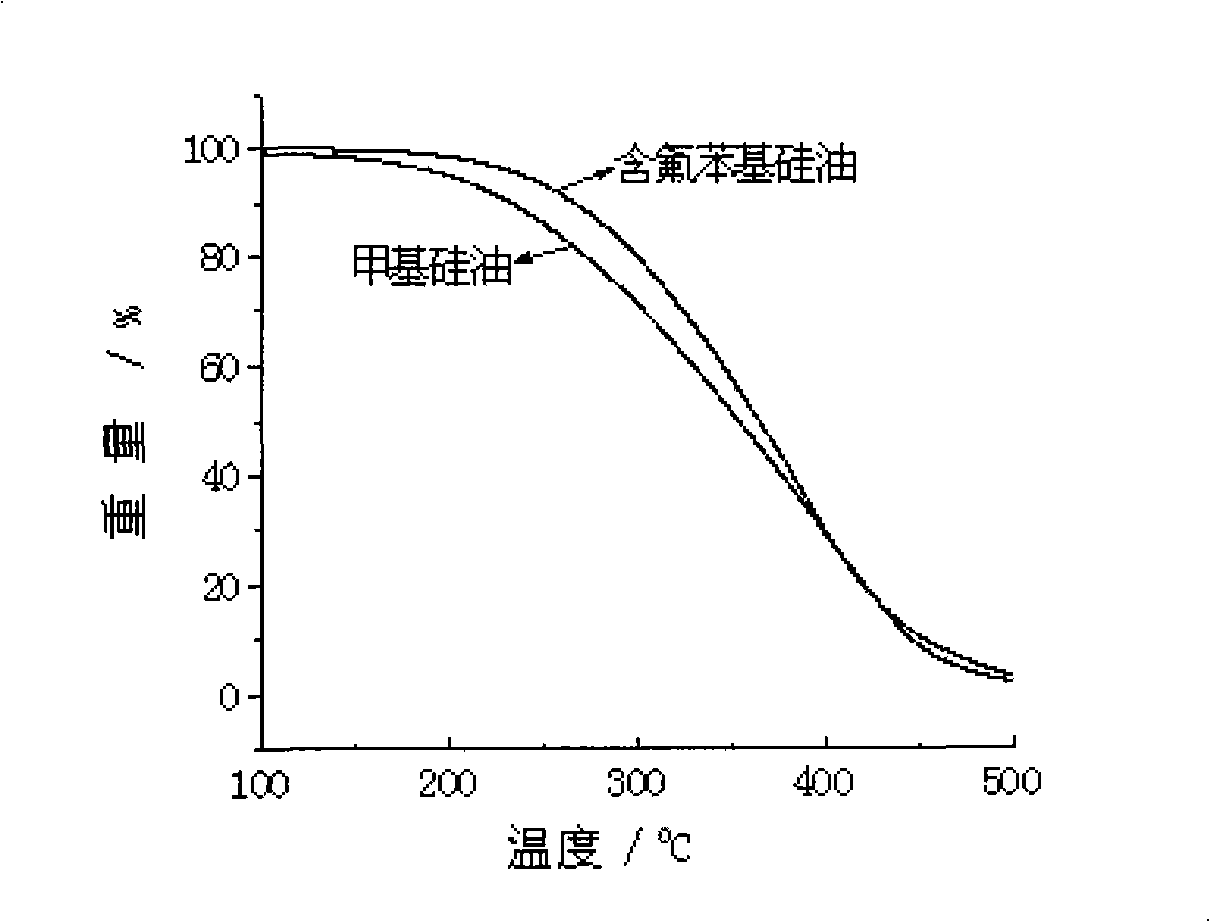
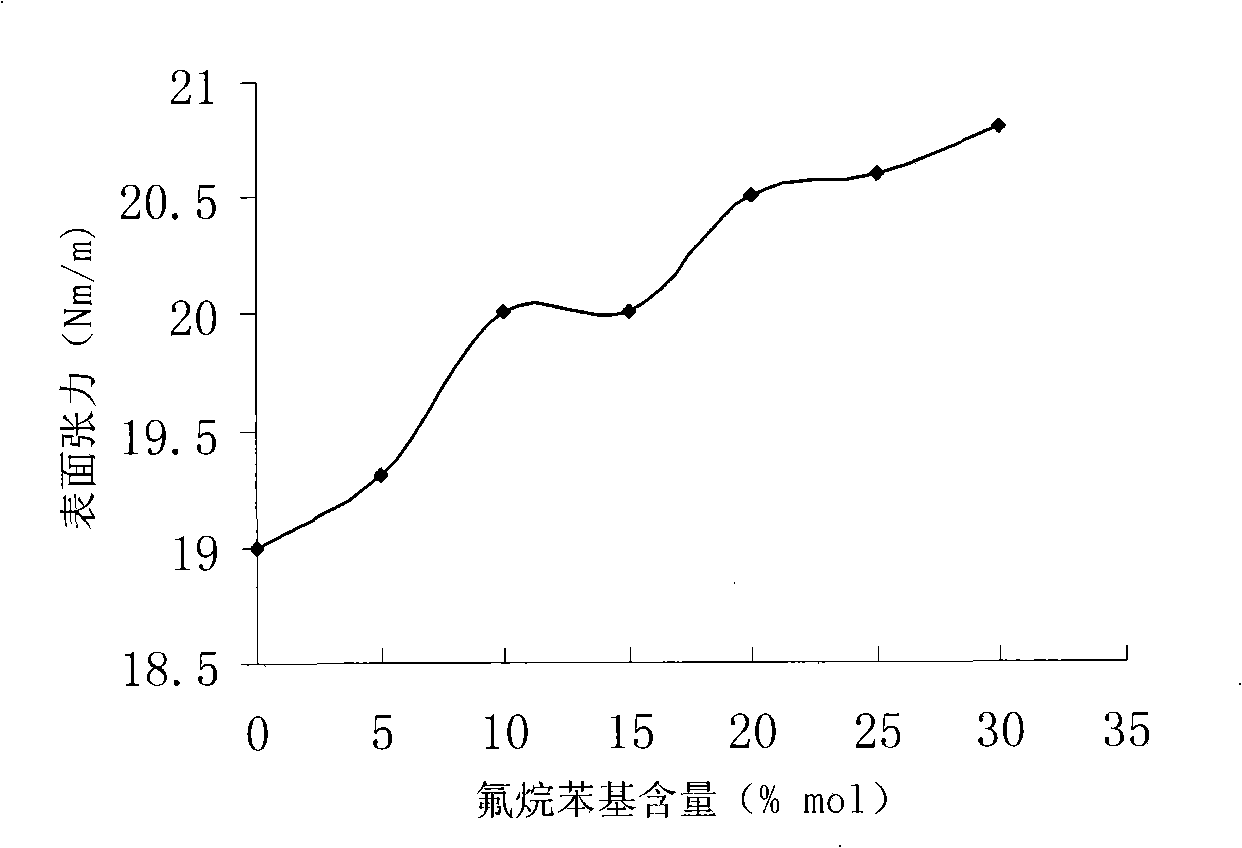
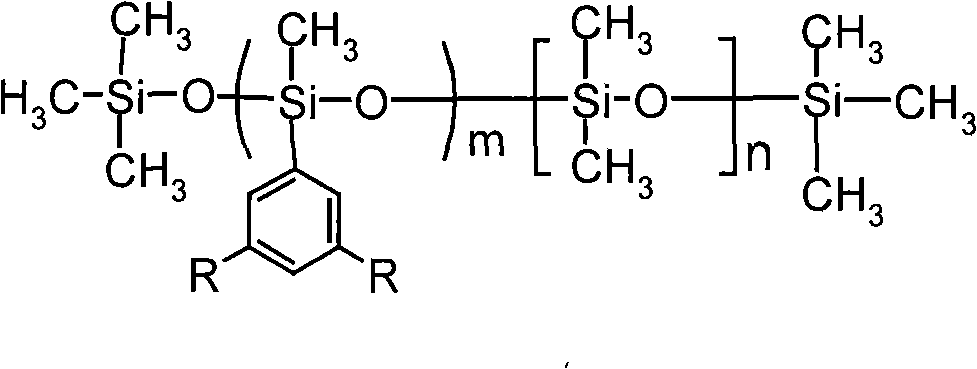
Novel fluorine phenyl-containing silicone oil and method of producing the same
A technology of phenyl silicone oil and fluorophenyl silicone, which is applied in the field of novel fluorine-containing phenyl silicone oil and its preparation, can solve the problems of poisoning, environmental pollution, bioaccumulative environmental hazards and the like, and achieves good heat resistance and easy preparation process. , the effect of promoting the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a 500mL three-neck flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, add 7.2g of magnesium chips and a certain amount of tetrahydrofuran (THF), and introduce N 2 , using a magnetic stirrer to stir, and heating the system to 30°C with a water bath, slowly added 58.6g of 3,5-bis(trifluoromethyl)bromobenzene, and continued to react for 1.5 hours to obtain the Grignard reagent after the addition was complete.
[0027] Equip a 1000mL three-neck flask with the same equipment as above, add 110.4g of methyltrimethoxysilane and THF, and then slowly add the prepared Grignard reagent after cooling, and continue the reaction for 2 hours after the dropwise addition. Suction filtration after the reaction was completed, the filtrate was rectified to obtain a colorless transparent liquid which was the product 3,5-bis(trifluoromethyl)phenylmethyldimethoxysilane, 31.8g of the product, 50% yield, 98.7% purity %. 1 H NMR (CDCl 3 -d 1 ): δ0...
