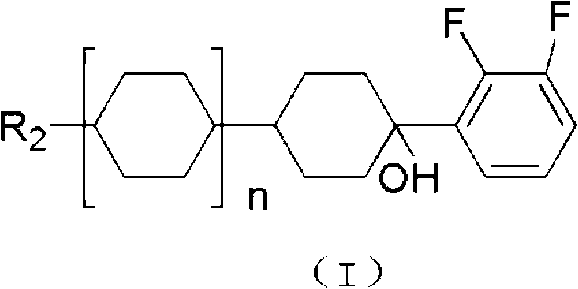
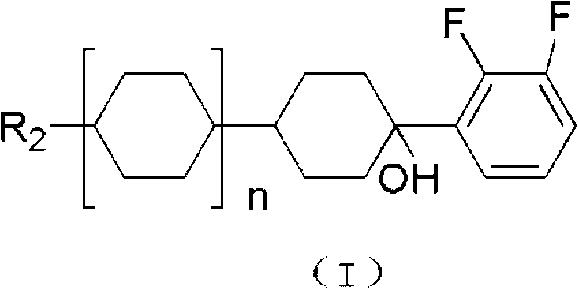
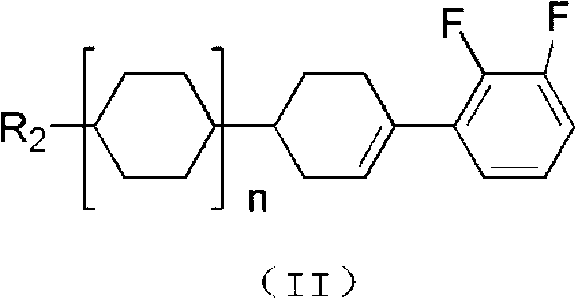
Preparation method for liquid crystal monomer of o-difluoroalkoxybenzene derivative
A technology of difluoroalkoxybenzene and liquid crystal monomers is applied in the preparation of ethers, the preparation of ethers by ester reaction, organic chemistry, etc. Conducive to large-scale production, overcoming inability to isomerize, and improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] When n=0, R 1 is n-butyl, R 2 When it is propyl, the product is 1-butoxy-4-(4-propyl-cyclohexyl)-benzene
[0045] 1) 1-(2,3-difluoro-phenyl)-4-propyl-cyclohexanol: add 100g (0.877mol) o-difluorobenzene, 300gTHF into a 2000ml three-necked flask, and store at -40℃ Add 256.425g (0.8335mol) of n-butyllithium solution dropwise, and after the dropwise addition, keep warm for 1 hour, add 116g 4-propylcyclohexanone (0.828mol), 116gTHF solution, keep warm for 2 hours, hydrolyze and separate, and wash the organic phase with water After neutralization, wait for the next step dehydration reaction; or,
[0046] 1-(2,3-Difluoro-phenyl)-4-propyl-cyclohexanol: Add 100g (0.877mol) o-difluorobenzene and 300gTHF to a 2000ml three-neck flask, add 22g (0.921mol ) Metal magnesium, after the addition is completed and kept warm for 1 hour, add 116g 4-propyl-cyclohexanone (0.828mol), 200gTHF solution, keep warm for 2 hours, hydrolyze and separate, and after the organic phase is washed with w...
Embodiment 2
[0054] When n=1, R 1 is propyl, R 2 When it is methyl, the product is 4-(2,3-difluoro-4-propoxy-phenyl)-4'-methyl-bicyclohexane
[0055] 1) 4-(2,3-difluoro-phenyl)-4'-methyl-bicyclohexyl alcohol: Add 100g (0.877mol) o-difluorobenzene, 400gTHF to a 2000ml three-necked bottle, and store at -50℃ Add 269.808g (0.877mol) sec-butyllithium solution dropwise, after the dropwise addition and keep warm for 2 hours, add 160.6g (0.828mol) 4-(4'-methyl-cyclohexyl) cyclohexanone, 116gTHF solution, keep warm After 2 hours, hydrolysis and separation, the organic phase was washed with water and neutralized, and then the next dehydration reaction was performed; or,
[0056] 4-(2,3-difluoro-phenyl)-4'-methyl-bicyclohexyl alcohol: add 100g (0.877mol) o-difluorobenzene, 400g THF to a 2000ml three-necked flask, add 23.15g ( 0.965mol) metal magnesium, after the heat preservation is completed for 1 hour, add 160.6g (0.828mol) 4-(4'-methyl-cyclohexyl) cyclohexanone and 200g THF solution, heat for 2...
Embodiment 3
[0064] When n=2, R 1 is ethyl, R 2 When it is n-butyl, the product is 4″-butyl-4-(4-ethoxy-2,3-difluoro-phenyl)-[1,1';4′,1″]tricyclohexane
[0065] 1) 4-(2,3-difluoro-phenyl)-4″-butyl-[1,1’;4’,1″]tricyclohexyl alcohol: add 100g (0.877 mol) o-difluorobenzene, 500gTHF, 275.204g (0.895mol) sec-butyllithium solution was added dropwise at -70°C, and after the addition was completed and kept warm for 3 hours, 263.3g (0.828mol) 4″-butyl- [1,1';4',1″]Tricyclohexanone, 120g THF solution, keep warm for 2 hours, hydrolyze and separate layers, wash the organic phase with water to be neutral, and wait for the next step of dehydration reaction; or,
[0066] 4-(2,3-difluoro-phenyl)-4″-butyl-[1,1’;4’,1″]tricyclohexyl alcohol: add 100g (0.877mol) to a 2000ml three-necked bottle o-difluorobenzene, 500gTHF, add 25.26g (0.921mol) magnesium metal at 30°C, after the dropwise addition is completed and keep warm for 1 hour, add 263.3g (0.828mol) 4″-butyl-[1,1';4', 1″]Tricyclohexanone, 120g THF so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com