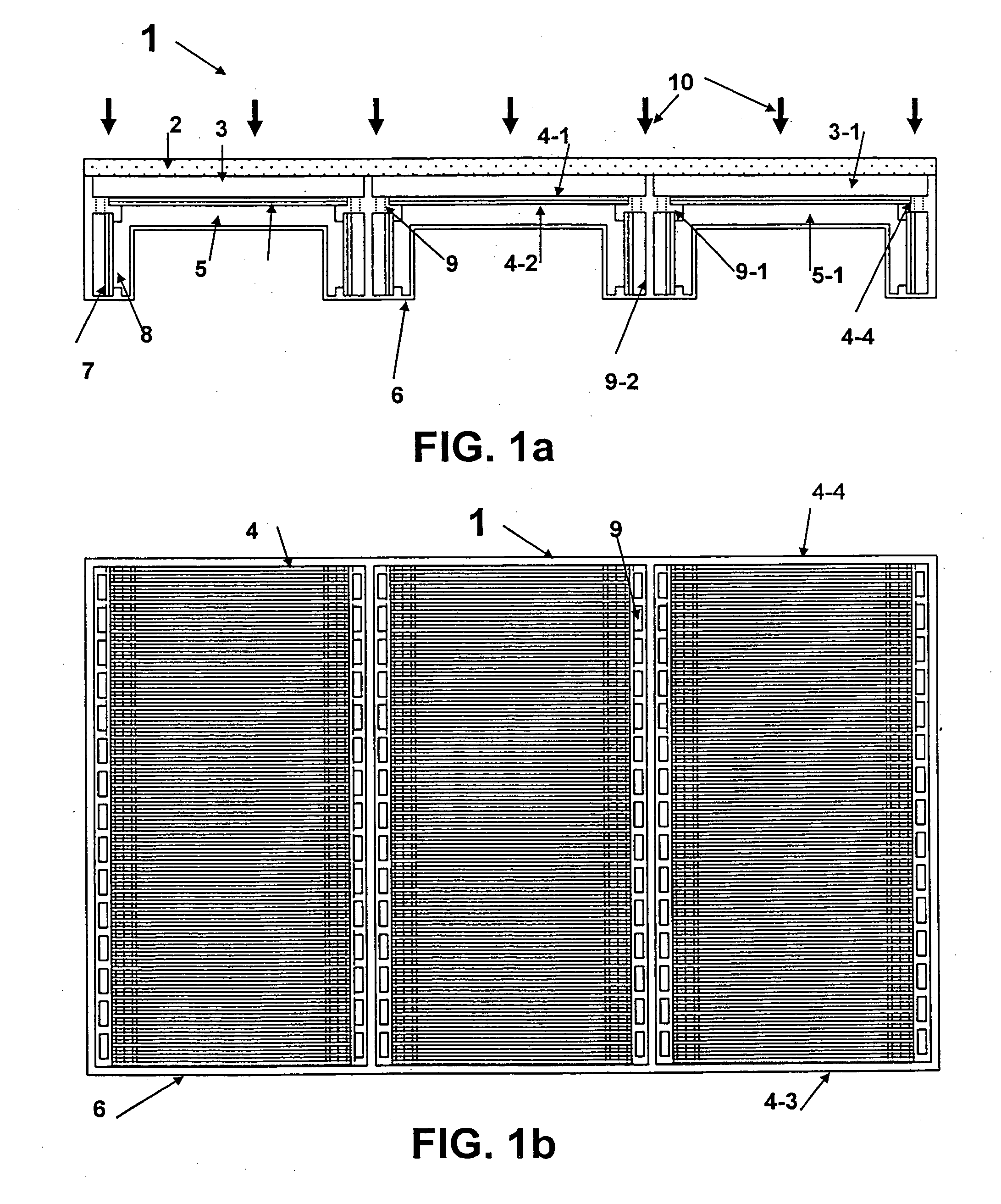
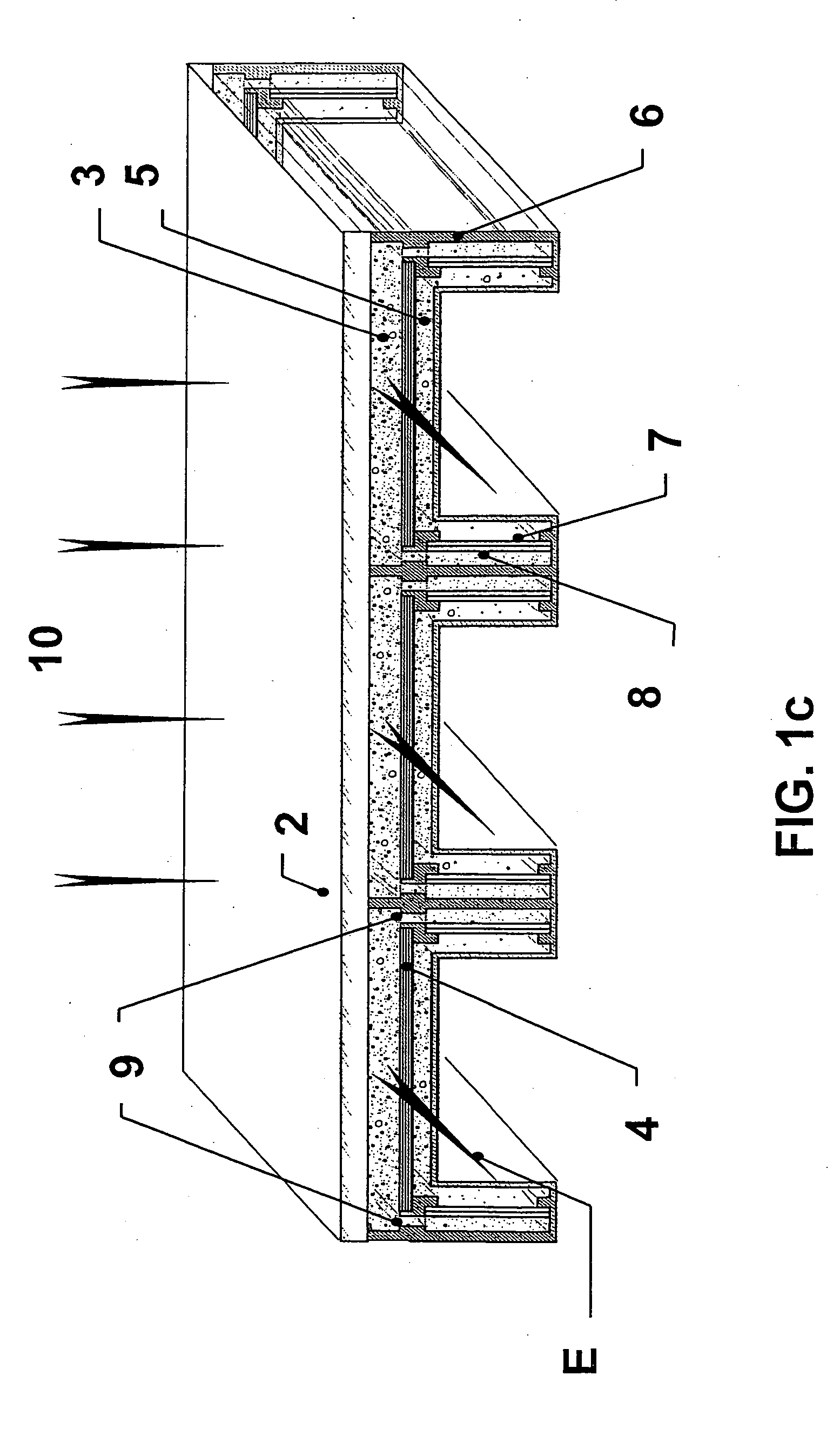
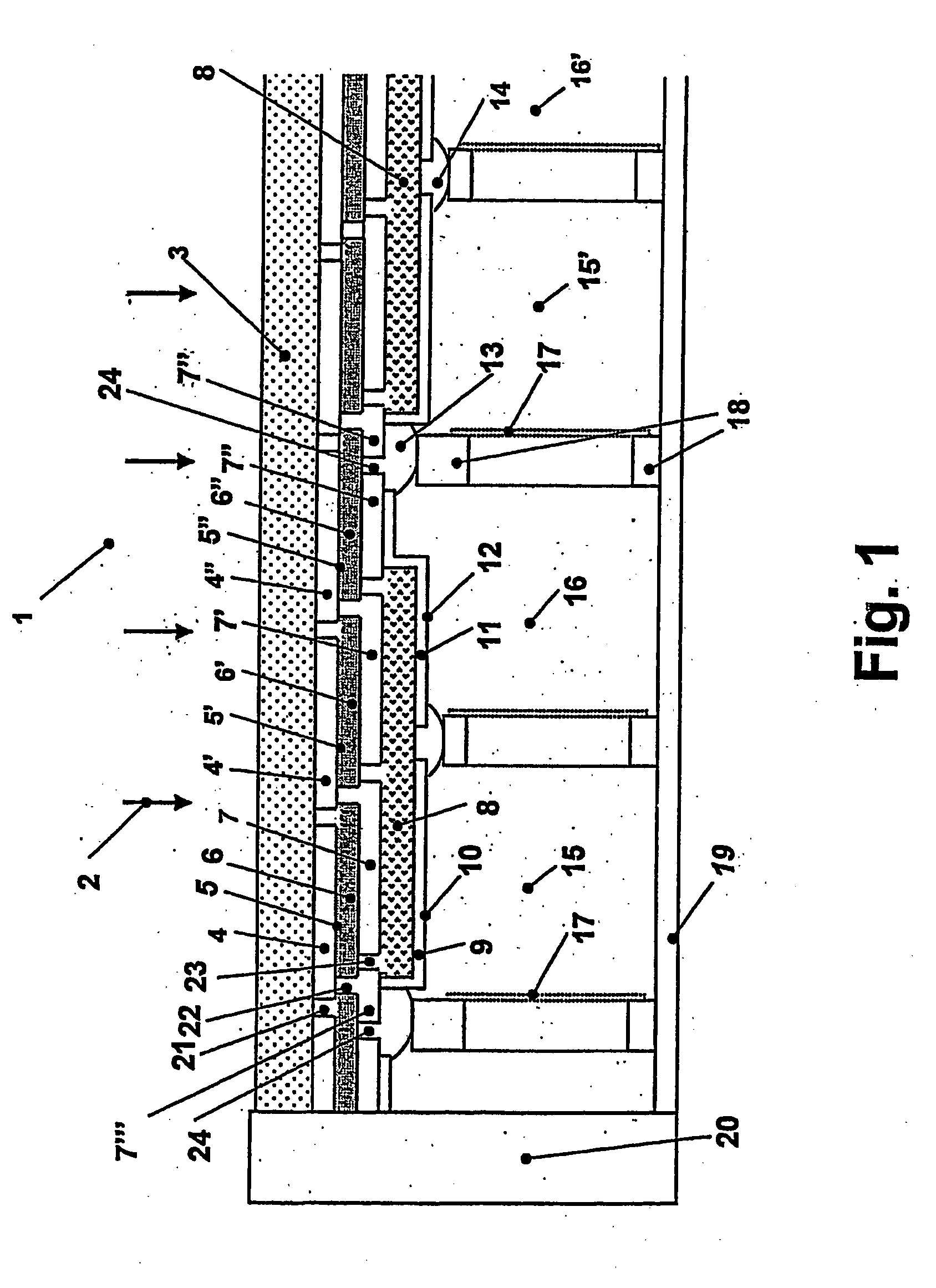
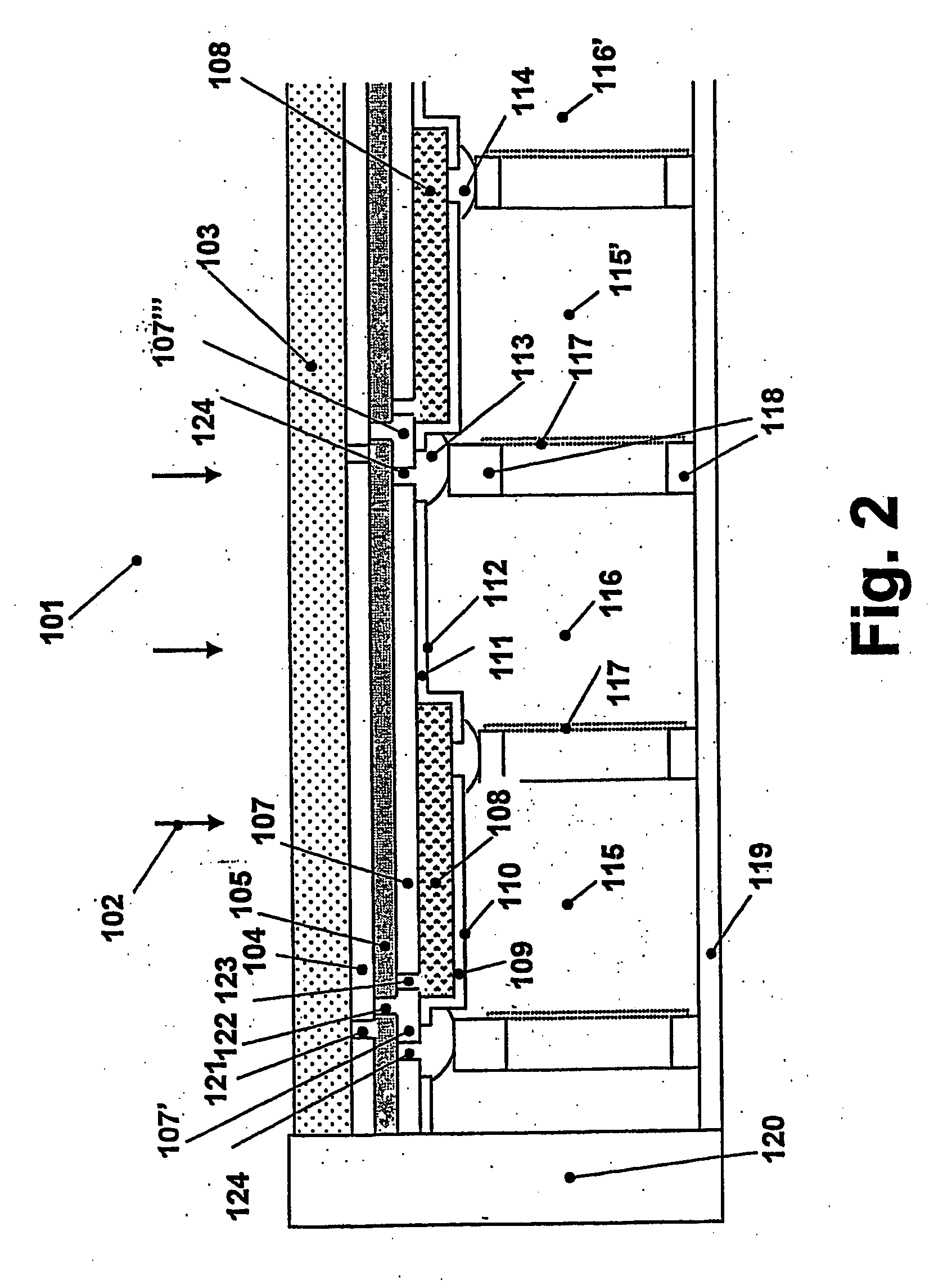
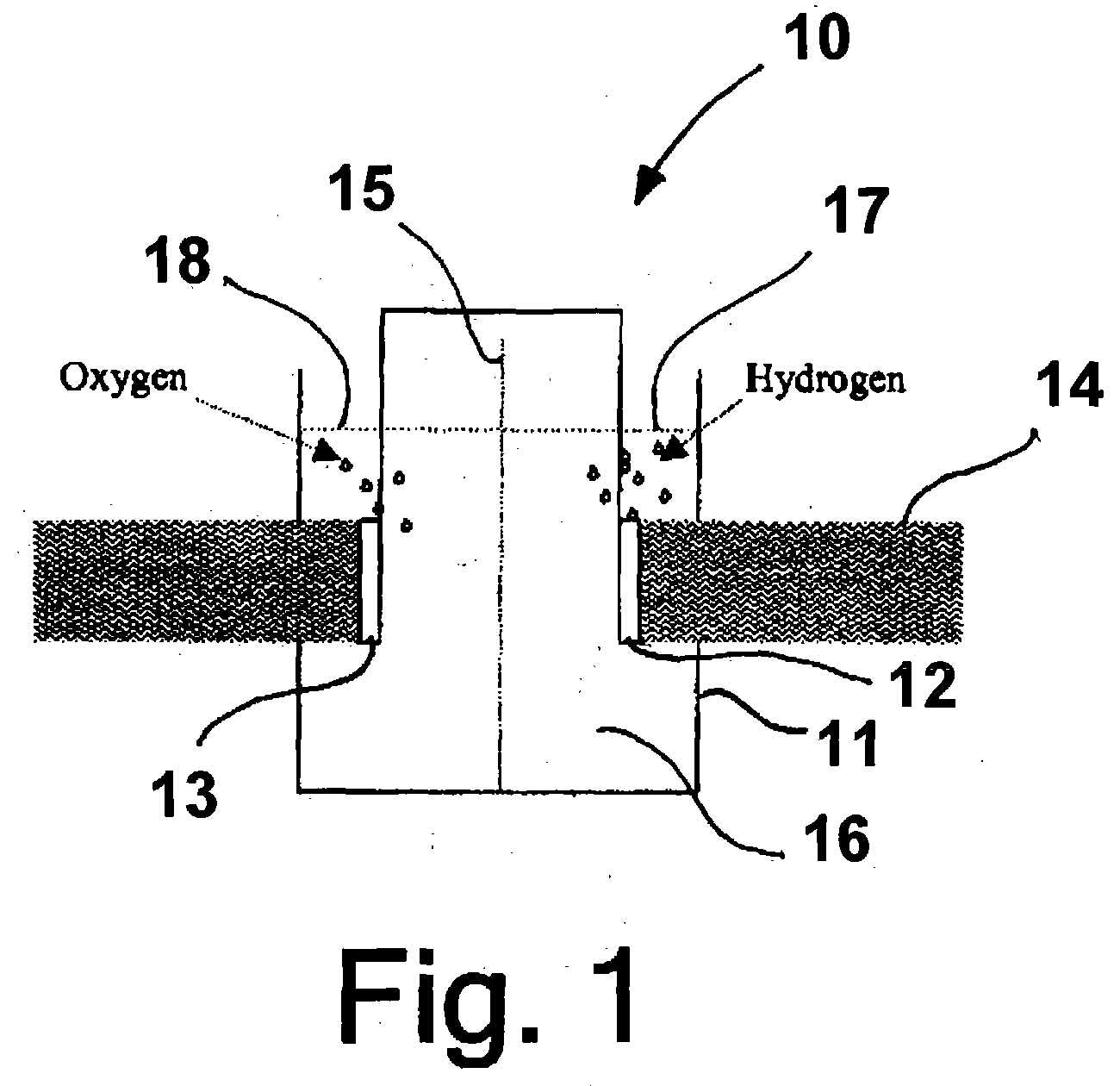
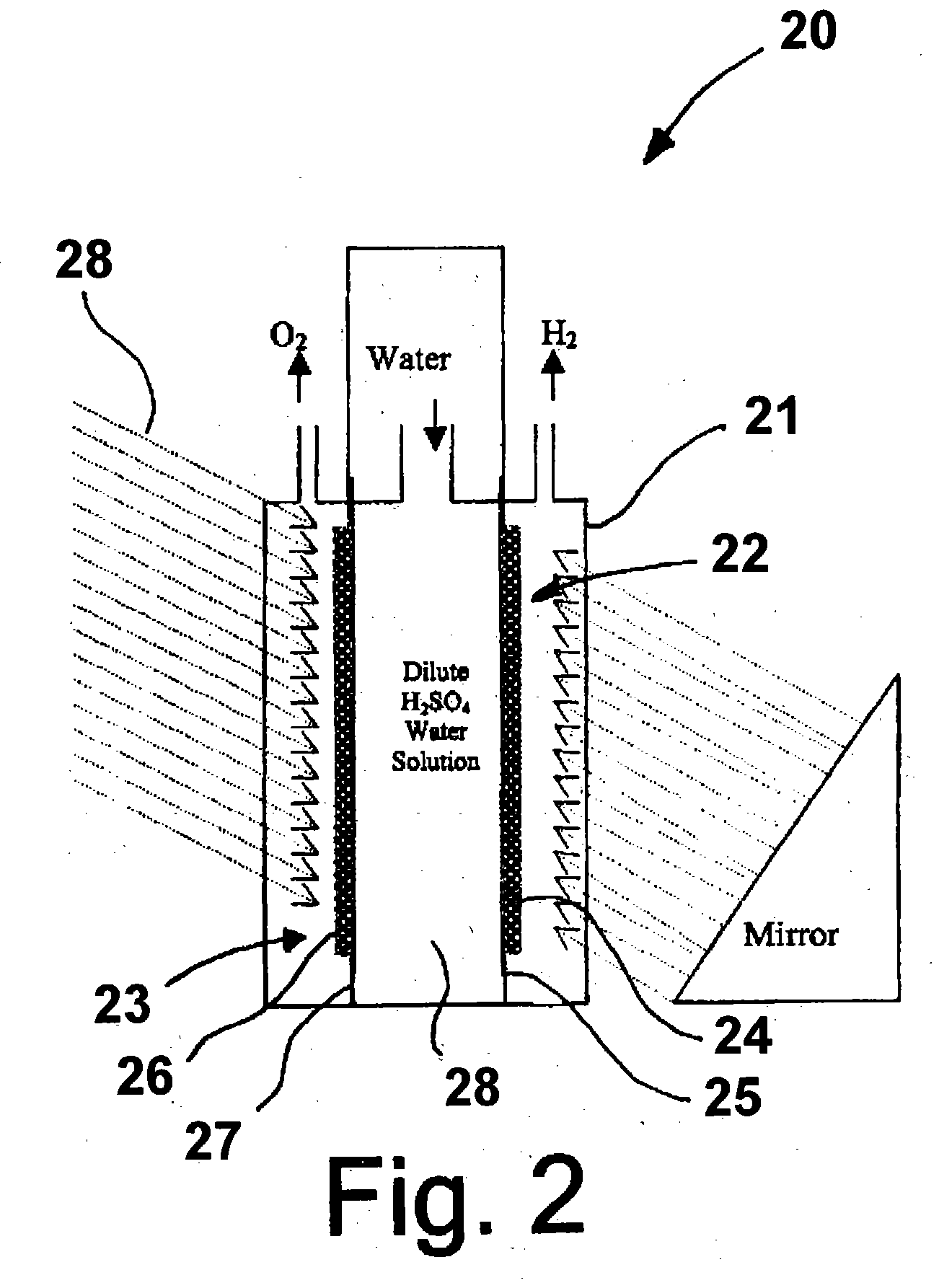
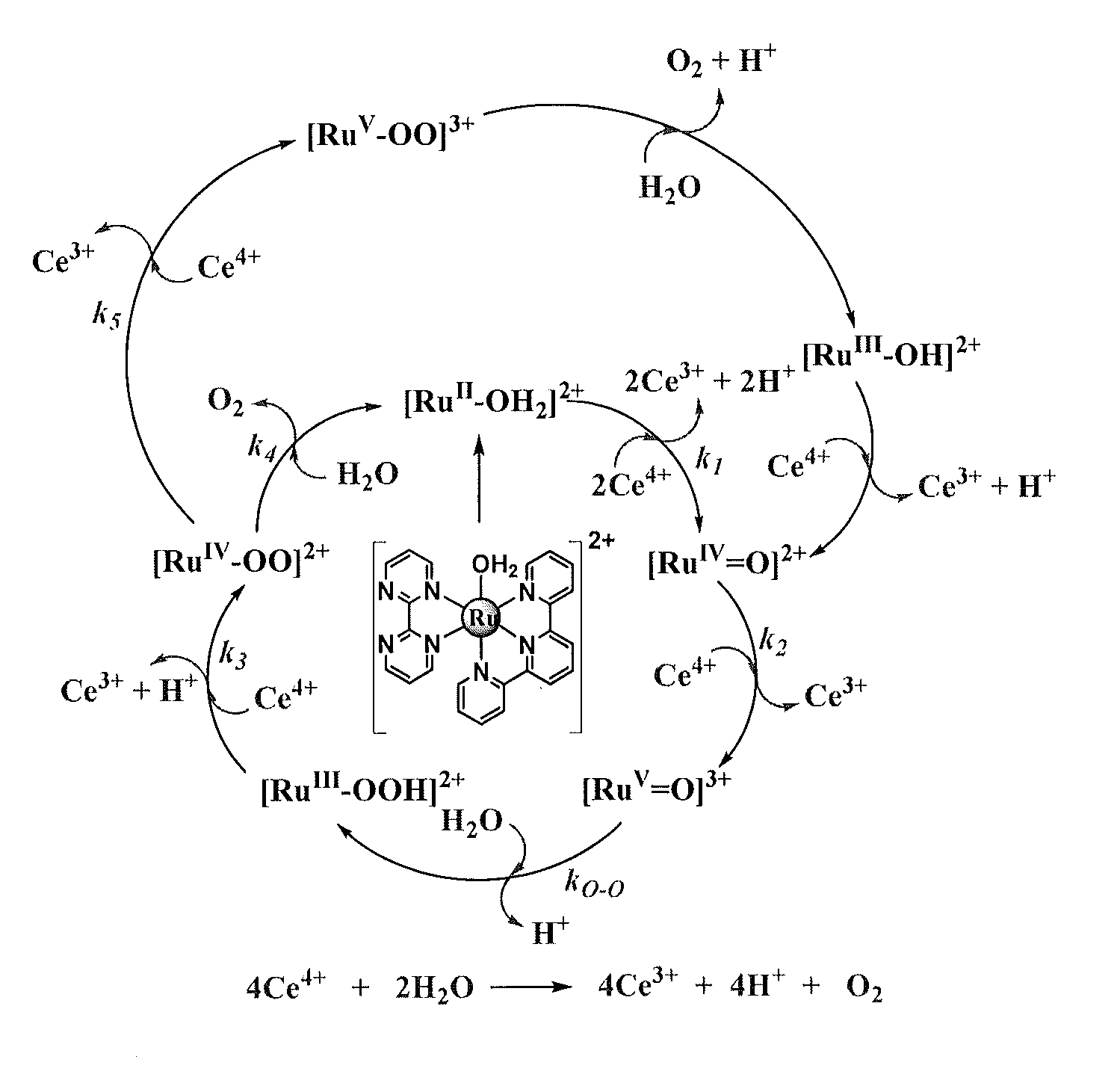
