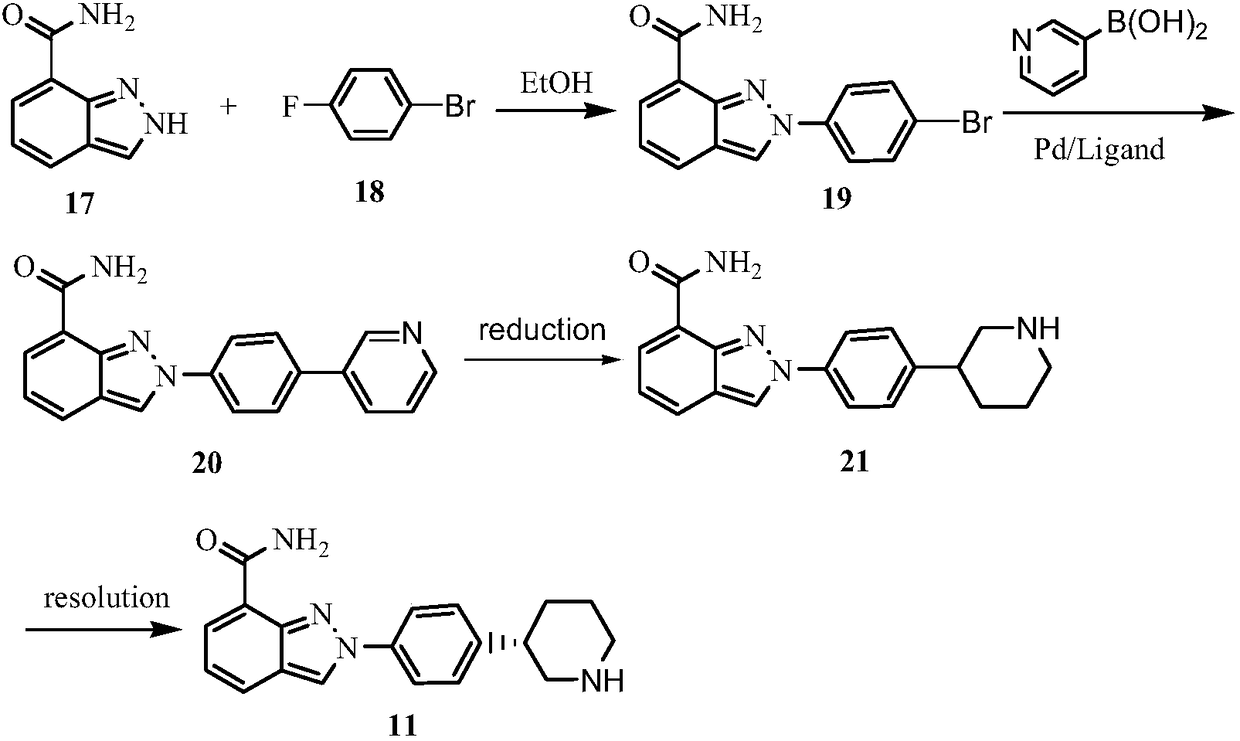
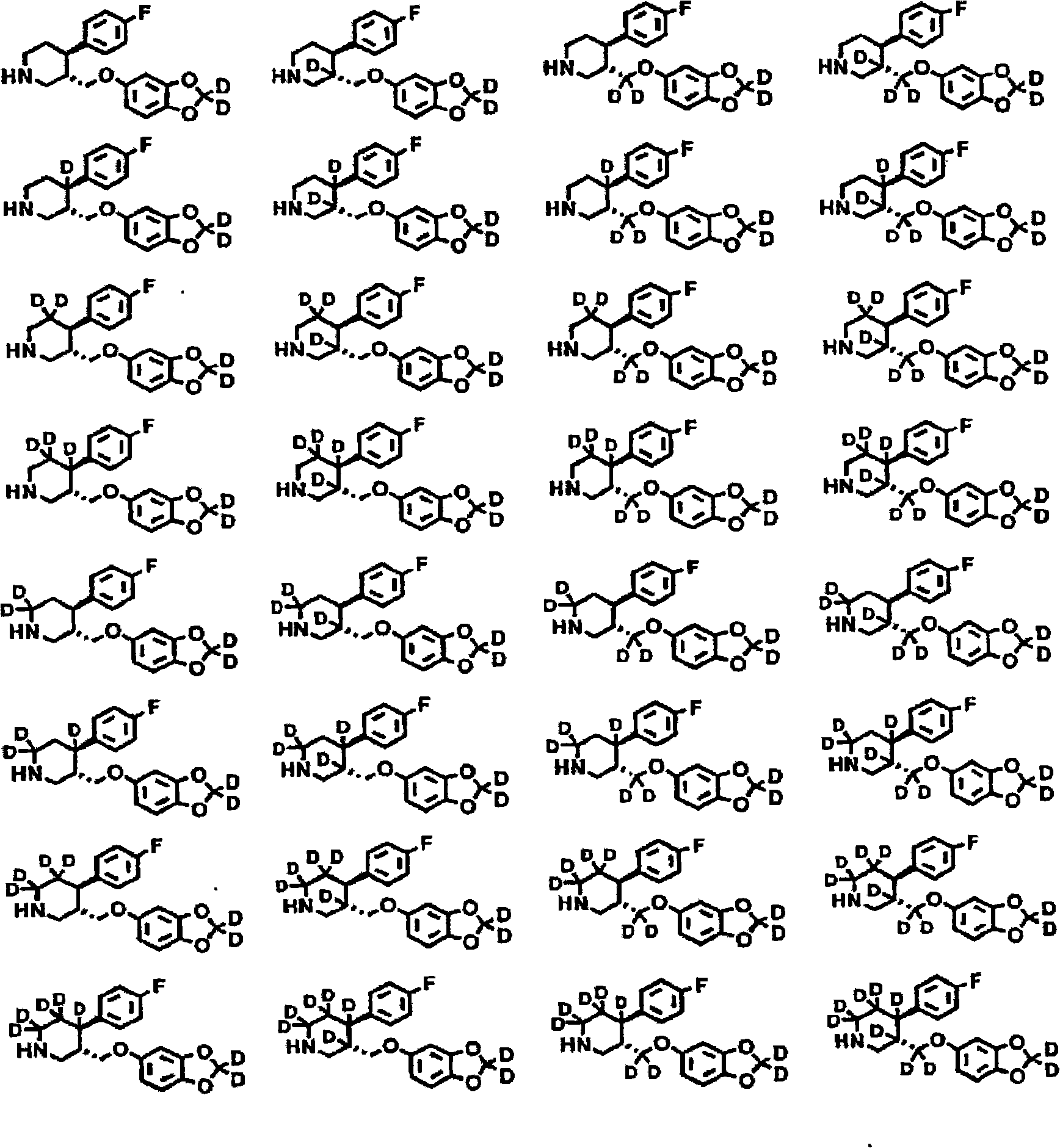
[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-(4-bromo-3-methyl-5-propoxy-thiophen-2-yl)-methanone hydrochloride as an inhibitor of mast cell tryptase
The present invention extends to the compound of formula I: or a prodrug, pharmaceutically acceptable salt, or solvate of said compound. Furthermore, the present invention is directed to a pharmaceutical composition comprising a pharmaceutically effective amount of the compound of formula I, and a pharmaceutically acceptable carrier. Furthermore, the present invention is directed to the use of a compound of formula I as an inhibitor of tryptase, comprising introducing the compound into a composition comprising tryptase. In addition, the present invention is directed to the use of a compound of formula I for treating a patient suffering from, or subject to, a physiological condition in need of amelioration of an inhibitor of tryptase comprising administering to the patient a therapeutically effective amount of the compound of claim 1 The present invention is directed also to the preparation of a compound of formula I.
Owner:AVENTIS PHARMA INC
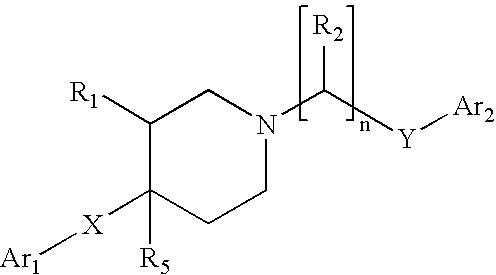
Disubstituted phenylpiperidines/piperazines as modulators of dopamine neurotransmission
The present invention relates to compounds which have therapeutic effects against disorders in the central nervous system, and in particular new 4-(ortho,meta-disubstituted phenyl)-1-alkypiperidines and piperazines. wherein R1, R2, R3 and X are as defined.
