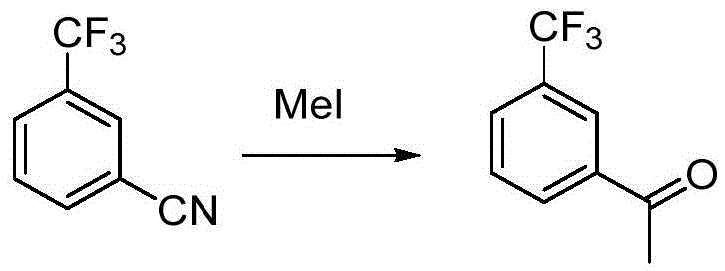
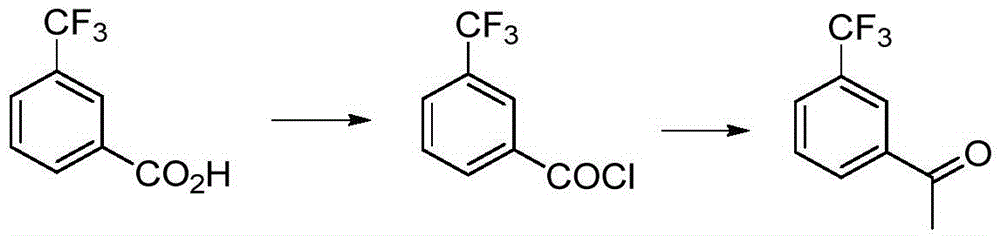
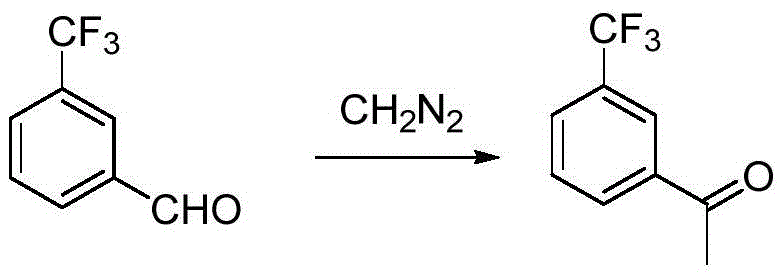Preparation method for m-trifluoromethyl acetophenone and intermediate thereof
A technology of trifluoromethyl acetophenone and toluene solution, which is applied in the field of preparation of trifluoromethyl acetophenone and its intermediates, can solve the problems of many reaction steps, easy flushing, and low yield, and achieve the goal of reaction The effect of short time and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 Grignard
[0062] Using m-chlorotrifluorotoluene (I) as raw material
[0063] Add 212 mg of anhydrous lithium chloride to a 100 mL four-necked flask equipped with a magnetic stirrer, a thermometer, and a condenser, and replace nitrogen three times. Then 18.1 g (0.1 mol) of m-chlorobenzotrifluoride (I) was added, and at room temperature, 40.0 mL of 2.5 mol / L (0.1 mol) isopropylmagnesium chloride tetrahydrofuran solution was added dropwise, and the drop was completed in 30 minutes. Heat to reflux (70° C.) and keep warm for 3 hours until the area content of the raw material m-chlorobenzotrifluoride is less than 5% (GC detection). At this time, the area content of benzotrifluoride was 94.0%.
Embodiment 2
[0064] Embodiment 2 Grignard
[0065] Using m-chlorotrifluorotoluene (I) as raw material
[0066] In a 100mL four-neck flask equipped with a magnetic stirrer, a thermometer, and a condenser tube, nitrogen was injected three times, and then 18.1g (0.1mol) of m-chlorobenzotrifluorotoluene (I) was added, and 2.5mol / L (0.1 mol) of isopropylmagnesium chloride tetrahydrofuran solution 40.0mL, dripped in 30 minutes. Heat to reflux (70° C.) and keep warm for 3 hours until the area content of the raw material m-chlorobenzotrifluoride is less than 5% (GC detection). At this time, the area content of benzotrifluoride was 89.3%.
Embodiment 3
[0067] Embodiment 3 Grignard
[0068] Using m-bromobenzotrifluoride (II) as raw material
[0069] Add 212 mg of anhydrous lithium chloride to a 100 mL four-necked flask equipped with a magnetic stirrer, a thermometer, and a condenser, and replace nitrogen three times. Then 22.5 g of m-bromobenzotrifluoride (II) was added, and 40.0 mL of 2.5 mol / L isopropylmagnesium chloride tetrahydrofuran solution was added dropwise at room temperature, and the dropwise was completed in 30 minutes. Heating to 70° C. and insulated for 3 hours, detected by GC, the area content of m-chlorobenzotrifluoride is less than 5%, and the area content of benzotrifluoride is 97.2%. (IV).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com