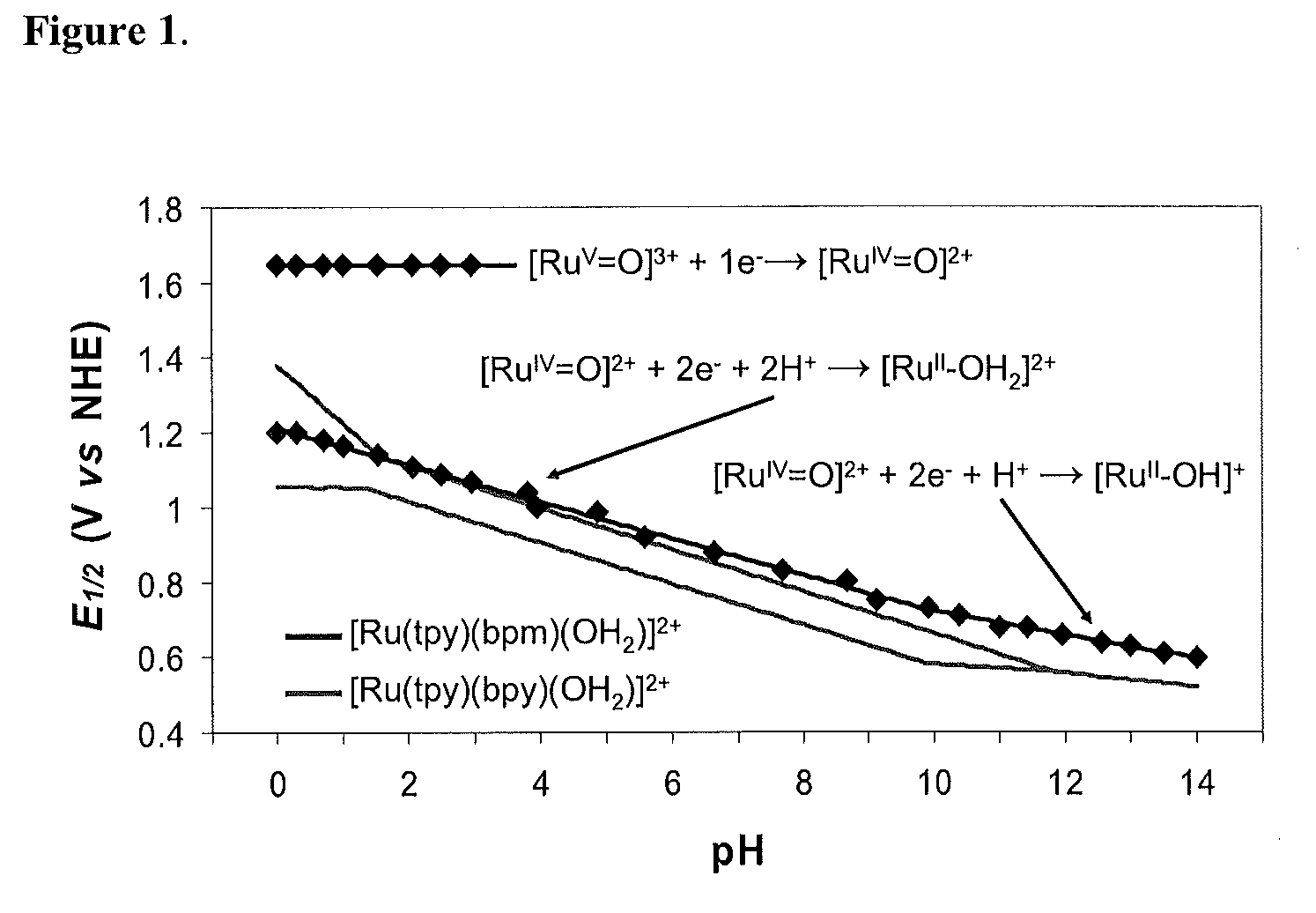
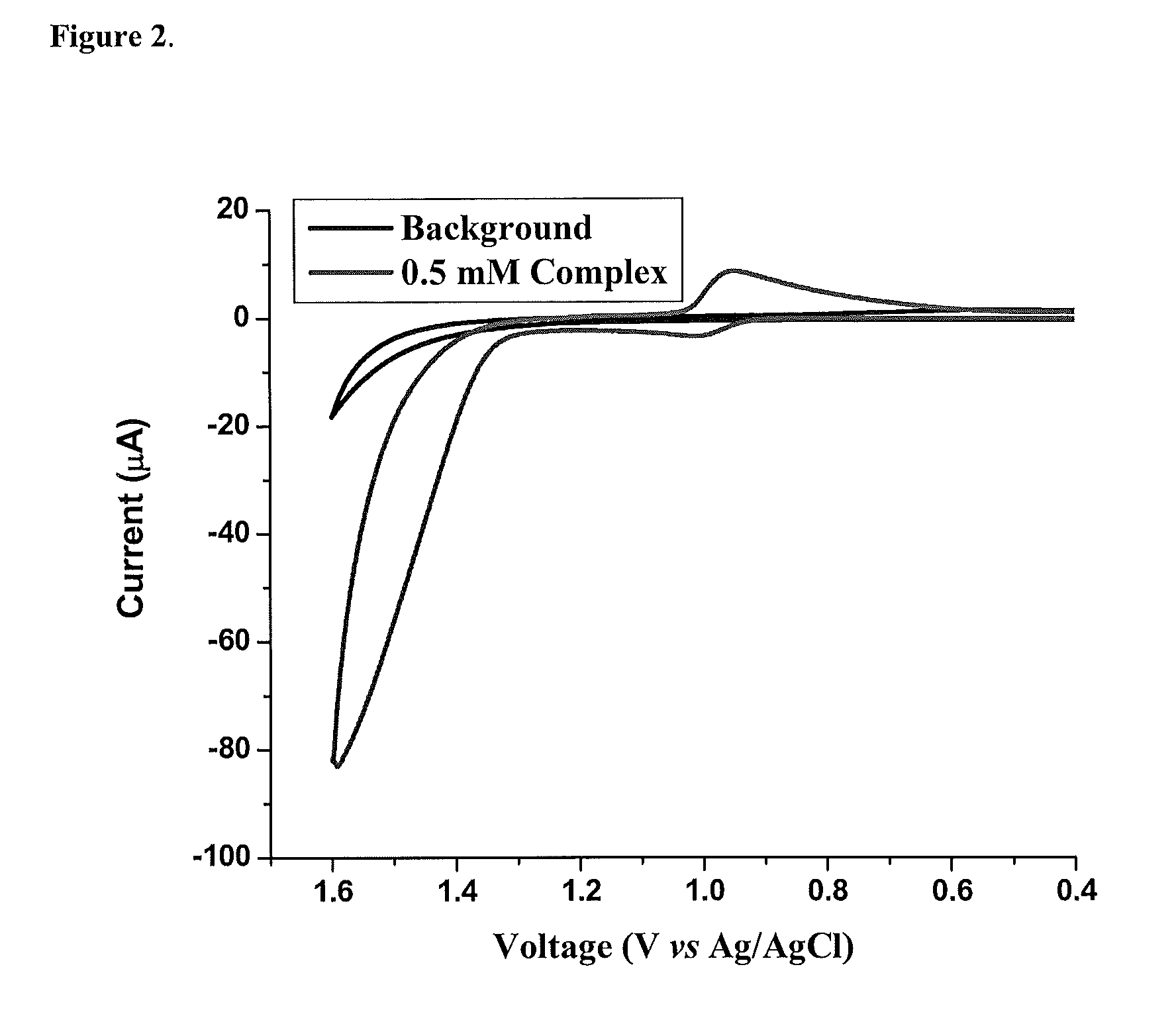
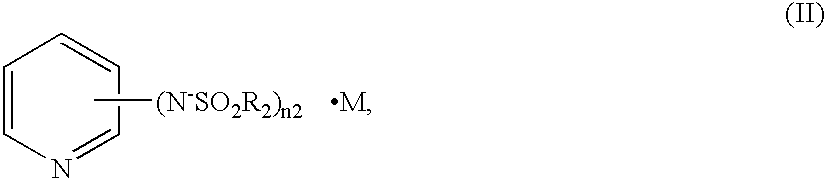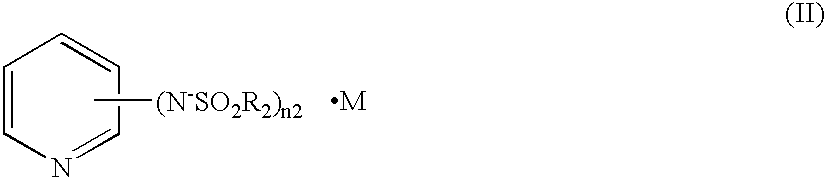Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
254 results about "Photoelectrochemical cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