Owner:SANIONA AB
Synthesis method for (R)-3-phenylpiperidine or/and (S)-3-phenylpiperidine and synthesis method for chiral intermediate of niraparib
InactiveCN108203404AHigh reaction yieldMild reaction conditionsOrganic chemistry methodsSynthesis methodsOrganic synthesis
The invention belongs to the technical field of organic synthesis. The synthesis method firstly provided by the invention takes benzyl-4-oxopiperidine as a starting material, and the starting materialis subjected to Grignard reaction, elimination reaction, hydrogenation reduction reaction and chiral resolution in sequence to successfully obtain a target product (R)-3-phenylpiperidine or / and (S)-3-phenylpiperidine. The synthesis method sencondly provided by the invention takes the same starting raw material for Grignard reaction, organic silicon reagent is used for removing a hydroxide radical, and benzyl is removed by catalytic hydrogenation reaction; finally, the chiral resolution is carried out to obtain a target product. The (S)-3-phenylpiperidine can be synthesized according to the synthesis method. (S)-3-p-aminosalicylic phenylpiperidine can be synthesized according to the third aspect; or according to the fourth aspect, (S)-3-p-bromophenyl piperidine is synthesized to serve asthe key intermediate for preparing the niraparib. According to the synthesis method for (R)-3-phenylpiperidine or / and (S)-3-phenylpiperidine and the synthesis method for chiral intermediate of niraparib, production cost is obviously lowered, and the synthesis methods are favorable for the large-scale industrial production of a niraparib medicine.
Owner:SHANGHAI BIOBOND PHARMA
Therapeutic agents useful for treating pain
4-Tetrazolyl-4-phenylpiperidine Compounds, compositions comprising an effective amount of a 4-Tetrazolyl-4-phenylpiperidine Compound, methods for treating or preventing pain or diarrhea in an animal comprising administering to an animal in need thereof an effective amount of a 4-Tetrazolyl-4-phenylpiperidine Compound and methods for stimulating opioid-receptor function in a cell comprising contacting a cell capable of expressing an opioid receptor with an effective amount of a 4-Tetrazolyl-4-phenylpiperidine Compound are disclosed.
Owner:PURDUE PHARMA LP
[4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase
The present invention is directed to an indole benzylamine compound of formula I:useful as an inhibitor of tryptase. In addition, the present invention is directed to the use of the compound for treating a patient suffering from, or subject to, a physiological condition in need of amelioration by inhibition of tryptase, comprising administering to the patient of a therapeutically effective amount of the compound, and to a pharmaceutical composition comprising a pharmaceutically effective amount of the compound of formula I, and a pharmaceutically acceptable carrier.
Owner:SANOFI SA
Crystalline forms of 4-[2-(4-methylphenylsulfanyl)-phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition and uses thereof
Crystalline forms of 4-[2-(4-methylphenylsulfanyl)-phenyl]piperidine and salts thereof are provided e.g. for the treatment of neuropathic pain.
Owner:H LUNDBECK AS
Disubstituted phenylpiperidines/piperazines as modulators of dopamine neurotransmission
The present invention relates to compounds which have therapeutic effects against disorders in the central nervous system, and in particular new 4-(ortho, meta-disubstituted phenyl)-1-alkypiperidines and piperazines.wherein R1, R2, R3 and X are as defined.
Owner:SANIONA AB
Substituted azaheterocyclecarboxylic acid
A compound of the chemical formula (I) and its pharmaceutically acceptable salt: ##STR1## wherein Y is C.sub.2 -C.sub.4 alkylene; Z is a valence bond or C.sub.1 -C.sub.6 alkylene; R.sup.1 is substituted or unsubstituted aryl (e.g., phenyl and naphthyl) or heteroaryl (e.g., thienyl and furyl); R.sup.2 is hydrogen or C.sub.1 -C.sub.6 alkyl; R.sup.3 is hydrogen, hydroxy or the like; R.sup.4 is substituted or unsubstituted benzyl or the like. Typical examples are (2S*,3S*,4R*,5R*)-4-Carboxy-3-[N-(5-Isopropyl-2-methoxy-benzyl)amino]-5-me thyl-2-phenylpyrrolidine and (2S*,3S*,5S*)-5-Carboxy-3-[N-(2-methoxy-5-trifluoromethoxybenzyl)amino]-2- phenylpiperidine. The novel substituted azaheterocyclecarboxylic acids in this invention have excellent substance P antagonistic activity and are thus useful for the treatment of gastrointestinal disorders, central nervous system disorders, allergy, inflammatory diseases, asthma, pain, emesis or migraine in mammals, especially humans.
Owner:PFIZER INC
Crystalline forms of 4- [2- (4-methylphenylsulfanyl) -phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition for the treatment of neuropathic pain
Crystalline forms of 4-[2-(4-methylphenylsulfanyl)-phenyl]piperidine and salts thereof are provided e.g. for the treatment of neuropathic pain.