Embodiment 2
[0029] Add water and hydrochloric acid to a three-necked flask, stir and heat to 55°C, then add 8.7 g of 3,5-bis(trifluoromethyl)phenylmethyldimethoxysilane monomer and dimethyldimethoxy The mixture of 62.4 g of silane monomers was stirred for 2.5 hours after the addition, separated, washed with water, and dried to obtain 35.6 g of hydrolyzed products.
[0030] Add the above hydrolyzate and 4.1g hexamethyldisiloxane (MM) into a single-necked flask, add 2.5g concentrated sulfuric acid to balance, stir at room temperature for 48 hours, separate the acid layer, wash the organic layer with water, dry, remove the solvent and low The distillate obtained 21.7 g of 3,5-bis(trifluoromethyl)phenyl silicone oil, and the total synthesis yield was 43.4%.
Embodiment 3
[0032] According to the processing condition of embodiment 2, by 3,5-bis (trifluoromethyl) phenylmethyl dimethoxysilane monomer 15.6g and dimethyl dimethoxy silane monomer 52.8g mixture, add Stirring was continued for 2.5 hours, the layers were separated, washed with water, and dried to obtain 37.0 g of hydrolyzate.
[0033] Add the above hydrolyzate and 4.1gMM into a single-necked flask, add 2.5g of concentrated sulfuric acid to balance, stir at room temperature for 48h, separate the acid layer, wash the organic layer with water, dry, and remove the solvent and low fraction to obtain 3,5-bis(trifluoro Methyl) phenyl silicone oil 22.0g, the total synthetic yield is 44.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com