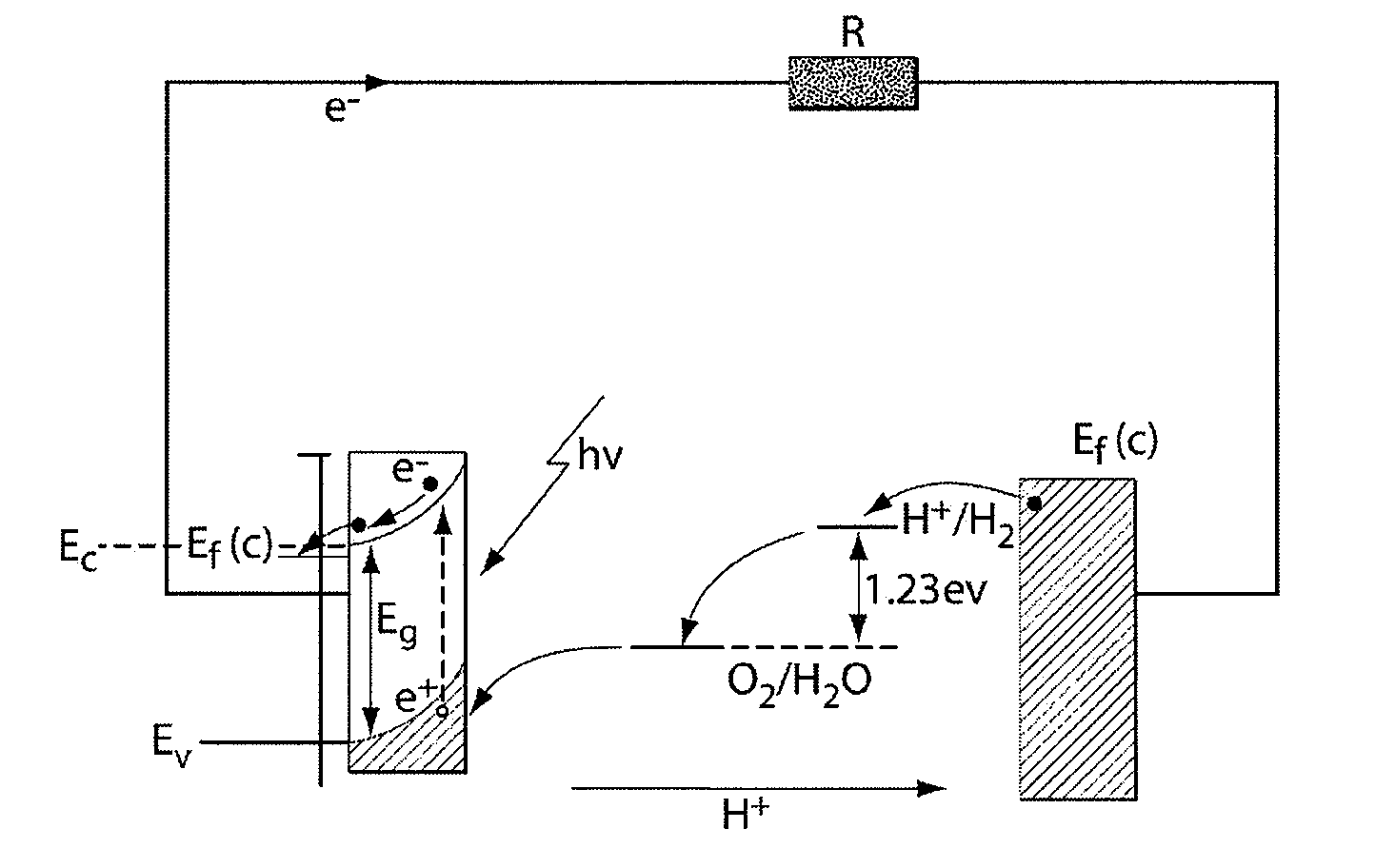
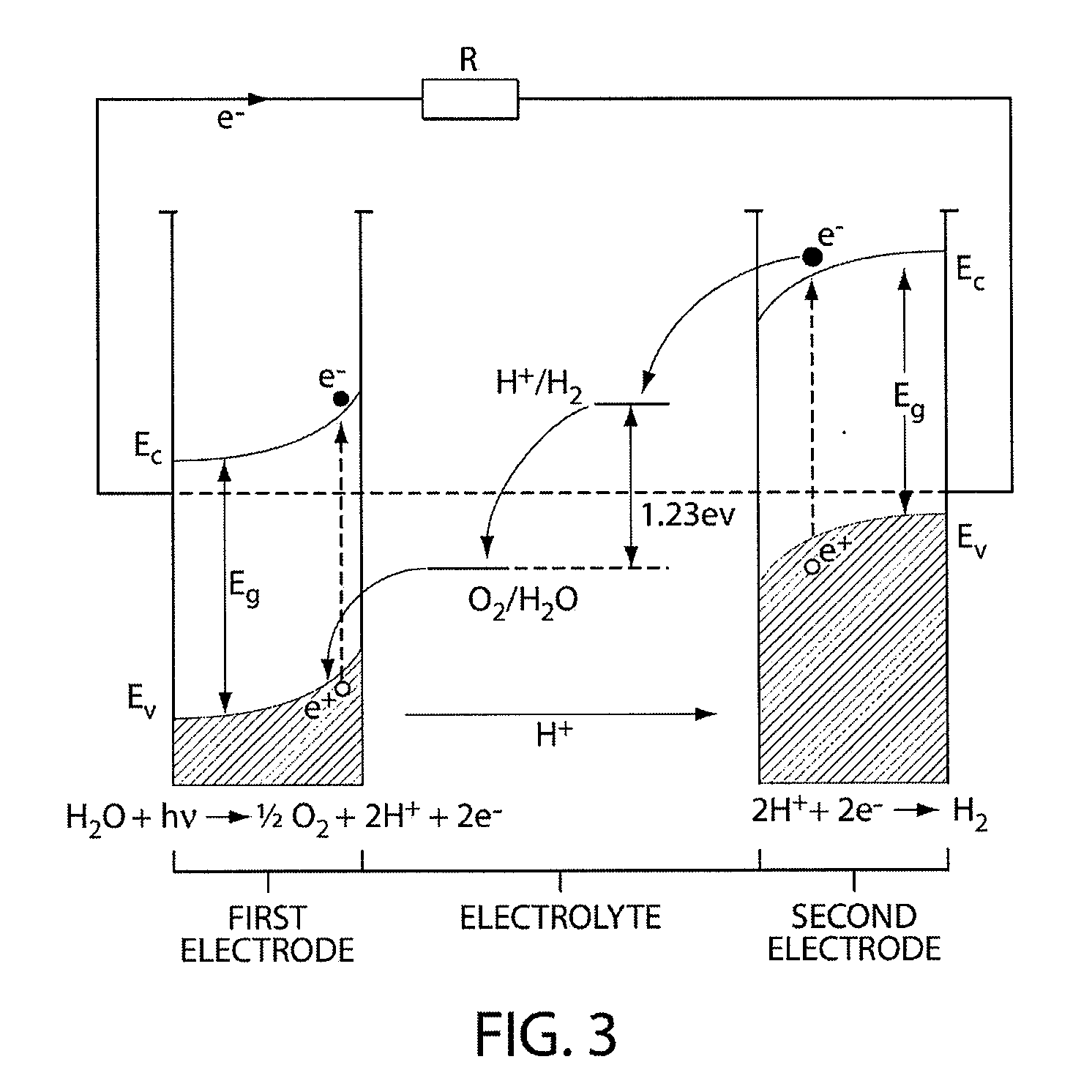
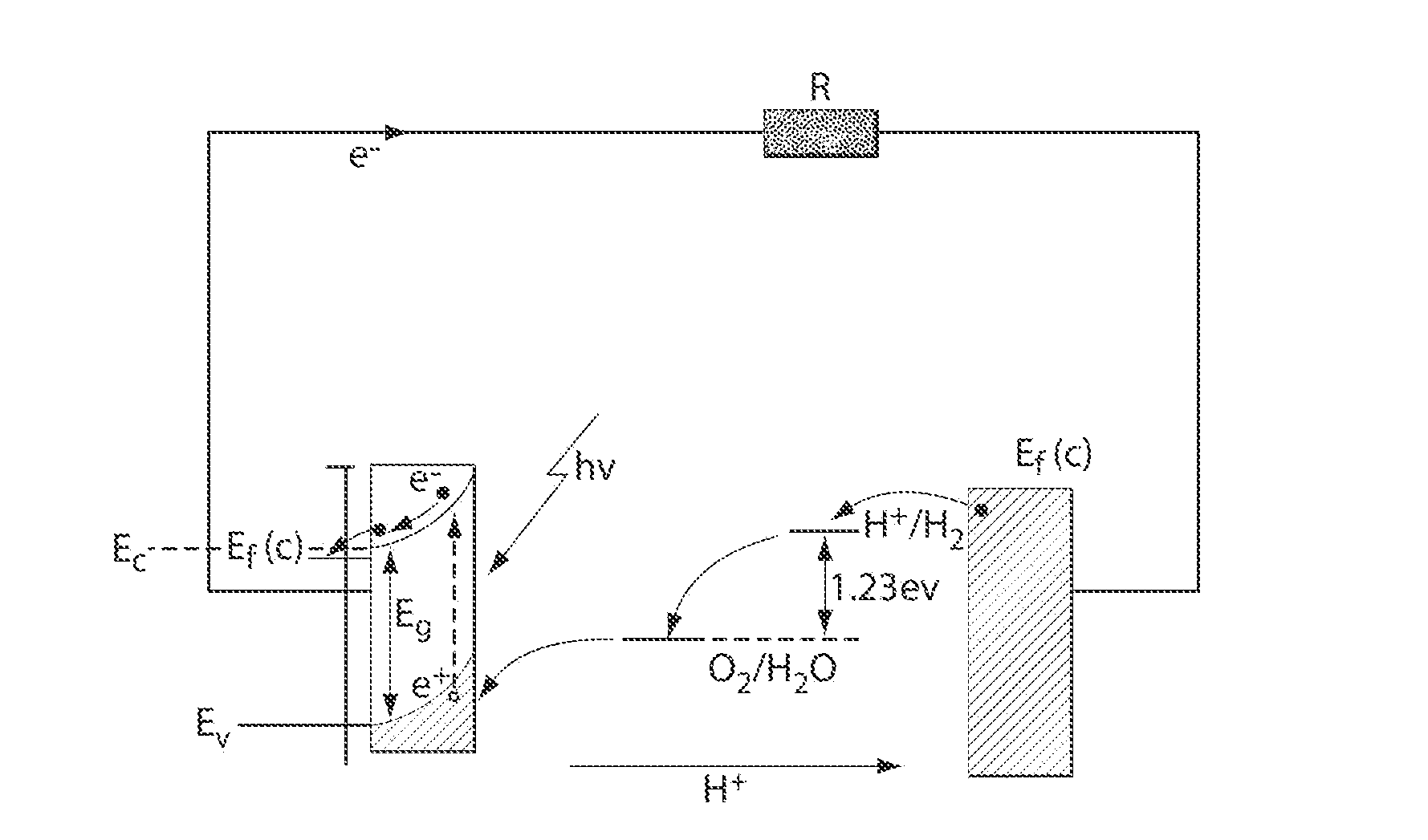
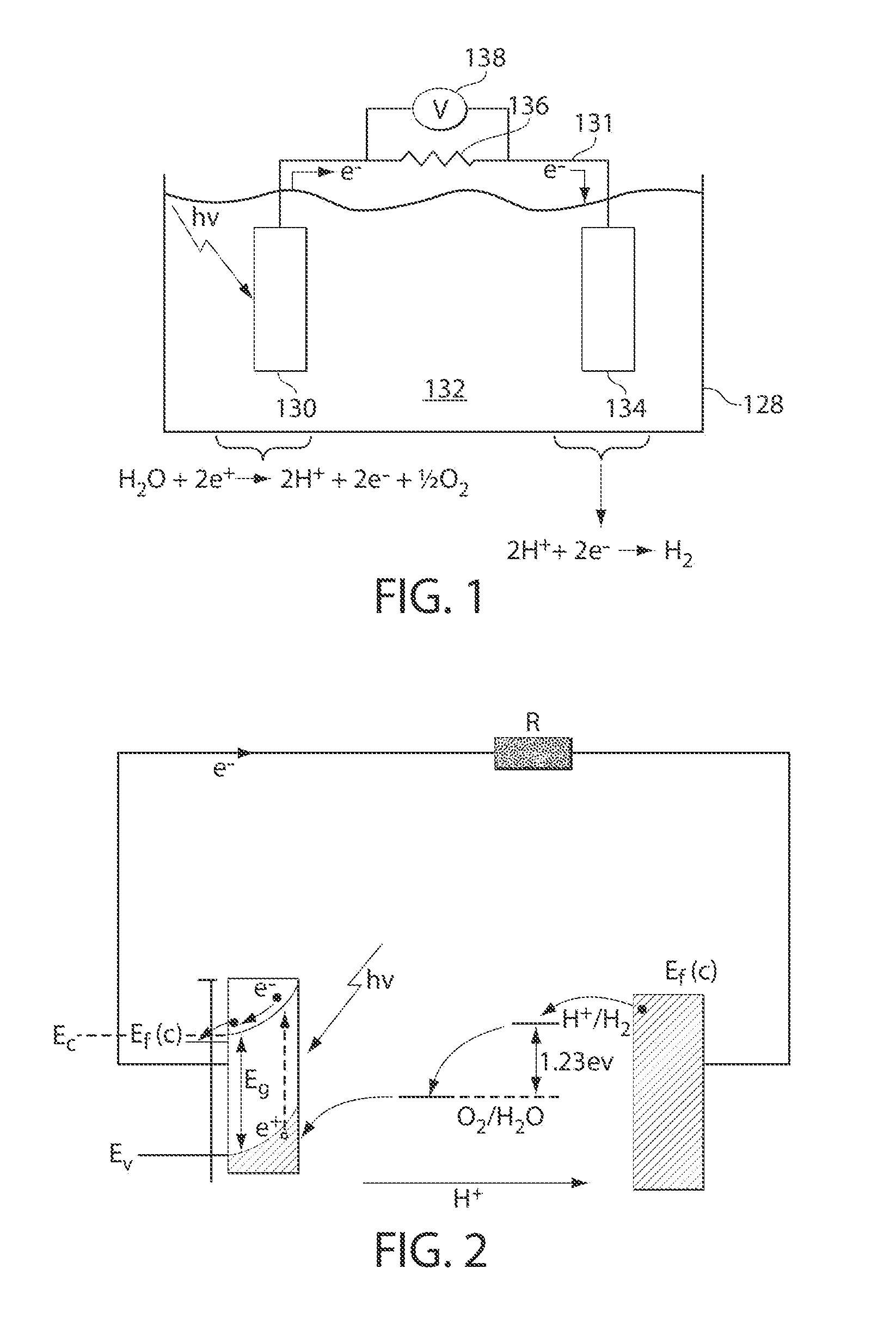
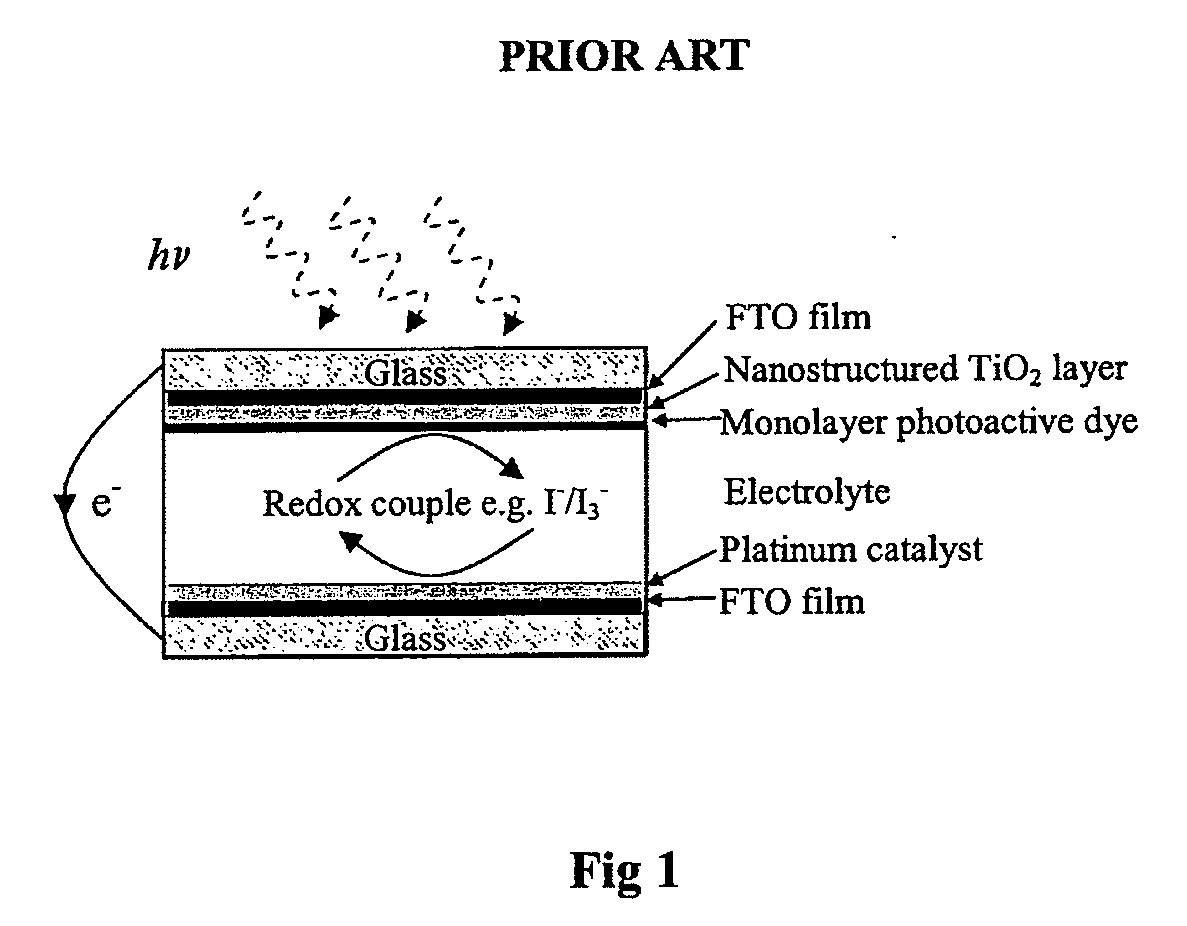
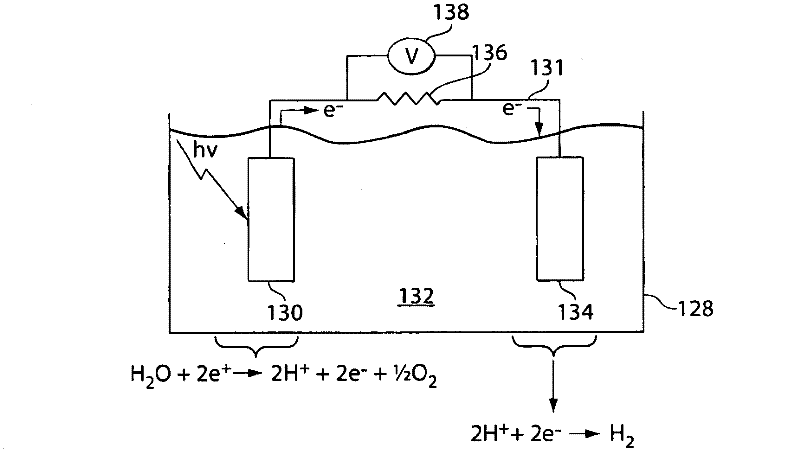