Owner:H LUNDBECK AS
Substituted phenylpiperidines with serotoninergic activity and enhanced therapeutic properties
InactiveCN101360742AOrganic active ingredientsNervous disorderCompulsive disordersDiabetic retinopathy
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive- compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:AUSPEX PHARMA INC
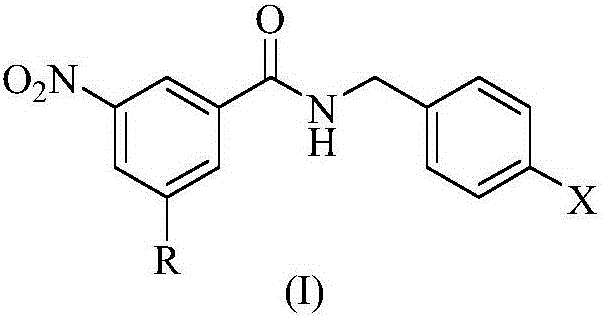
N-benzylbenzamide compounds and preparation method thereof
ActiveCN106543106AGood curative effectHigh activityAntibacterial agentsOrganic chemistryAntituberculous drugsPhenyl group
The invention relates to N-benzylbenzamide compounds represented by formula (I), a preparation method and a medicinal use thereof, and an anti-tuberculosis medicinal composition adopting the compounds as an effective component. The N-benzylbenzamide compounds are concretely characterized in that a substituent group in the para-position of a benzyl group of the N-benzylbenzamide compounds is a nitrogen-containing heterocyclic segment; and in the formula (I), R represents a trifluoromethyl group or a nitro group, and X represents a 4-thiomorpholinyl group, an octahydro-2H-isoindole-2-yl group, an isoindoline-2-yl group, a 4-substituted piperidine-1-yl group, a (4-substituted phenyl)piperidine-1-yl group or a (4-substituted phenyl)piperazine-1-yl group.
Owner:ZHEJIANG STARRY PHARMA +1
Libraries of n-(2-oxo-1-phenylpiperidin-3-yl)sulfonamides for the identification of biological and pharmacological activity
InactiveUS20120122708A1Organic active ingredientsOrganic chemistryFunctional activityBiological target
New compounds are continually sought after for the treatment and prevention of disorders. The invention relates to N-(2-oxo-1-phenylpiperidin-3-yl)sulfonamides which can be biologically and pharmacologically traced, in order to be used in the search for, and identification of, new lead compounds that can modulate the functional activity of a biological target.
Owner:INST UNIV DE CIENCIA I TECH SA
Synthesis method of (R)-3-phenylpiperidine or/and (S)-3-phenylpiperidine and synthesis method of chiral intermediates of niraparib
InactiveCN109810047AHigh reaction yieldMild reaction conditionsOrganic chemistry methodsSynthesis methodsCombinatorial chemistry
The invention provides a synthesis method on the first aspect, and by means of the synthesis method, a target product, namely (R)-3-phenylpiperidine or / and (S)-3-phenylpiperidine is / are successfully prepared with N-protected 3-piperidone as a starting material; the invention provides a synthesis method on the second aspect, and by means of the synthesis method, the target product, namely the (R)-3-phenylpiperidine or / and the (S)-3-phenylpiperidine is / are successfully prepared with the same N-protected 3-piperidone as the starting material and by ingeniously utilizing an organic silicon reagent. Chiral intermediates alpha, beta and gamma of niraparib are synthesized from the (S)-3-phenylpiperidine correspondingly according to the third aspect, the fourth aspect and the fifth aspect of the invention. In one word, according to the synthesis method of the (R)-3-phenylpiperidine or / and the (S)-3-phenylpiperidine and the synthesis method of the chiral intermediates alpha, beta and gamma of the niraparib, the production cost is obviously lowered, and large-scale industrial production of niraparib medicines is facilitated.
Owner:SHANGHAI BIOBOND PHARMA
(4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use
The present invention pertains generally to the field of therapeutic compounds. More specifically the present invention pertains to certain (4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds that, inter alia, inhibit 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit 11β-hydroxysteroid dehydrogenase type 1; to treat disorders that are ameliorated by the inhibition of 11β-hydroxysteroid dehydrogenase type 1; to treat the metabolic syndrome, which includes disorders such as type 2 diabetes and obesity, and associated disorders including insulin resistance, hypertension, lipid disorders and cardiovascular disorders such as ischaemic (coronary) heart disease; to treat CNS disorders such as mild cognitive impairment and early dementia, including Alzheimer's disease; etc.
Owner:THE UNIV OF EDINBURGH
New disubstituted phenylpiperidines as modulators of dopamine neurotransmission
The present invention relates to compounds which have therapeutic effects against disorders in the central nervous system, and in particular new 4-(ortho, meta-disubstituted phenyl)-1-alkypiperidines and piperazines, wherein R1, R2, R3 and Y are as defined.
