Siliceous aromatic ether and aryne polymer and preparation method thereof
A polymer and aryl ether technology, applied in the field of silicon-containing aryl ether aryne polymers and their preparation, can solve the problems of poor toughness, poor overall mechanical properties, and high viscosity of silicon-containing aryl alkyne resins, and achieves good performance, Excellent thermal stability and mechanical properties, easy to store
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
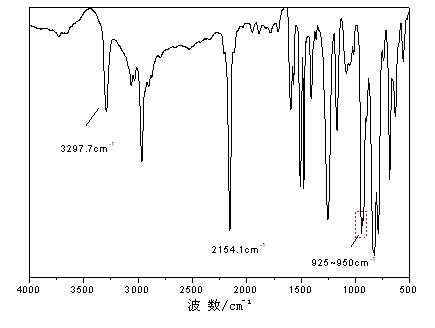
[0030] In an anhydrous, oxygen-free nitrogen atmosphere, add 6.18g (0.26mol) of magnesium powder and THF (50ml) into a four-necked flask, and drop about 1 / 3 of ethyl chloride 26.16g (0.24mol) / THF at one time (50ml) solution in the system, after triggering, add the remaining ethyl chloride (0.16mol) dropwise after cooling in an ice-water bath, and keep warm at 40-60°C for 1-2h. Cool to room temperature, dropwise add bisphenol A (4.56g, 0.02mol) / THF (40ml) solution and diethynylbenzene (12.6g, 0.10mol) / THF (60ml) solution successively, control the dropping rate, 60~70℃ Under the reaction 1 ~ 3h. Cool down to room temperature, add dimethyldichlorosilane (12.9g, 0.10mol) / THF (50ml) dropwise, and react at 60-70°C for 2-5h. Acetic acid / THF solution terminated the reaction, precipitated after suction filtration, and stood overnight to desalt by suction filtration and rotary evaporation to remove the solvent to obtain a brown-yellow viscous liquid. Its infrared spectrum is as figur...
Embodiment 2
[0032] In an anhydrous, oxygen-free nitrogen atmosphere, add 3.09g (0.13mol) of magnesium powder and THF (20ml) into a four-necked flask, and drop about 1 / 3 of the amount of ethyl chloride 7.80g (0.12mol) / THF (50ml) solution in the system, after being triggered, drop the remaining ethyl chloride (0.08mol) after cooling down in an ice-water bath, and keep warm at 40-60°C for 1-3h. Cool to room temperature, successively add bisphenol A (2.28g, 0.01mol) / THF (50ml) solution and diethynylbenzene (6.3g, 0.05mol) / THF (50ml) solution dropwise, control the dropping rate, 60~70℃ The reaction time is 1-2 hours. Cool down to room temperature, add dimethyldichlorosilane (10.35g, 0.09mol) / THF (50ml) dropwise, and react at 60-70°C for 2-5 hours. Acetic acid / THF solution terminated the reaction, precipitated after suction filtration, stood overnight to remove salt by suction filtration, and removed the solvent by rotary evaporation to obtain a brown-yellow viscous liquid. It has a molecular...
Embodiment
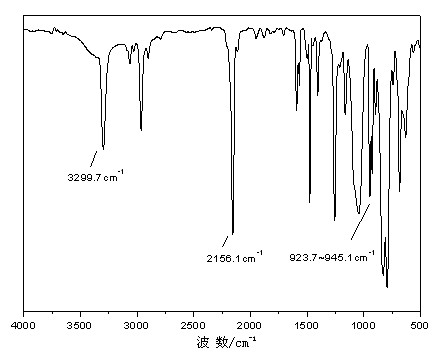
[0034] In an anhydrous, oxygen-free nitrogen atmosphere, add magnesium powder 6.18g (0.26mol) / THF (50ml) to the four-necked flask; drop about 1 / 3 of the amount of vinyl chloride 15.00g (0.24mol) / THF (50ml) ) solution in the system, after being triggered, drop the remaining vinyl chloride (0.16mol) after cooling down in an ice-water bath, and keep warm at 40-50°C for 1-2h. After cooling to room temperature, 4,4'-dihydroxydiphenyl ether (4.04g, 0.02mol) / THF (50ml) solution and diethynylbenzene (12.6g, 0.10mol) / THF (50ml) solution were added dropwise successively, controlling Dropping rate, 60~70°C reaction time 1~2h. Cool down to room temperature, add methylhydrogendichlorosilane (11.50g, 0.10mol) / THF (50ml) dropwise, and react at 60-70°C for 2-5 hours. Acetic acid / THF solution terminated the reaction, precipitated after suction filtration, stood overnight to remove salt by suction filtration, and removed the solvent by rotary evaporation to obtain a brown-yellow viscous liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com