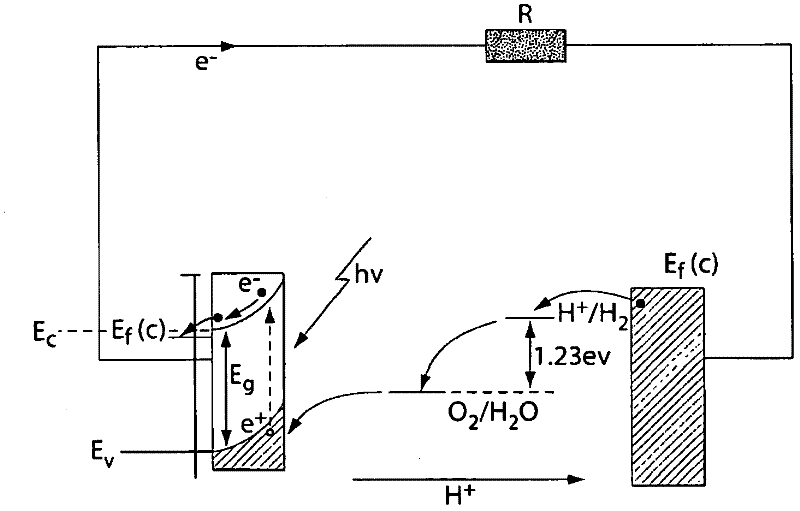
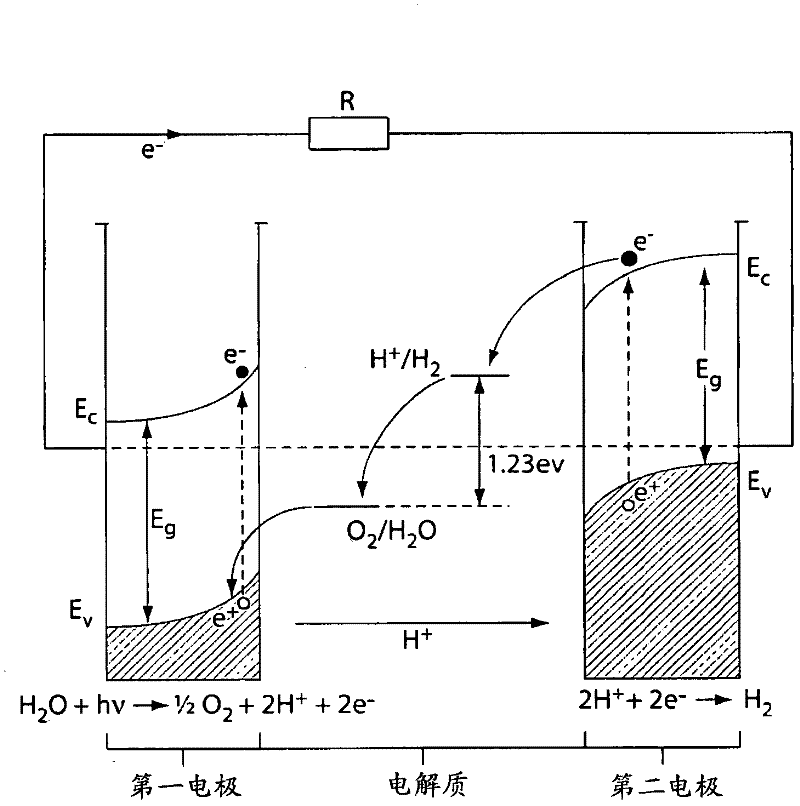
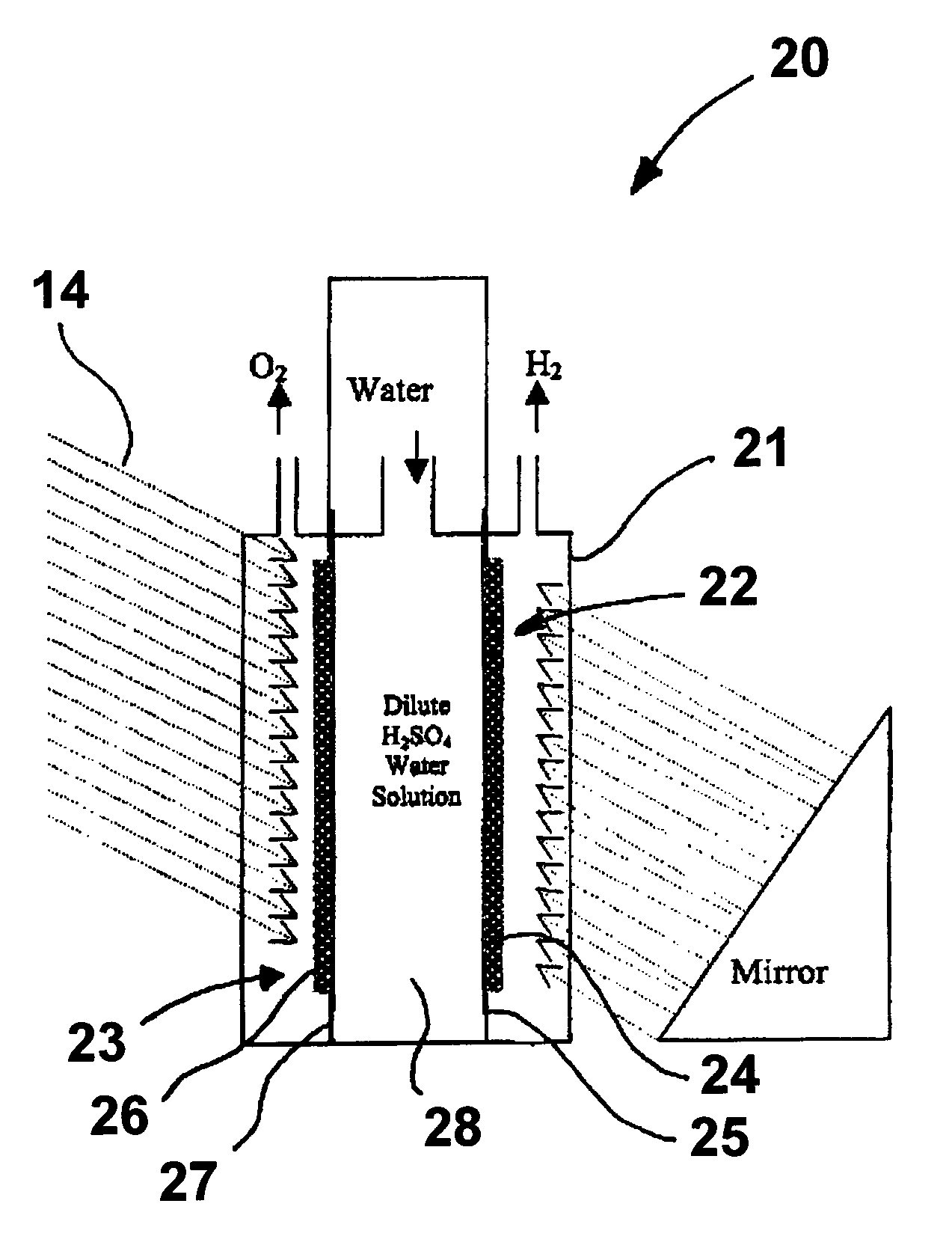
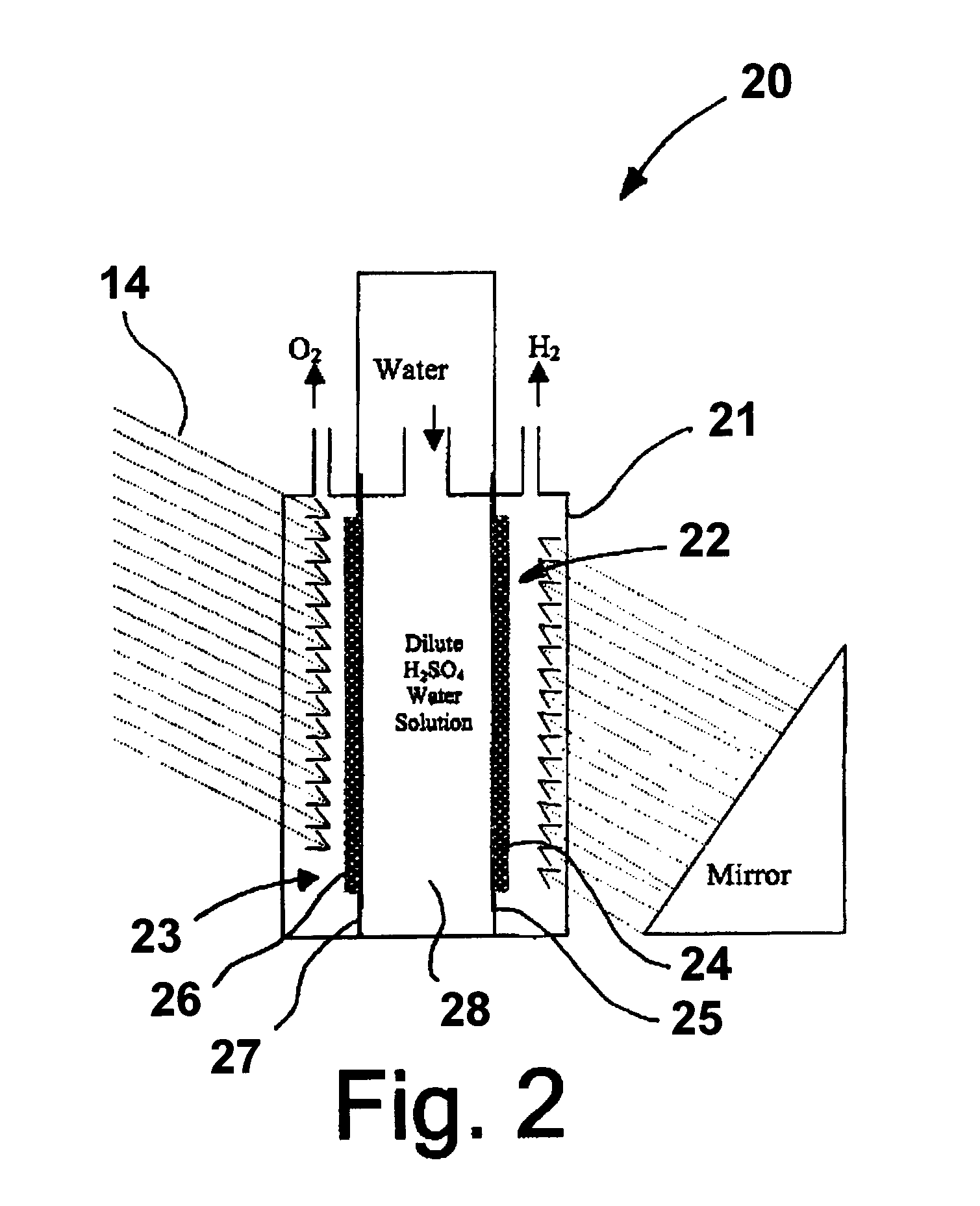
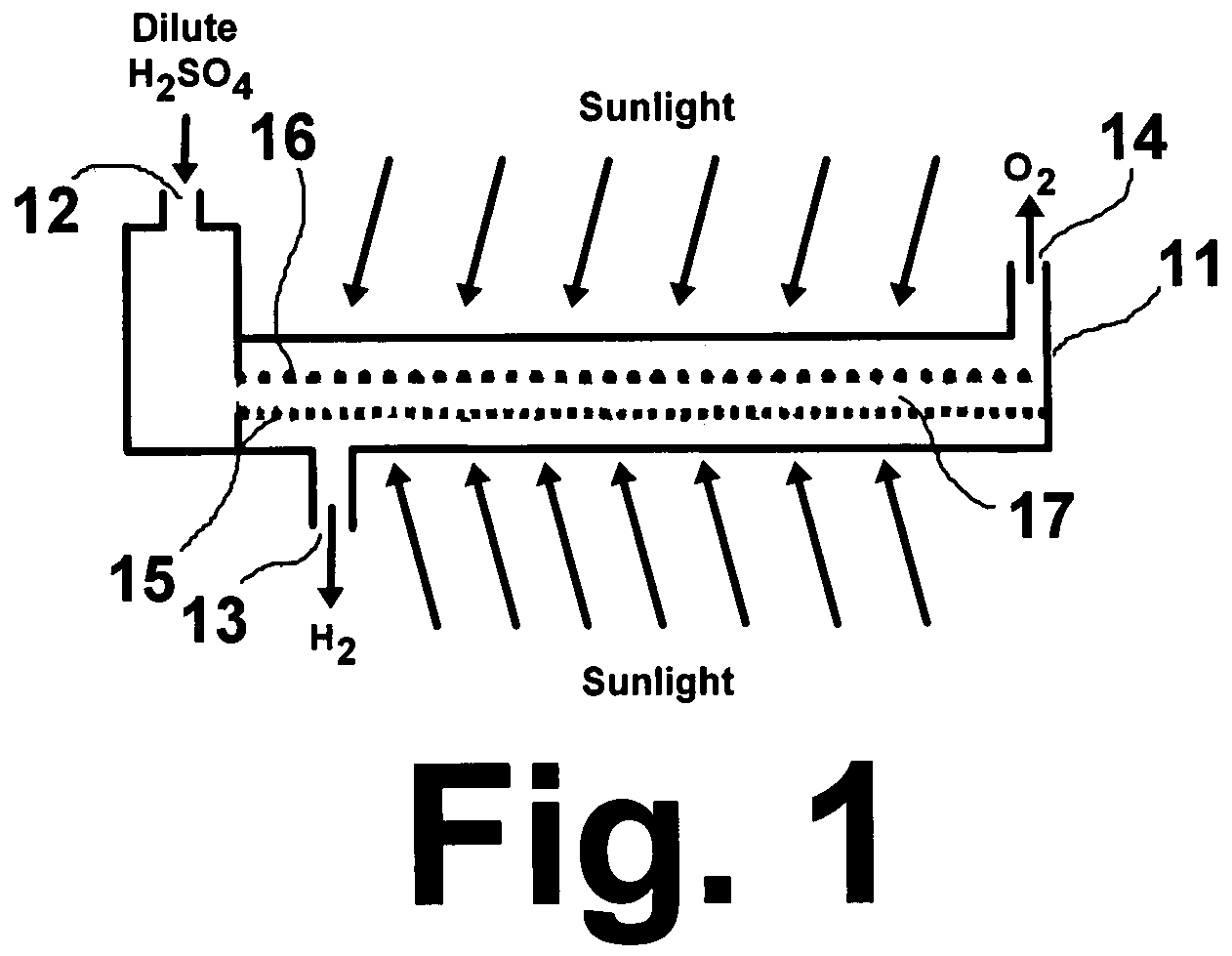
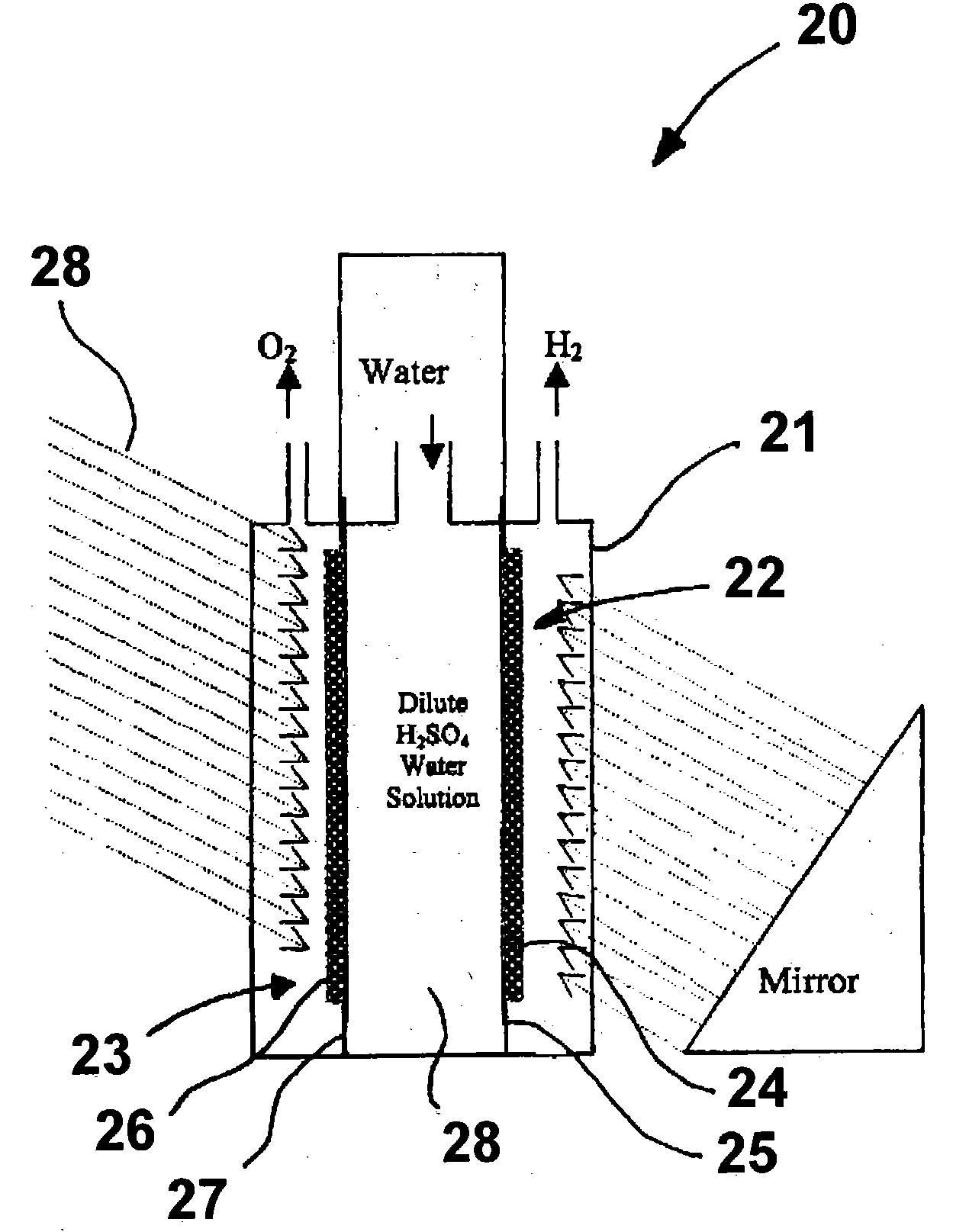
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water.