Owner:NSAB FILIAL AF NEUROSEARCH SVERIGE
Preparation method for 2-(alpha-hydroxyl aryl) benzimidazole compound
The invention discloses a preparation method for an optically pure 2-(alpha-hydroxyl aryl) benzimidazole compound. The preparation method comprises the specific steps that 1-butyloxycarboryl-2-arylcyclopropane-benzimidazole is mixed with a photosensitizer, N-phenylpiperidine, a chiral catalyst and an organic solvent, under the condition that the temperature is equal to or lower than -78 DEG C, degassing is conducted 2-3 times by using a vacuum pump, degassing is conducted for 5-10 minutes at a time, and then the mixture is exposed to a 3-10 W blue lamp at the temperature of -28 DEG C--32 DEG C, a reaction is carried out for 20-48 hours, after the reaction is finished, column chromatography separation, rotary evaporation and vacuum drying are conducted in sequence, and then the product is obtained. By means of the method, the optically pure 2-(alpha-hydroxyl aryl) benzimidazole compound can be directly, efficiently and generally synthesized. The method has the advantages of being mild in reaction condition, high in yield and high in substrate universality.
Owner:HENAN NORMAL UNIV
Preparation method of ALK inhibitor Brigatinib
ActiveCN111138492AEasy to operateReaction raw materials are readily availableGroup 5/15 element organic compoundsPhenylpiperidineAniline
The invention relates to the technical field of medicines, and relates to a preparation method of a medicine ALK inhibitor AP26113. The method comprises the following steps: (1) carrying out substitution reaction of aromatic amine on 2,4,5-trichloropyrimidine and 2-(dimethyloxyphosphate)aniline under the action of an acid applying agent to obtain an intermediate 1 (2,5-dichloro-N-(2-(dimethylphosphine)phenyl)pyrimidine-4-amine), and pulping the intermediate; (2) carrying out aromatic amine substitution reaction on 2-nitro-5-fluoroanisole and 1-methyl-4-(4-piperidyl)-piperazine under the actionof an acid applying agent to obtain a compound 1-(1-(3-methoxy-4-nitrophenyl)piperidin-4-yl)4-methylpiperazine, dissolving the compound with an organic solvent, and extracting the compound with an acidic solvent; (3) carrying out palladium-carbon catalytic hydrogenation on the product obtained in the step (2) to obtain an intermediate 2 (2-methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperazinyl]-aniline); (4) carrying out amine substitution reaction on the intermediate 1 and the intermediate 2 under an acidic catalytic condition to obtain a crude product of Brigatinib. The yield of the Brigatinibreaches 50% or above, and the purity of the Brigatinib reaches 99.9% or above.
Owner:SHENYANG PHARMA UNIVERSITY
Phenylpiperidines and phenylpyrrolidines
Substituted phenylpiperidines and phenylpyrrolidines of formula (I), compositions containing them, and methods of making and using them to treat histamine-mediated conditions.
Owner:JANSSEN PHARMA NV
Preparation method of niraparib intermediate (S)-3-(4-bromophenyl) piperidine
PendingCN111808016ALow costAvoid Chiral SwitchingOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention discloses a preparation method of a niraparib intermediate that is (S)-3-(4-bromophenyl) piperidine, which comprises the following steps: by using chiral 1-phenylethylamine as a raw material, carrying out alkylation, condensation, cyclization and reduction, and finally removing an amino protection group to obtain the (S)-3-(4-bromophenyl) piperidine. The preparation method of the (S)-3-(4-bromophenyl) piperidine has the advantages of short route, few steps, simple and convenient process operation, avoidance of use of dangerous reagents, highly toxic reagents and irritant malodorous reagents, reduction of potential safety hazards to operators, reduction of the operation safety level of production, environmental protection, and facilitation of industrialization.
Owner:成都正善达生物医药科技有限公司
3,5-disubstituted phenyl-piperidines as modulators of dopamine neurotransmission
ActiveUS8501777B2Improve effectivenessReduction tendencyBiocideNervous disorderTherapeutic effectPhenylpiperidine
The present invention relates to compounds having therapeutic effects against disorders in the central nervous system, and in particular substituted phenylpiperidines of the formula 1:wherein R is as defined herein.
Owner:SANIONA AB
Disubstituted phenylpiperidines as modulators of dopamine and serotonin neurotransmission
InactiveUS7851629B2Improve effectivenessIncrease neurotransmissionBiocideOrganic chemistryDiseaseMedicine
The present invention relates to compounds which have therapeutic effects against disorders in the central nervous system, and in particular new 4-(ortho,meta-disubstituted phenyl)-1-alkypiperidines.wherein R1, R2, and R3 are as defined.
Owner:NSAB FILIAL AF NEUROSEARCH SVERIGE
Duraglutin artificial antigen and preparation method thereof
InactiveCN111423358AKeep featuresStrong specificityOvalbuminSerum albuminImmune profilingButanedioic acid
The invention provides a duramine artificial antigen and a preparation method thereof. The nitrile group of the 4-phenylpiperidine-4-carbonitrile is ingeniously reduced into the amino group; carboxylgroups capable of being used for crosslinking are introduced, so the characteristic original appearance of a duraglutin main ring is maintained; butanedioic anhydride is introduced, so acetyl functional groups on a duraglutin side chain is maintained on the said chain; the hapten synthesized by the process can maintain the characteristic group original appearance of duraglutin, and the synthesizedcorresponding artificial antigen has good specificity. The preparation process is simple, and the raw material source is not limited; the prepared artificial antigen can be used for animal immunization to obtain a corresponding duraglutin antibody, has strong antibody specificity, can be used in immunoassay, is suitable for research of various duraglutin immunoassay methods, and can be used as akey raw material for production of a duraglutin immunochromatographic kit.
Owner:HANGZHOU BIOTEST BIOTECH CO LTD
4-(penyl-piperidin-4-ylidene-methyl)-benzamide derivatives and their use for the treatment of pain, anxiety or gastrointestinal disorders
InactiveCN1527828AImprove system performanceGood pain reliefOrganic active ingredientsNervous disorderDiseaseFuran
Compounds of general formula I[Chemical formula should be inserted here. Please see paper copy] R1 is selected from any one of phenyl, pyridinyl, pyrrolyl, thienyl, furanyl, imidazolyl, triazolyl, and pyridine N-oxide; where each R1 phenyl ring and R1 heteroaromatic ring may optionally and independently be further substituted by 1,2 or 3 substituents selected from straight and branched C1-C6 alkyl, NO2, CF3, C1-C6 alkoxy, chloro, fluoro, bromo, and iodo. The substitutions on the phenyl ring and on the heteroaromatic ring may take place in any position on said ring systems; are disclosed and claimed in the present application, as well as their pharmaceutically acceptable salts and pharmaceutical compositions comprising the novel compounds and their use in therapy, in particular in the management of pain, anxiety and functional gastrointestinal disorders.
Owner:ASTRAZENECA AB
New disubstituted phenylpiperidines as modulators of dopamine and serotonin neurotransmission
InactiveUS20070179185A1Reduce depressionHigh activityBiocideOrganic chemistryMedicineTherapeutic effect
The present invention relates to compounds which have therapeutic effects against disorders in the central nervous system, and in particular new 4-(ortho,meta-disubstituted phenyl)-1-alkypiperidines. wherein R1, R2, and R3 are as defined.
Owner:NSAB FILIAL AF NEUROSEARCH SVERIGE
Application of Heterocyclic Substituted Phenylpiperazine or Phenylpiperidine Derivatives in Antidepressants
ActiveCN112209917BStrong inhibitory activityStrong antidepressant activityOrganic active ingredientsNervous disorderPhenylpiperazineReceptor
The invention discloses the application of a heterocyclic substituted phenylpiperazine (pyridine) derivative in antidepressant drugs. 1A and 5‑HT 7 The two receptors have good inhibitory activity, the compound has good antidepressant activity in vivo, has the effect of improving cognitive function and is well tolerated in animals. The heterocyclic substituted phenylpiperazine (pyridine) derivatives are compounds represented by general structural formula (I) or pharmaceutically acceptable salts thereof:
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
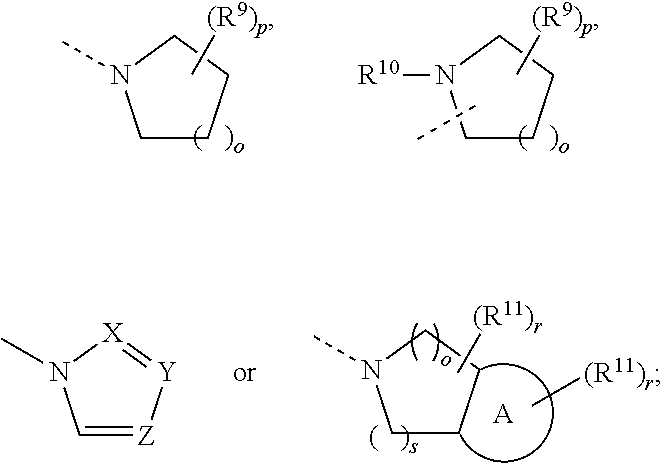
Serotonin 5-HI1A and dopamin D2 receptor ligands
The present invention relates to a novel series of 4-phenylpiperazines, 4-phenyl-piperidines and 4-phenyl-1,2,3,6-tetrahydropyridines compound of general formula (I) wherein A is alkylene, alkenylene, alkynylene, and C3-7 cycloalkylene; R<1> is a C3-10 alkyl, alkenyl, or alkynyl group, cycloalk(en)yl, cycloalk(en)yl-alk(en / yn)yl, trifluoromethylsulfonyl, or alkylsulfonyl, R<2> - R<5> are optional substituents; R<9> and R<10> are hydrogen, alkyl or together form an ethylene or propylene bridge; W is O or S; V is O, S, CR<6>R<7>, or NR<8> wherein R<6>, R<7>, and R<8> are hydrogen or alkyl, alkenyl, cycloalkyl, cycloalkylalkyl, optionally substituted arylalkyl or aryl, or R<6> and R<7> constitute a 3-7 membered spiroring; Z is -(CH2)m-, m being 2 or 3 or Z is -CH=CH-; X is N, C or CH; show effects on central serotonin 5-HT1A and dopamine D2 receptors. Thus the novel compounds are useful in the treatment of certain psychic and neurologic disorders in particular psychosis.