Electrolyte composition, photoelectric conversion device and photo-electrochemical cell
InactiveUS6376765B1Improve rendering capabilitiesIncreased durabilityLight-sensitive devicesOrganic chemistryPhotoelectrochemical cellHydrogen atom
Owner:FUJIFILM HLDG CORP +1
Catalytic materials, photoanodes, and photoelectrochemical cells for water electrolysis and other electrochemical techniques
InactiveUS20100133111A1Photography auxillary processesCell electrodesChemistryPhotoelectrochemical cell
Catalytic materials, photoanodes, and systems for electrolysis and / or formation of water are provided which can be used for energy storage, particularly in the area of solar energy conversion, and / or production of oxygen and / or hydrogen. Compositions and methods for forming photoanodes and other devices are also provided.
Owner:SUN CATALYTIX CORP +1
Photoelectrochemical determination of chemical oxygen demand
ActiveUS20060240558A1High sensitivityWide linear rangeChemical analysis using catalysisChemical analysis using combustionSupporting electrolytePotential measurement
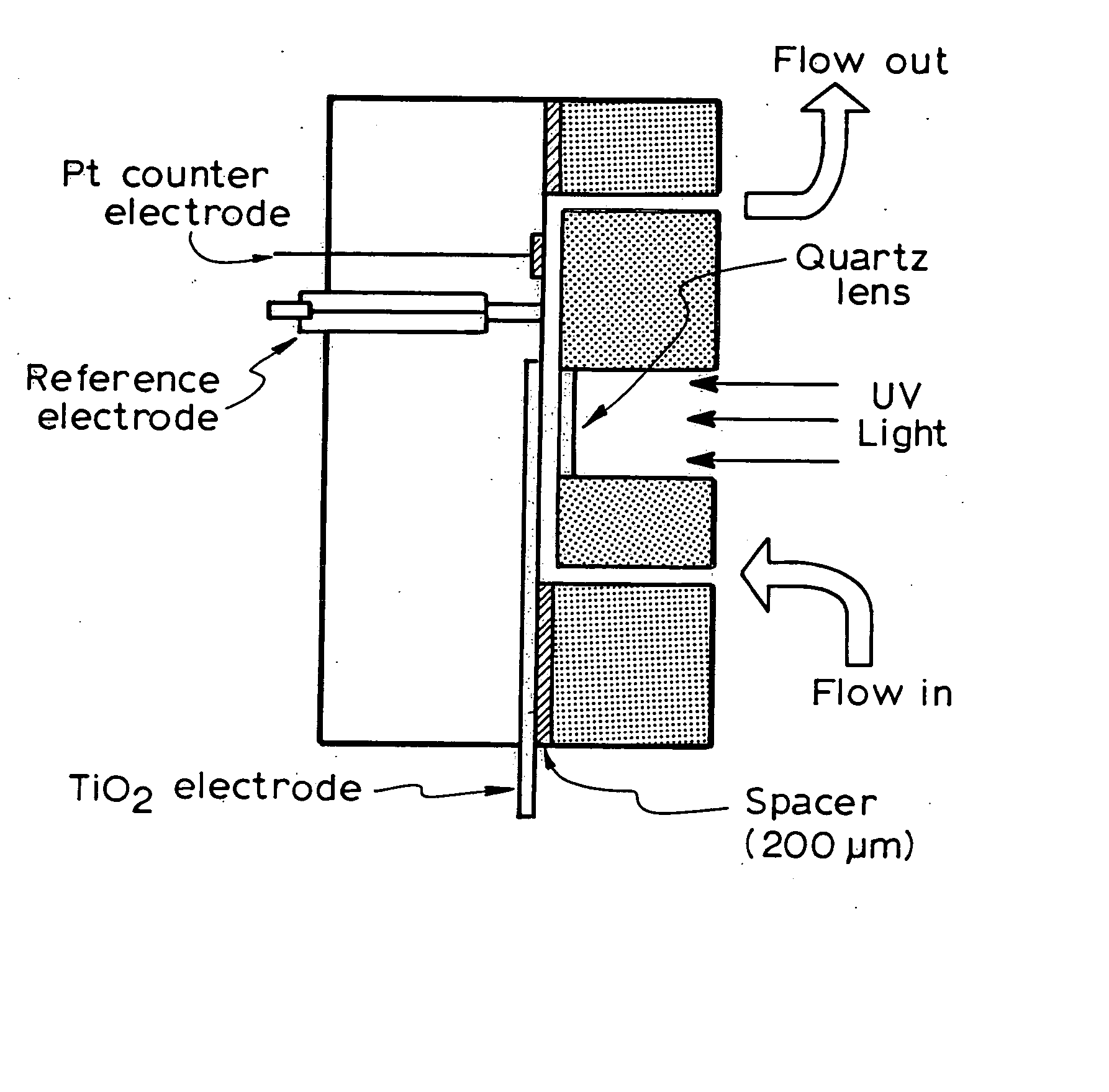
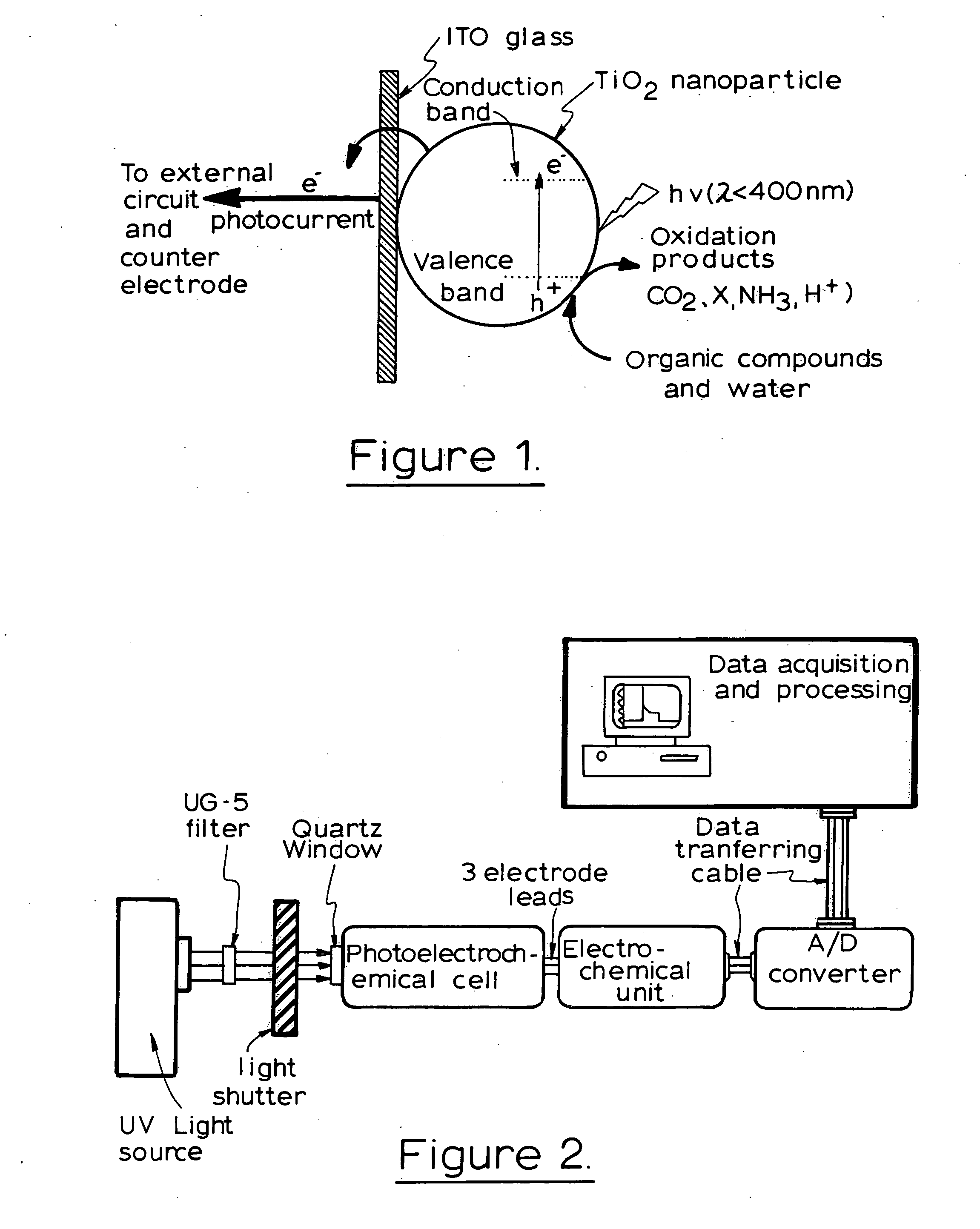
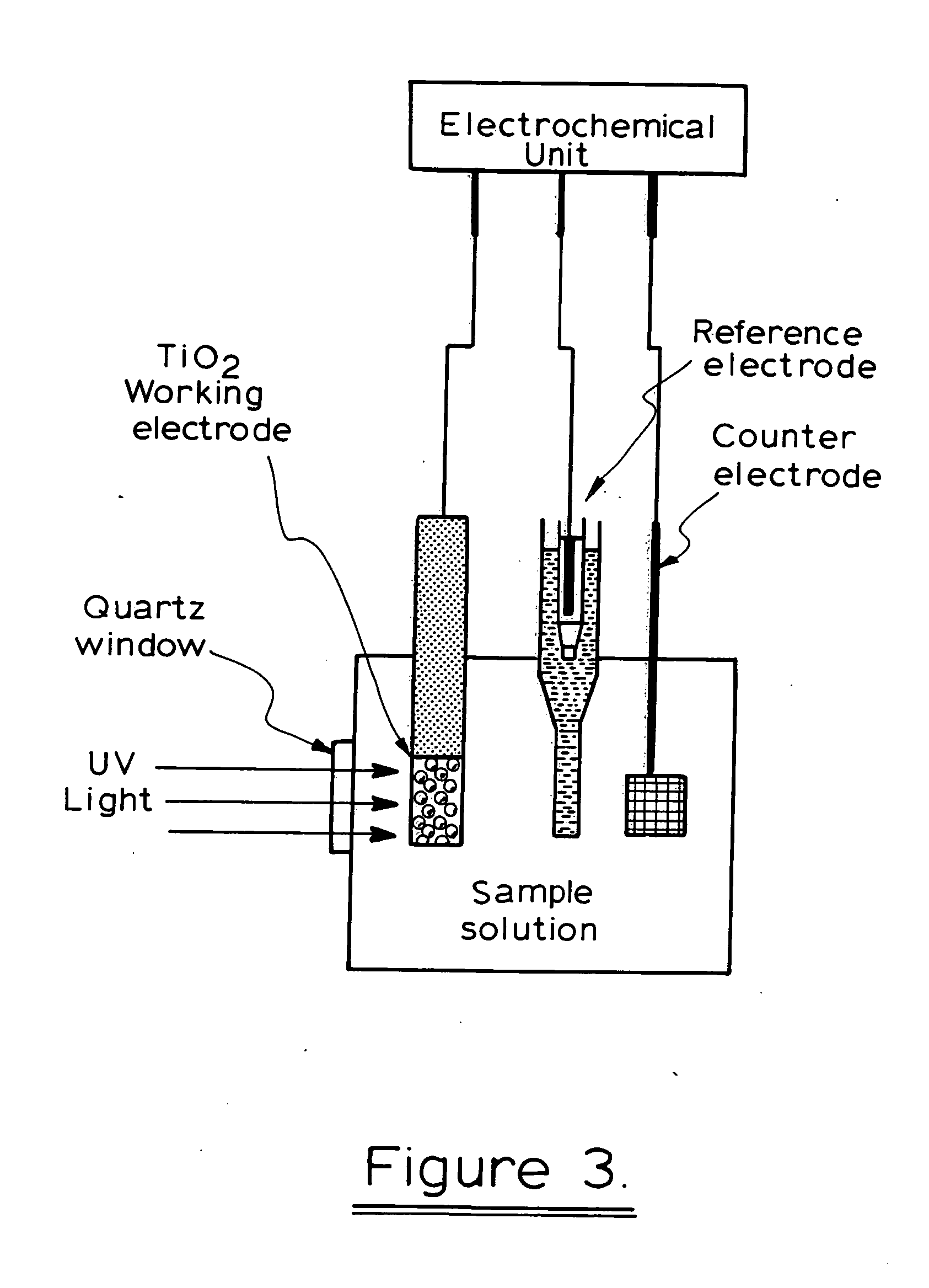
A photoelectrochemical assay apparatus for determining chemical oxygen demand (COD) of a water sample which consists of a) a measuring cell for holding a sample to be analysed b) a titanium dioxide nanoparticle photoelectric working electrode and a counter electrode disposed in said cell, c) a UV light source adapted to illuminate the photoelectric working electrode d) control means to control the illumination of the working electrode e) potential measuring means to measure the electrical potential at the working and counter electrodes f) analysis means to derive a measure of oxygen demand from the measurements made by the potential measuring means. The method of determining chemical oxygen demand of a water sample, comprises the steps of a) applying a constant potential bias to a photoelectrochemical cell, containing a supporting electrolyte solution; b) illuminating the working electrode with a UV light source and recording the background photocurrent produced at the working electrode from the supporting electrolyte solution; c) adding a water sample, to be analysed, to the photoelectrochemical cell; d) illuminating the working electrode with a UV light source and recording the total photocurrent produced; e) determining the chemical oxygen demand of the water sample according to the type of degradation conditions employed. The determination may be under exhaustive degradation conditions, in which all organics present in the water sample are oxidised or under non-exhaustive degradation conditions, in which the organics present in the water sample are partially oxidised.