Owner:H LUNDBECK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-(4-bromo-3-methyl-5-propoxy-thiophen-2-yl)-methanone hydrochloride as an inhibitor of mast cell tryptase [4-(5-Aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-(4-bromo-3-methyl-5-propoxy-thiophen-2-yl)-methanone hydrochloride as an inhibitor of mast cell tryptase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/17f95333-8855-4f54-942f-9a821f112b41/US20070142435A1-20070621-D00001.png)
![[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-(4-bromo-3-methyl-5-propoxy-thiophen-2-yl)-methanone hydrochloride as an inhibitor of mast cell tryptase [4-(5-Aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-(4-bromo-3-methyl-5-propoxy-thiophen-2-yl)-methanone hydrochloride as an inhibitor of mast cell tryptase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/17f95333-8855-4f54-942f-9a821f112b41/US20070142435A1-20070621-D00002.png)
![[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-(4-bromo-3-methyl-5-propoxy-thiophen-2-yl)-methanone hydrochloride as an inhibitor of mast cell tryptase [4-(5-Aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-(4-bromo-3-methyl-5-propoxy-thiophen-2-yl)-methanone hydrochloride as an inhibitor of mast cell tryptase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/17f95333-8855-4f54-942f-9a821f112b41/US20070142435A1-20070621-C00001.png)






![[4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase [4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c1d2ed5a-8f5b-4813-997c-fef82533a849/US20110201647A1-20110818-D00001.png)
![[4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase [4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c1d2ed5a-8f5b-4813-997c-fef82533a849/US20110201647A1-20110818-D00002.png)
![[4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase [4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c1d2ed5a-8f5b-4813-997c-fef82533a849/US20110201647A1-20110818-C00001.png)
![Crystalline forms of 4-[2-(4-methylphenylsulfanyl)-phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition and uses thereof Crystalline forms of 4-[2-(4-methylphenylsulfanyl)-phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/db084815-2b02-4f91-af5a-11374af03865/US08299095-20121030-D00000.png)
![Crystalline forms of 4-[2-(4-methylphenylsulfanyl)-phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition and uses thereof Crystalline forms of 4-[2-(4-methylphenylsulfanyl)-phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/db084815-2b02-4f91-af5a-11374af03865/US08299095-20121030-D00001.png)
![Crystalline forms of 4-[2-(4-methylphenylsulfanyl)-phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition and uses thereof Crystalline forms of 4-[2-(4-methylphenylsulfanyl)-phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/db084815-2b02-4f91-af5a-11374af03865/US08299095-20121030-D00002.png)



![Crystalline forms of 4- [2- (4-methylphenylsulfanyl) -phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition for the treatment of neuropathic pain Crystalline forms of 4- [2- (4-methylphenylsulfanyl) -phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition for the treatment of neuropathic pain](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b43c2aad-2ff5-49ef-adde-a476a199ed6e/US20090264465A1-20091022-D00001.png)
![Crystalline forms of 4- [2- (4-methylphenylsulfanyl) -phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition for the treatment of neuropathic pain Crystalline forms of 4- [2- (4-methylphenylsulfanyl) -phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition for the treatment of neuropathic pain](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b43c2aad-2ff5-49ef-adde-a476a199ed6e/US20090264465A1-20091022-D00002.png)
![Crystalline forms of 4- [2- (4-methylphenylsulfanyl) -phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition for the treatment of neuropathic pain Crystalline forms of 4- [2- (4-methylphenylsulfanyl) -phenyl] piperidine with combined serotonin and norepinephrine reuptake inhibition for the treatment of neuropathic pain](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b43c2aad-2ff5-49ef-adde-a476a199ed6e/US20090264465A1-20091022-D00003.png)
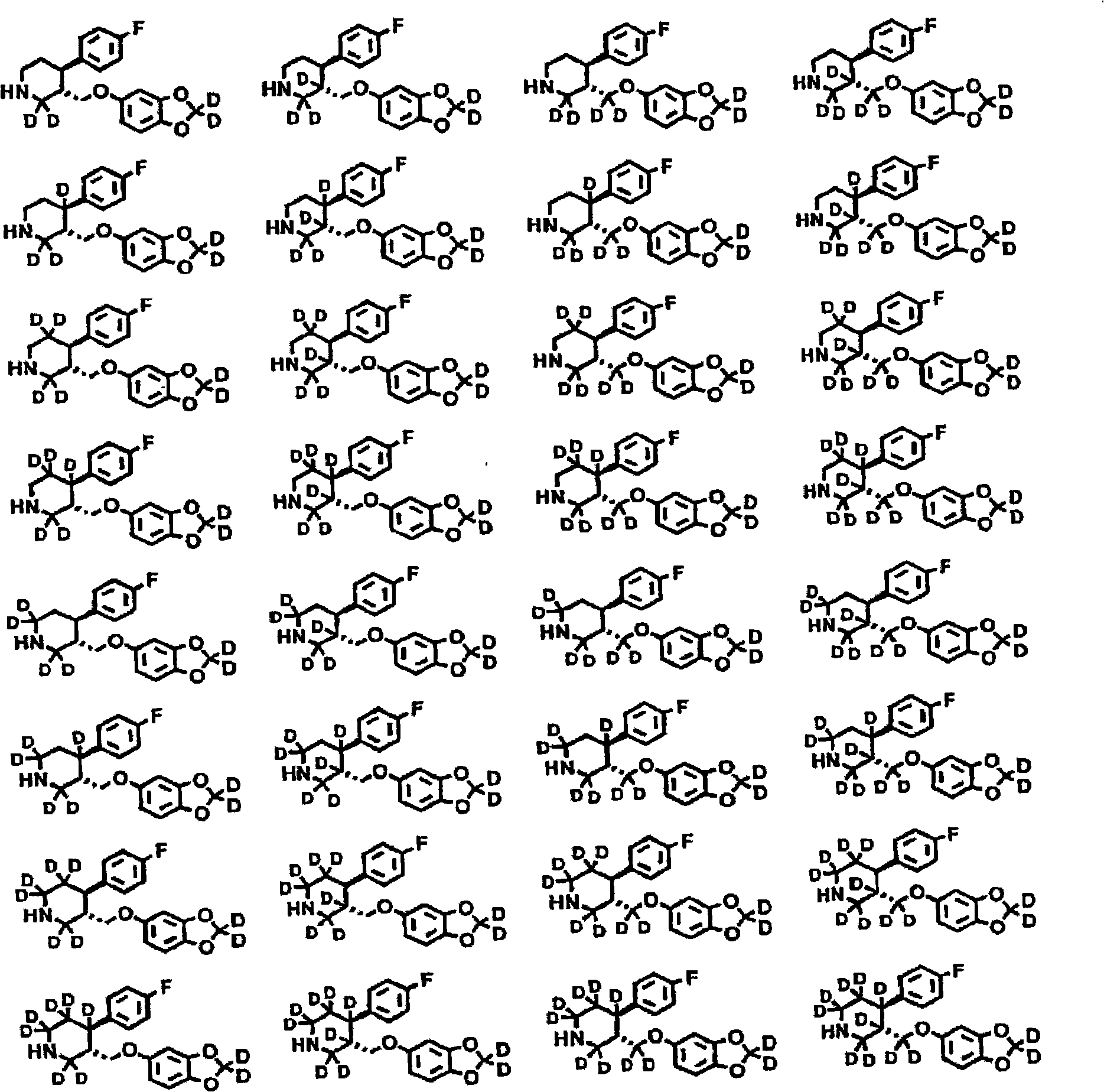







![(4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use (4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6bf0a989-bb3d-45b0-9cbb-64f93c11bc1e/US08642621-20140204-C00001.png)
![(4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use (4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6bf0a989-bb3d-45b0-9cbb-64f93c11bc1e/US08642621-20140204-C00002.png)
![(4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use (4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6bf0a989-bb3d-45b0-9cbb-64f93c11bc1e/US08642621-20140204-C00003.png)