Owner:579453 ONTARIO INC
Catalytic materials, photoanodes, and photoelectrochemical cells for water electrolysis and other, electrochemical techniques
InactiveUS20100133110A1Photography auxillary processesPhysical/chemical process catalystsHydrogenPhotoelectrochemical cell
Catalytic materials, photoanodes, and systems for electrolysis and / or formation of water are provided which can be used for energy storage, particularly in the area of solar energy conversion, and / or production of oxygen and / or hydrogen. Compositions and methods for forming photoanodes and other devices are also provided.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +2
Electric connection of electrochemical and photoelectrochemical cells
InactiveUS6657119B2Equipment is cheapReduce needLight-sensitive devicesPV power plantsPhotoelectrochemical cellEngineering
Owner:FORSKARPATENT I UPPSALA
Photoelectrochemical Cell
InactiveUS20080286643A1Reduced current efficiencyCurrent efficiency downElectrolysis componentsElectrolytic capacitorsPhotoelectrochemical cellChemical species
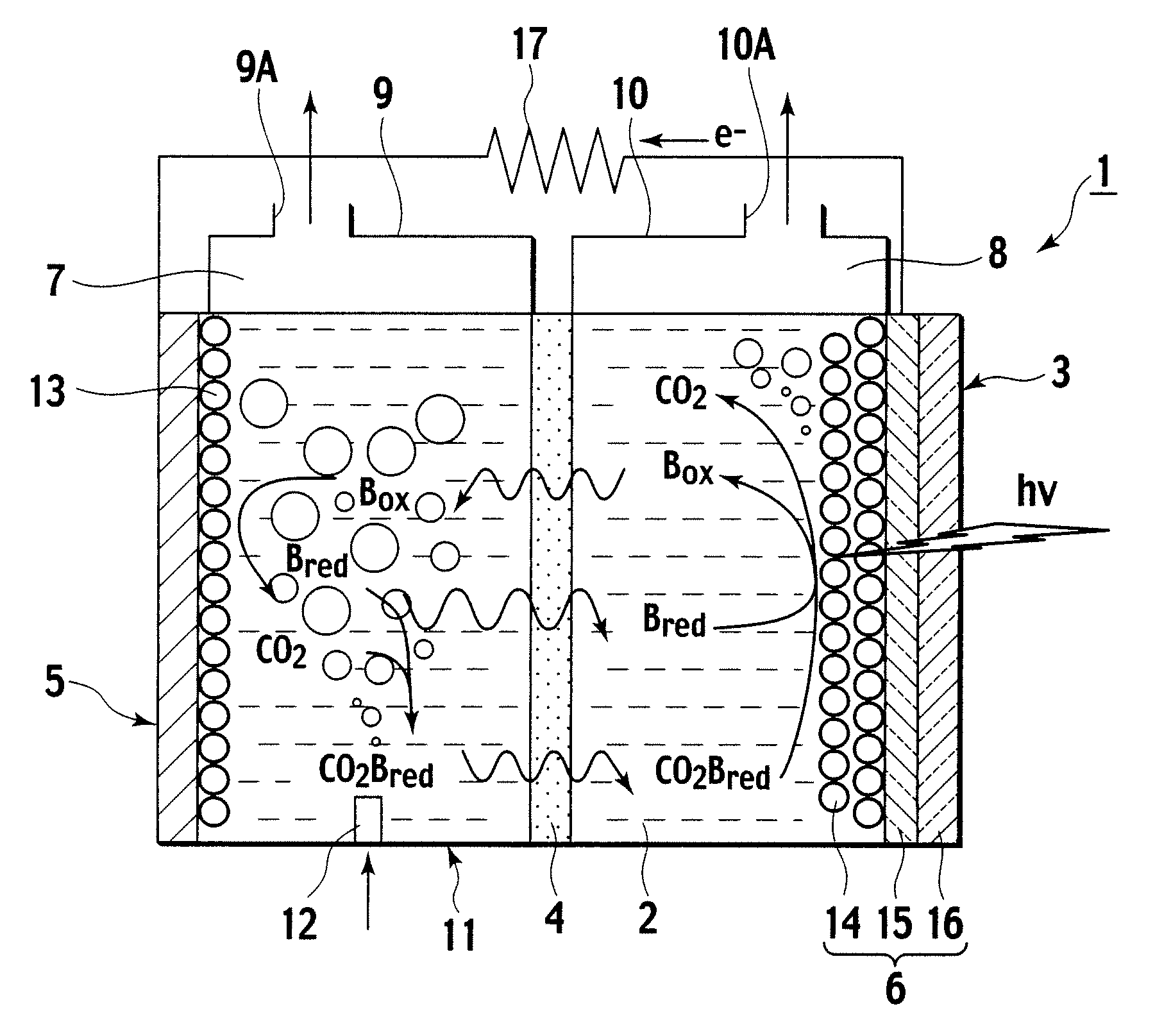
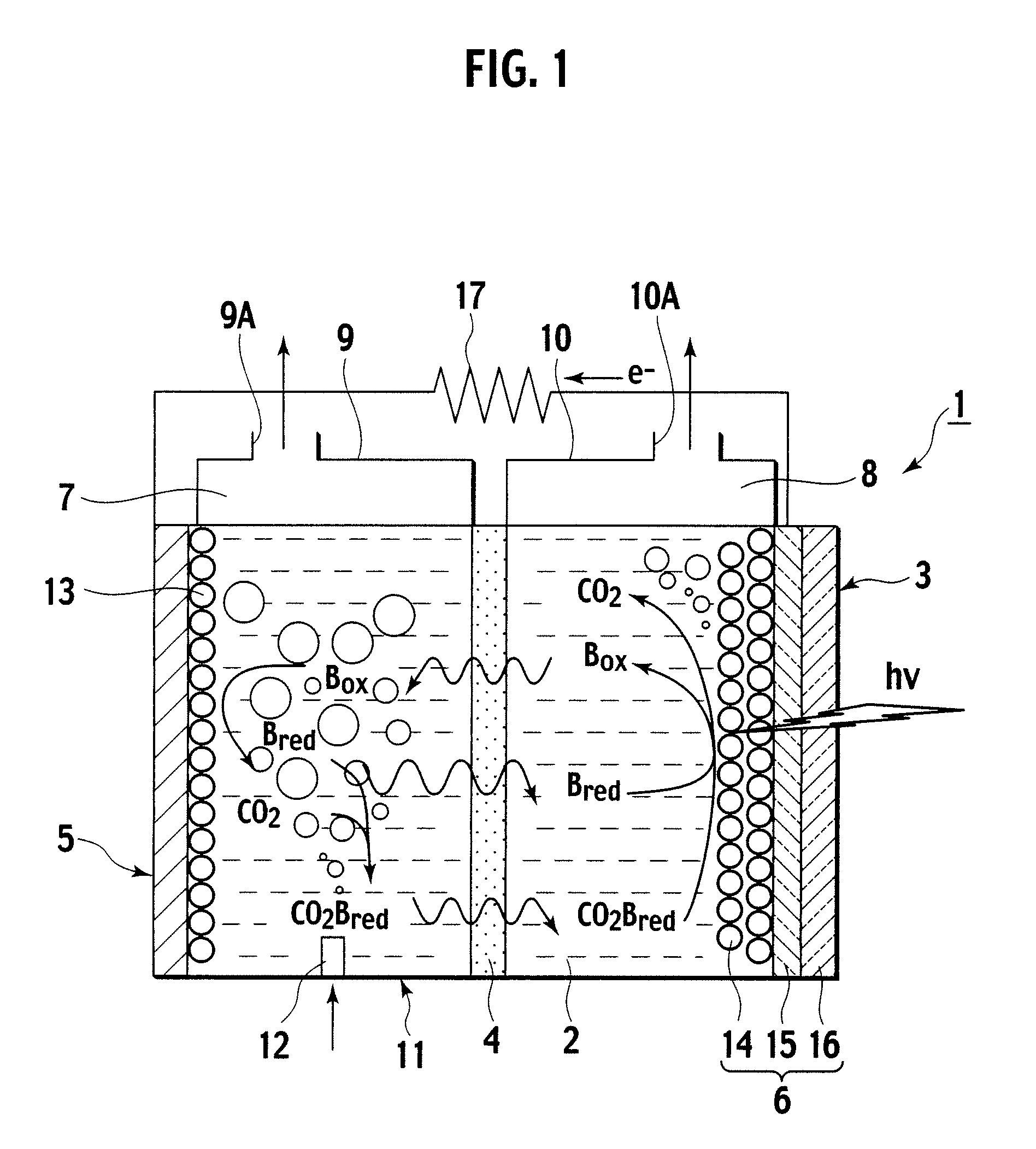
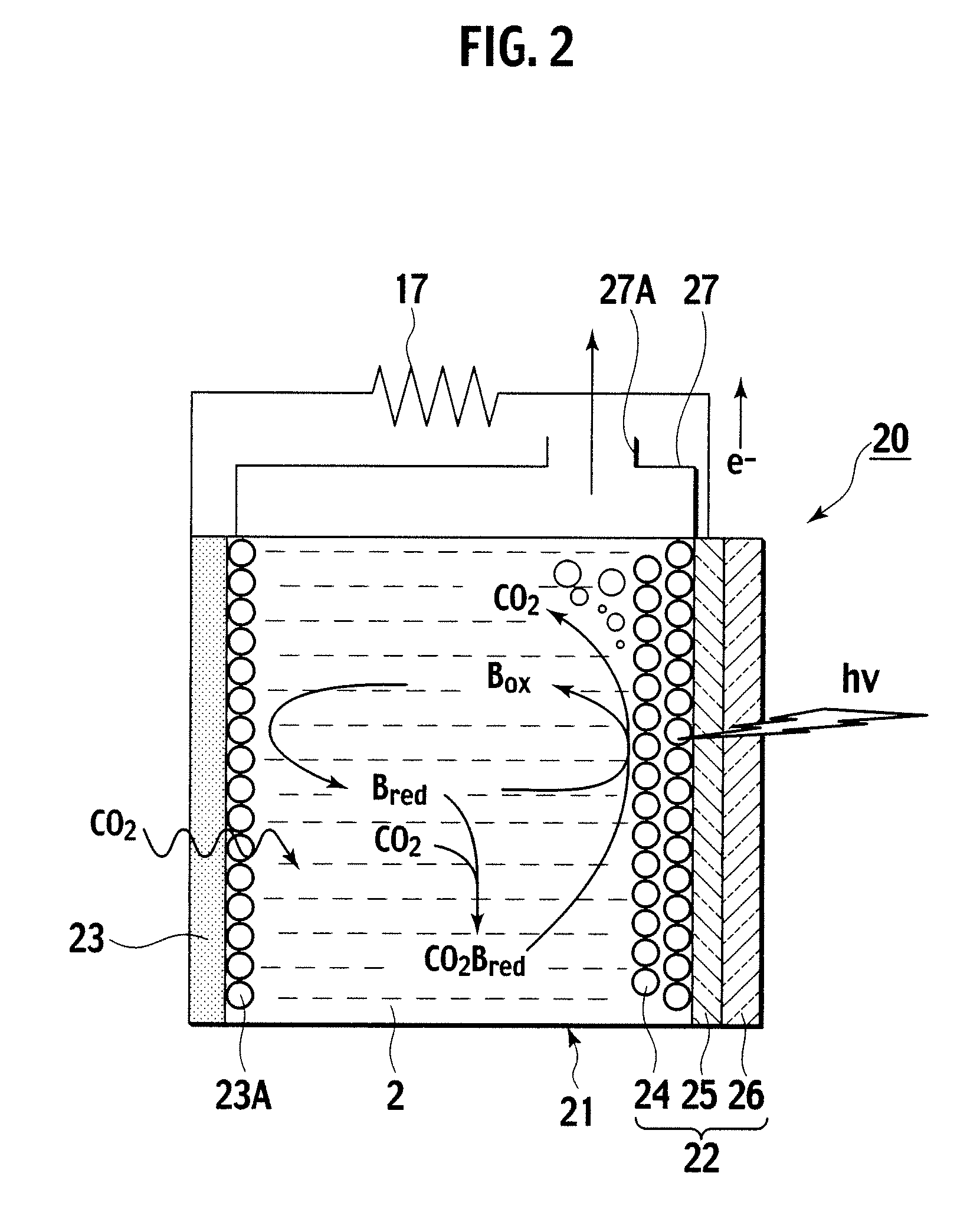
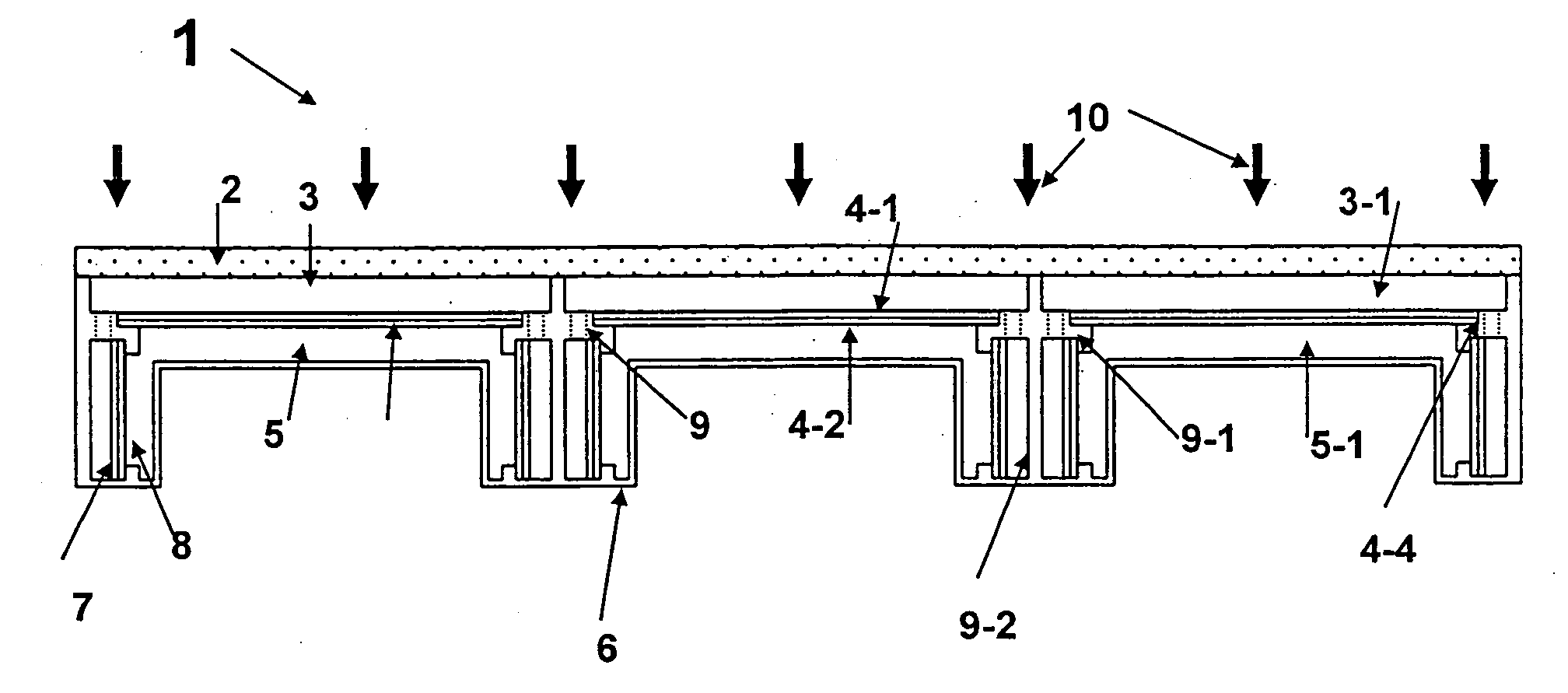
A photoelectrochemical cell (1) includes an electrolyte container (3) containing an ionic liquid (2), and a partitioning membrane (4) dividing an interior of the electrolyte container (3) into two being a CO2 capturing chamber (7) and a CO2 releasing chamber (8), having side walls opposing each other, with the partitioning membrane (4) in between, either as a carbon electrode (5) and the other as a photoelectrode (6). A redox mediator (B) has different bonding forces to carbon dioxide, as it appears as an oxidant Box and a reductant Bred, of which that one which has a greater bonding force serves as an intermediary chemical species carrying carbon dioxide to one of the paired electrodes (5, 6). Over the CO2 releasing chamber (10), an upper wall portion (10) is formed, which has a CO2 take-out port (10A) formed therein, for making use of oxidation and reduction of the redox mediator to achieve separation and concentration of carbon dioxide, converting photo energy of sunlight into electric power.
Owner:NISSAN MOTOR CO LTD
Spinel catalysts for water and hydrocarbon oxidation
ActiveUS20130040806A1Electrolytic capacitorsElectrical-based machining electrodesElectric energyPhotoelectrochemical cell
A catalyst for the electrolysis of water molecules and hydrocarbons, the catalyst including catalytic groups comprising A1-xB2−yB′yO4 spinels having a cubical M4O4 core, wherein A is Li or Na, B and B′ are independently any transition metal or main group metal, M is B, B′, or both, x is a number from 0 to 1, and y is a number from 0 to 2. In photo-electrolytic applications, a plurality of catalytic groups are supported on a conductive support substrate capable of incorporating water molecules. At least some of the catalytic groups, supported by the support substrate, are able to catalytically interact with water molecules incorporated into the support substrate. The catalyst can also be used as part of a photo-electrochemical cell for the generation of electrical energy.
Owner:RUTGERS THE STATE UNIV
Integrated photoelectrochemical cell and system having a liquid electrolyte
InactiveUS20050211290A1Improve hydrogen efficiencyImprove oxygen production efficiencyCellsLight-sensitive devicesHydrogenPhotoelectrochemical cell
An integrated photoelectrochemical (PEC) cell generates hydrogen and oxygen from water while being illuminated with radiation. The PEC cell employs a liquid electrolyte, a multi-junction photovoltaic electrode, and a thin ion-exchange membrane. A PEC system and a method of making such PEC cell and PEC system are also disclosed.
Owner:UNIVERSITY OF TOLEDO
Interconnected Photoelectrochemical Cell
InactiveUS20080223439A1Light-sensitive devicesElectrolysis componentsHydrogenPhotoelectrochemical cell
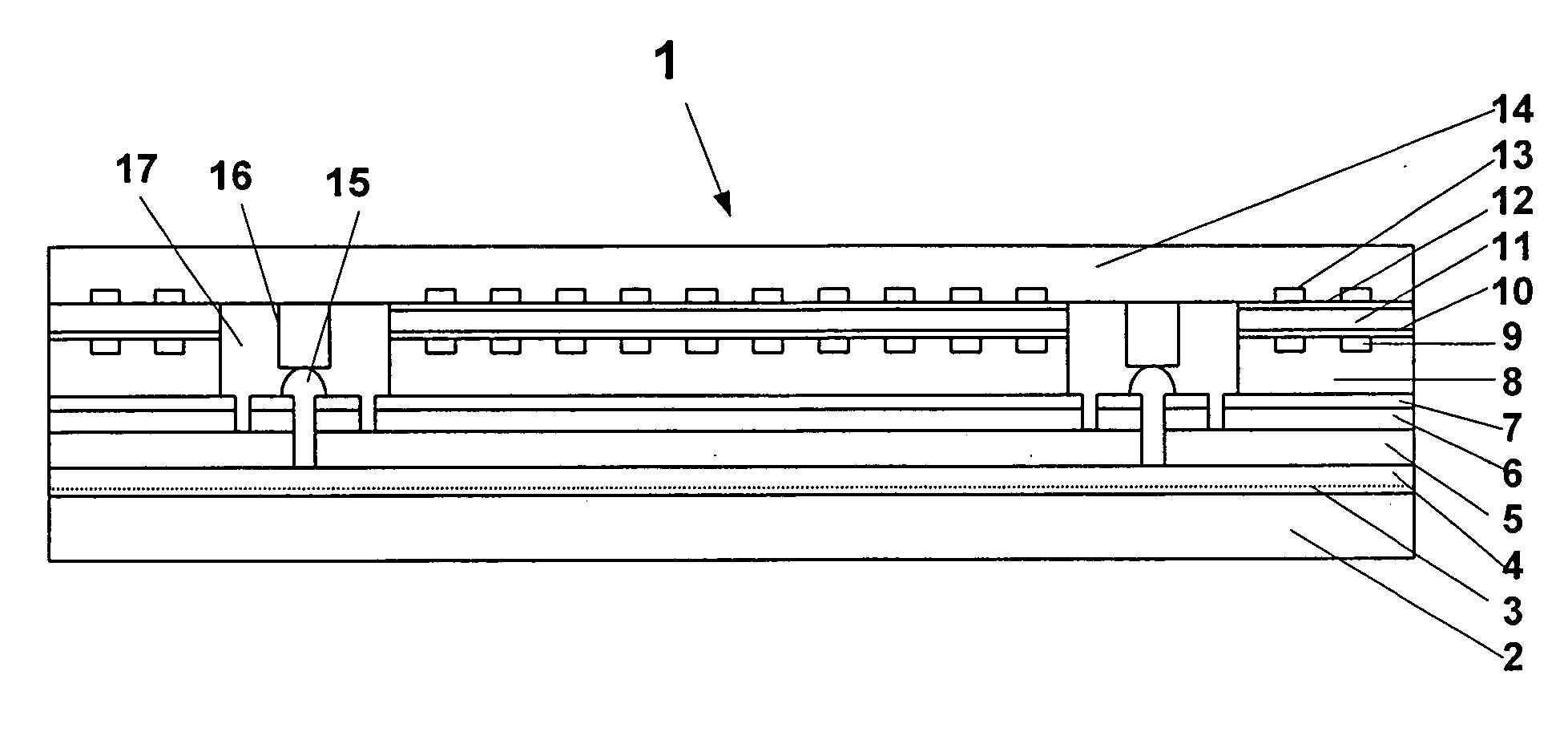
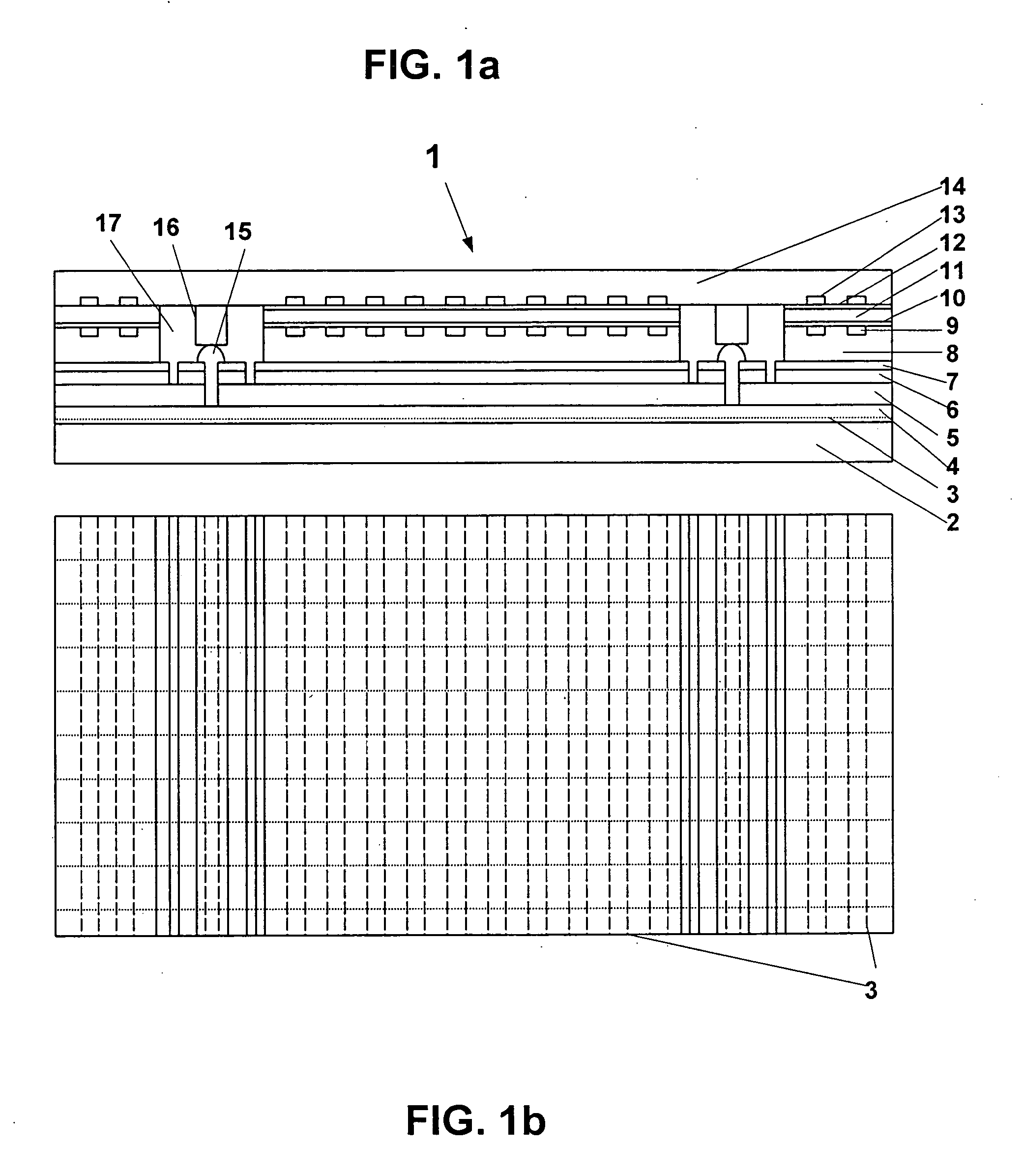
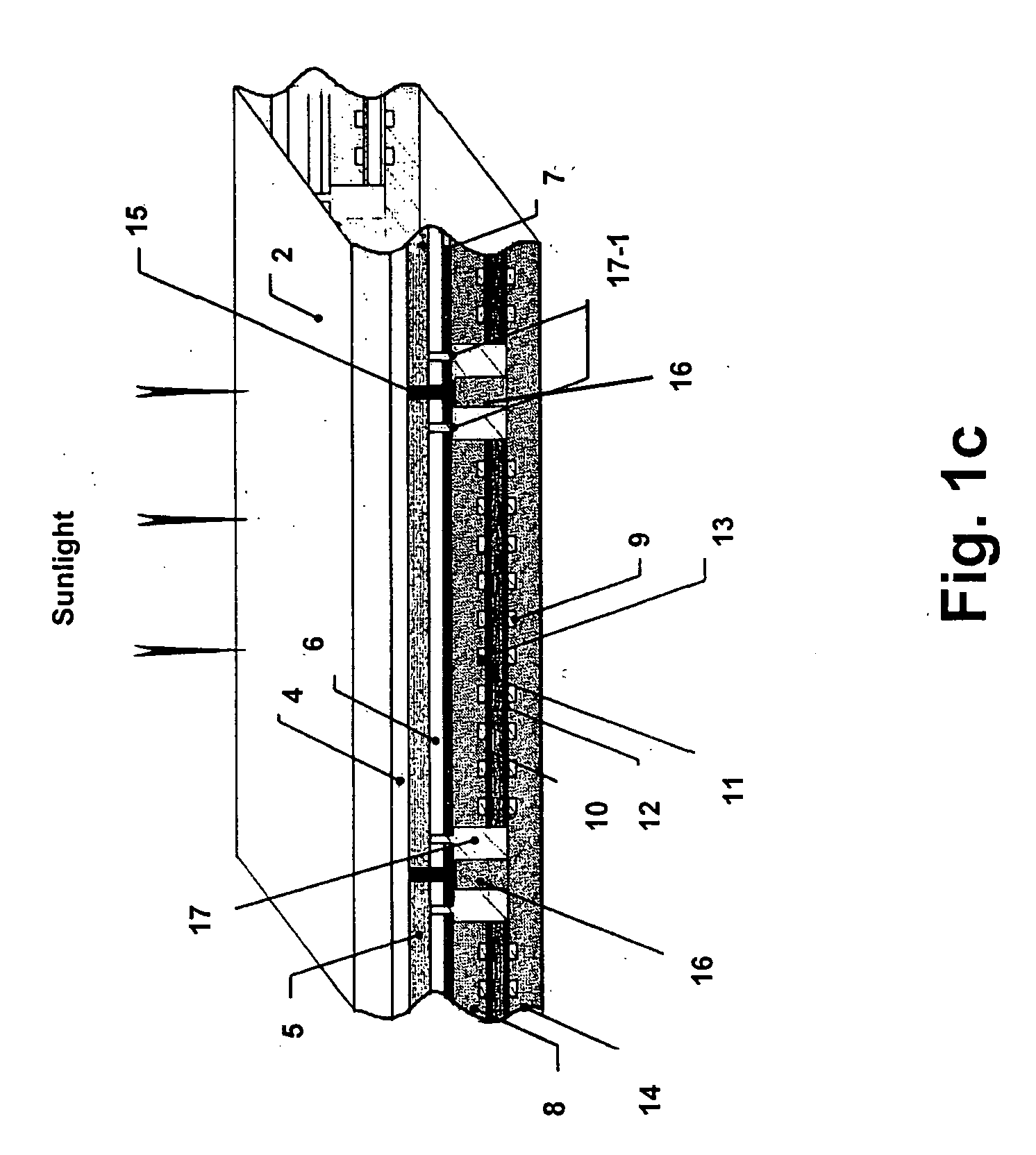
An interconnected photoelectrochemical (PEC) cell (1) generates hydrogen and oxygen from water while being illuminated with radiation such as sunlight. The photovoltaic structure in the photoelectrode is deposited on a transparent and insulating substrate (also called superstrate) (3) that is covered with a transparent conducting layer (front electrode) (4). The front electrode is electrically connected to the back side of the photovoltaic structure such that the PLC cell can be made with high efficiency and high durability and at low cost. Three types of photoelectrodes and phtoelectrochemical cells are illustrated as examples.
Owner:UNIVERSITY OF TOLEDO +1
Integrated photoelectrochemical cell and system having a solid polymer electrolyte
InactiveUS20050205128A1Improve conversion efficiencyLow costElectrode manufacturing processesElectrolysis componentsPolymer electrolytesPhotoelectrochemical cell
A photoelectrochemical (PEC) cell includes a photovoltaic electrode that generates voltage under radiation; a solid membrane electrode assembly that includes at least one solid polymer electrolyte and first and second electrodes; a mechanism that collect gases from oxidation and reduction reactions; and an electrical connection between the photovoltaic electrode and the solid membrane electrode assembly. A PEC system and a method of making such PEC cell and PEC system are also disclosed.
Owner:UNIVERSITY OF TOLEDO
Charge transfer material, and photoelectric conversion device and photoelectric cell using same, and pyridine compound
InactiveUS6911595B2Increased durabilityGood transportabilityOrganic chemistryLight-sensitive devicesCompound aPhotoelectrochemical cell
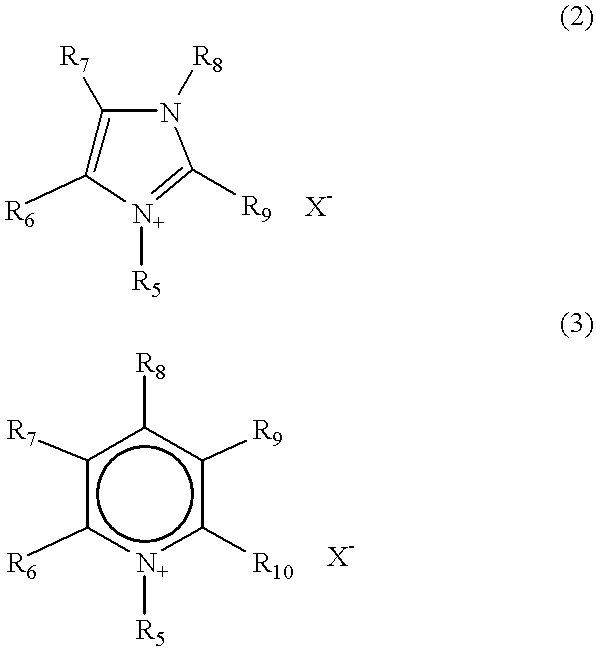
A charge transfer material comprising a basic compound having negative charge and represented by the following general formula (I):(A1-L)n1-A2·M (I), wherein A1 represents a group having negative charge; A2 represents a basic group; M represents a cation for neutralizing the negative charge of (A1-L)n1-A2; L represents a divalent linking group or a single bond; and n1 represents an integer of 1 to 3. A photoelectric conversion device and a photo-electrochemical cell comprising the charge transfer material. A new nonvolatile pyridine compound, which is a low-viscosity liquid at room temperature, is preferably used for A2.
Owner:FUJIFILM CORP
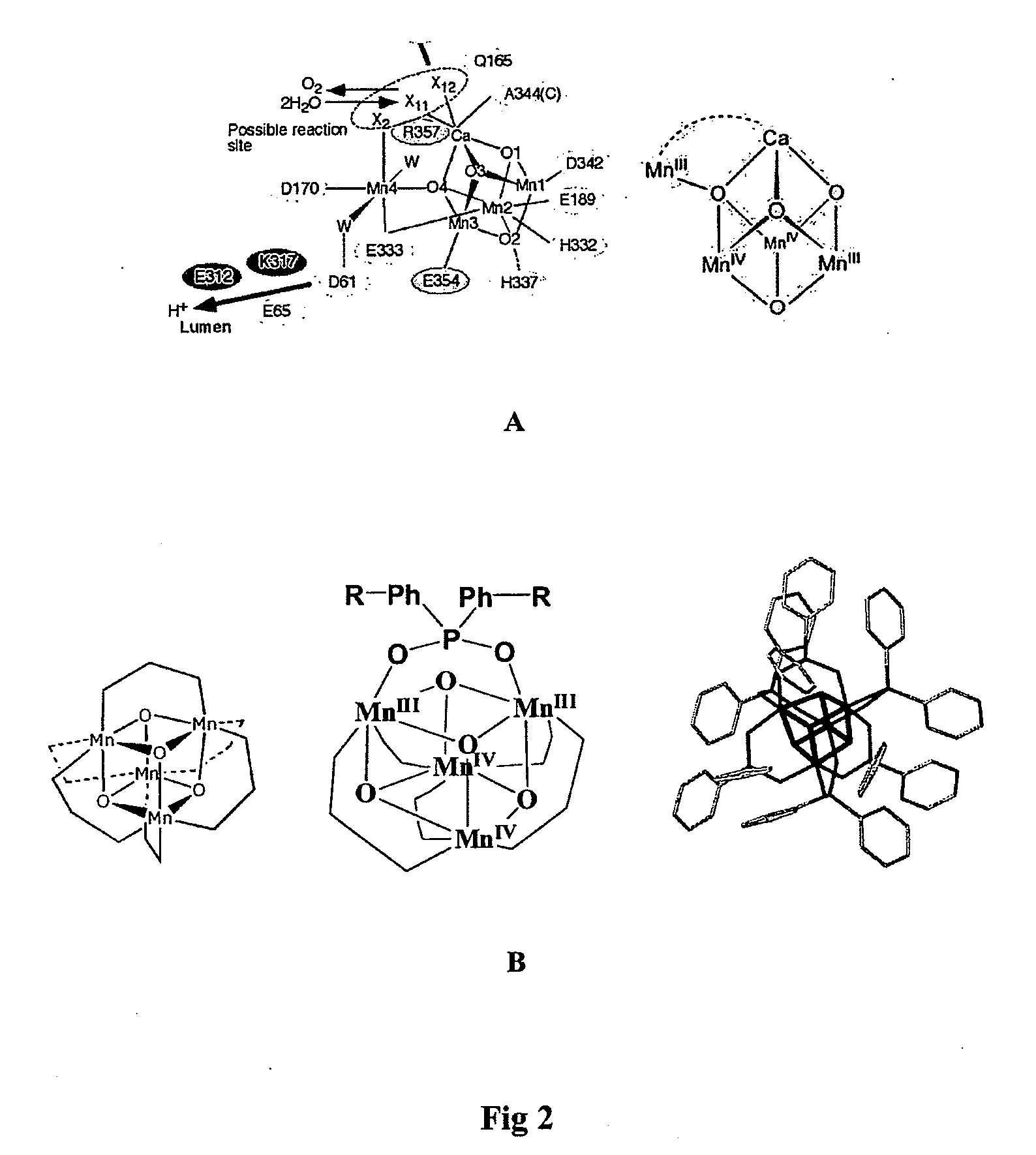
Water Oxidation Catalyst
InactiveUS20100143811A1Rapid reformationTrend downMachining electrodesCellsPhotoelectrochemical cellElectrolysis of water
A catalyst for the photo-electrolysis of water molecules, the catalyst including catalytic groups comprising tetra-manganese-oxo clusters. A plurality of the catalytic groups are supported on a conductive support substrate capable of incorporating water molecules. At least some of the catalytic groups, supported by the support substrate, are able to catalytically interact with water molecules incorporated into the support substrate. The catalyst can be used as part of photo-electrochemical cell for the generation of electrical energy.
Owner:COMMONWEALTH SCI & IND RES ORG +2
Catalytic materials, photoanodes, and photoelectrochemical cells for water electrolysis and other electrochemical techniques
Catalytic materials, photoanodes, and systems for electrolysis and / or formation of water are provided which can be used for energy storage, particularly in the area of solar energy conversion, and / or production of oxygen and / or hydrogen. Compositions and methods for forming photoanodes and other devices are also provided.
Owner:MASSACHUSETTS INST OF TECH +1
Photoelectrolysis of water using proton exchange membranes
ActiveUS7037414B2Improve efficiencyImprove stabilityMachining electrodesCellsElectro catalystWater use
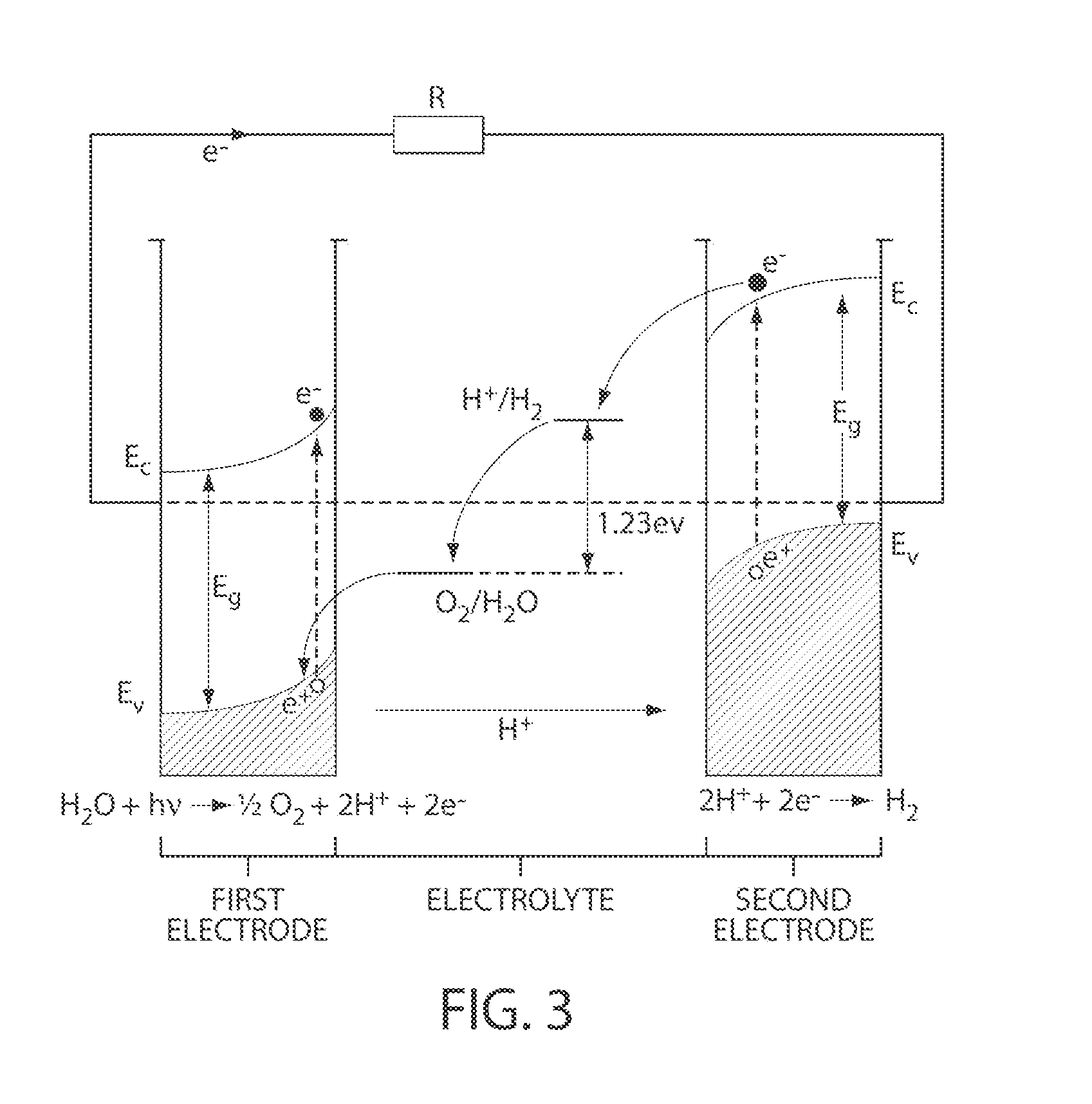
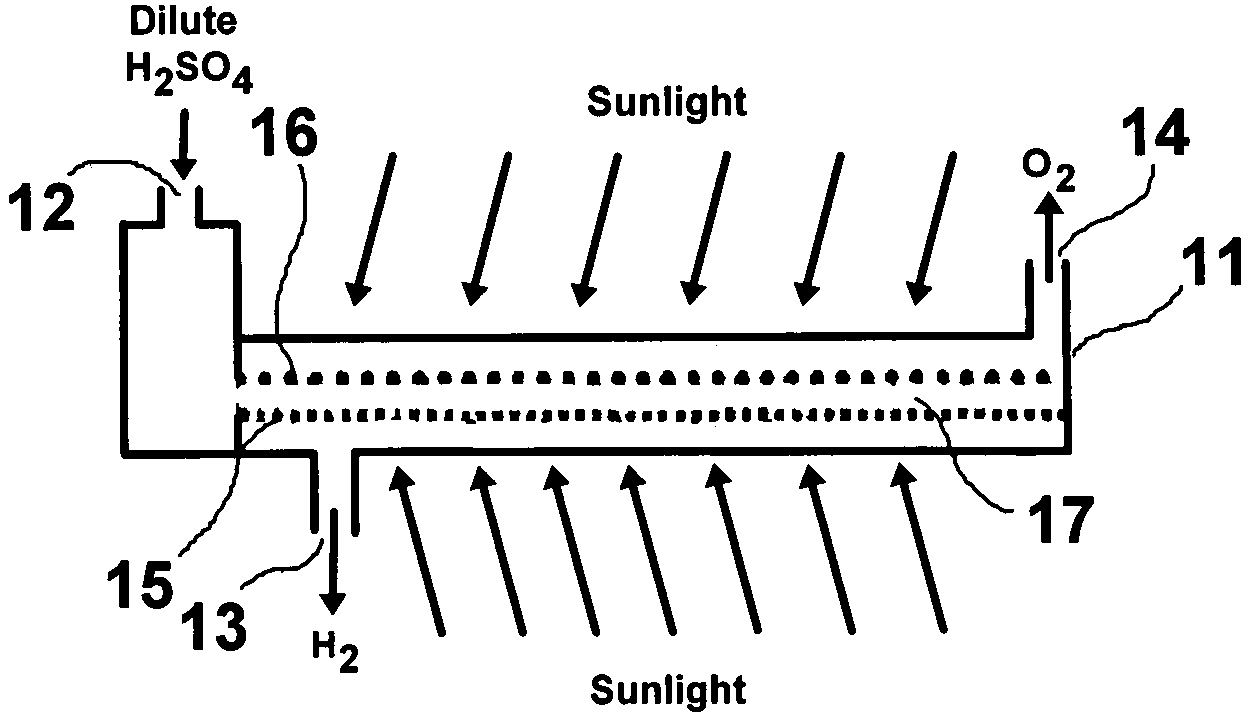
A photoelectrochemical cell which includes a light transmissive enclosure, a semiconductor photoanode disposed within the light transmissive enclosure, a semiconductor photocathode disposed within the light transmissive enclosure, and an electrolytic solution disposed entirely between the semiconductor photoanode and the semiconductor photocathode. This is achieved by the use of semiconductor photoelectrodes (photoanodes and photocathodes) which include a proton exchange membrane having an electrolyte facing surface in contact with the electrolytic solution and a light transmissive wall facing surface, and having a photo electro-catalyst disposed on the light transmissive wall facing surface.
Owner:GAS TECH INST
Compound, photoelectric converter and photoelectrochemical cell
A complex compound (I) obtained by coordinating a compound represented by the following formula (II), hereinafter abbreviated as compound (II), to a metal atom. In the formula, R1, R2 and R3 each independently represent a substituent represented by the following formula (III), formula (IV), formula (V) or formula (VI) and at least one of them is a substituent represented by the formula (III); a, b and c each independently represent an integer of 0 to 2 and a+b+c≧1; here, L represents a linking group represented by the following formula (VII) or formula (VIII); Ar represents an aryl group which may have a substituent; A represents an acidic group or a salt thereof; Y represents a halogen atom or a substituent; Q1 and Q2 each independently represent a hydrogen atom, an alkyl group having 1 to 20 carbon atoms, an alkoxy group having 1 to 20 carbon atoms, an aryl group having 6 to 20 carbon atoms or a cyano group; and p and q each represent an integer of 1 to 3.
Owner:SUMITOMO CHEM CO LTD
Porous membrane semiconductor optical electrode having visible light response and photoelectrochemical reaction equipment and preparation thereof
InactiveCN1542998AGuaranteed mobilityShorten the diffusion distanceCell electrodesFuel cellsLight responseTin
A porous film semiconductor photoemitter with visible light response formed by a porous structure complex metal oxide semiconductor is used in photoelectric chemical cell reacted in the energy storage. The said photoemitter is composed of more than two kinds of elements: an is one of Bi, Ag, Cu, Sn, Pb, V, In, Pr Cr and Ni, B is selected from Ti, Nb, Ta, Zr, Hf, Mo, W, Zn, Ga, In, Ge and Sn. This invented simple device alters the visible light such as the sunlight to hydrogen chemical energy effectively, even if the photoemitter has very low quantum absorptivity yet it will approach to 100% so long as the filming method is perfect.
Owner:NANJING UNIV
Gold nanoparticle-modified dendritic titanium dioxide nanorod array electrode, as well as preparation method and application of hydrogen production by photocatalytic water splitting
InactiveCN103354283AInhibitory complexGuaranteed normal transmissionNanostructure manufactureElectrode manufacturing processesElectron holePhotocatalytic water splitting
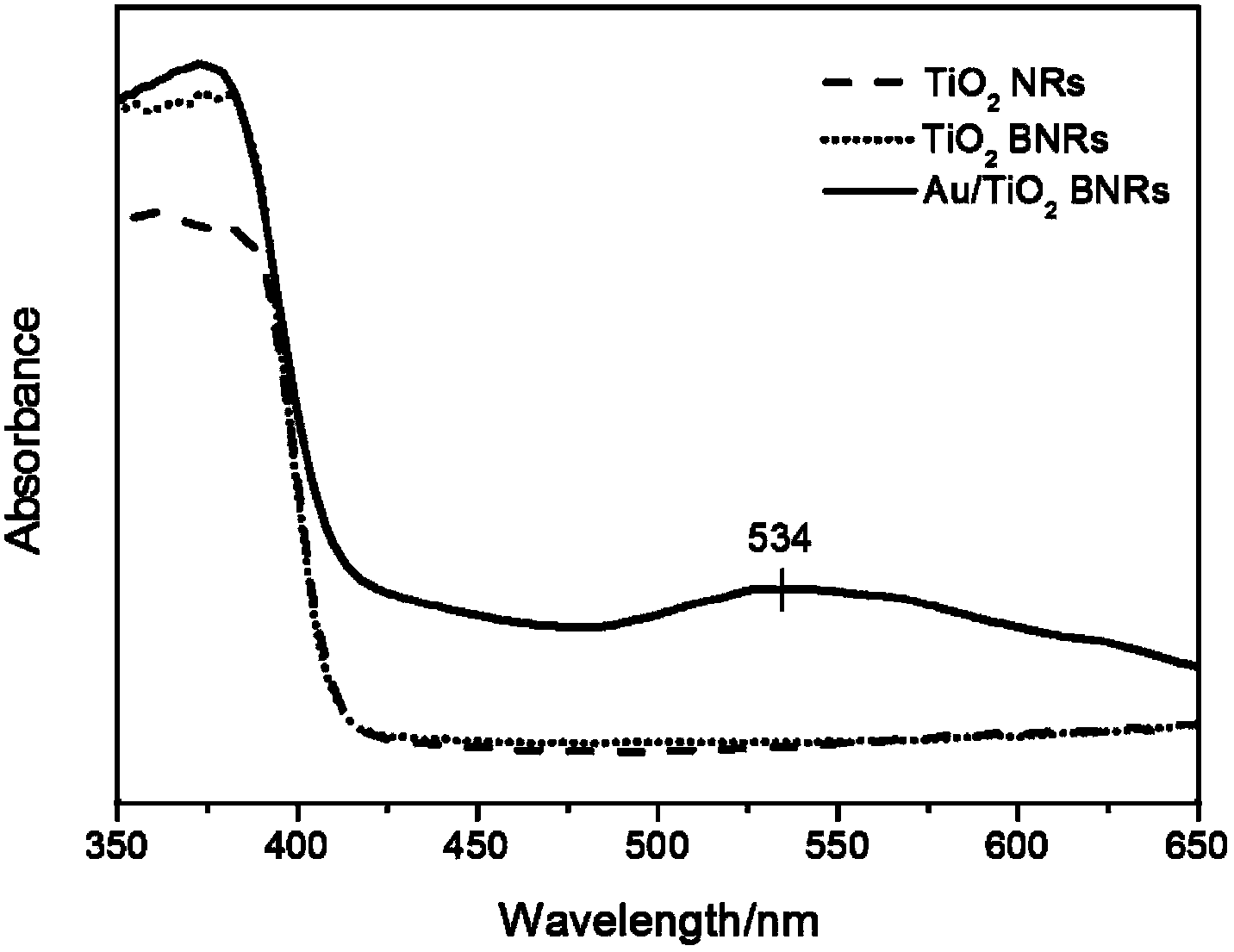
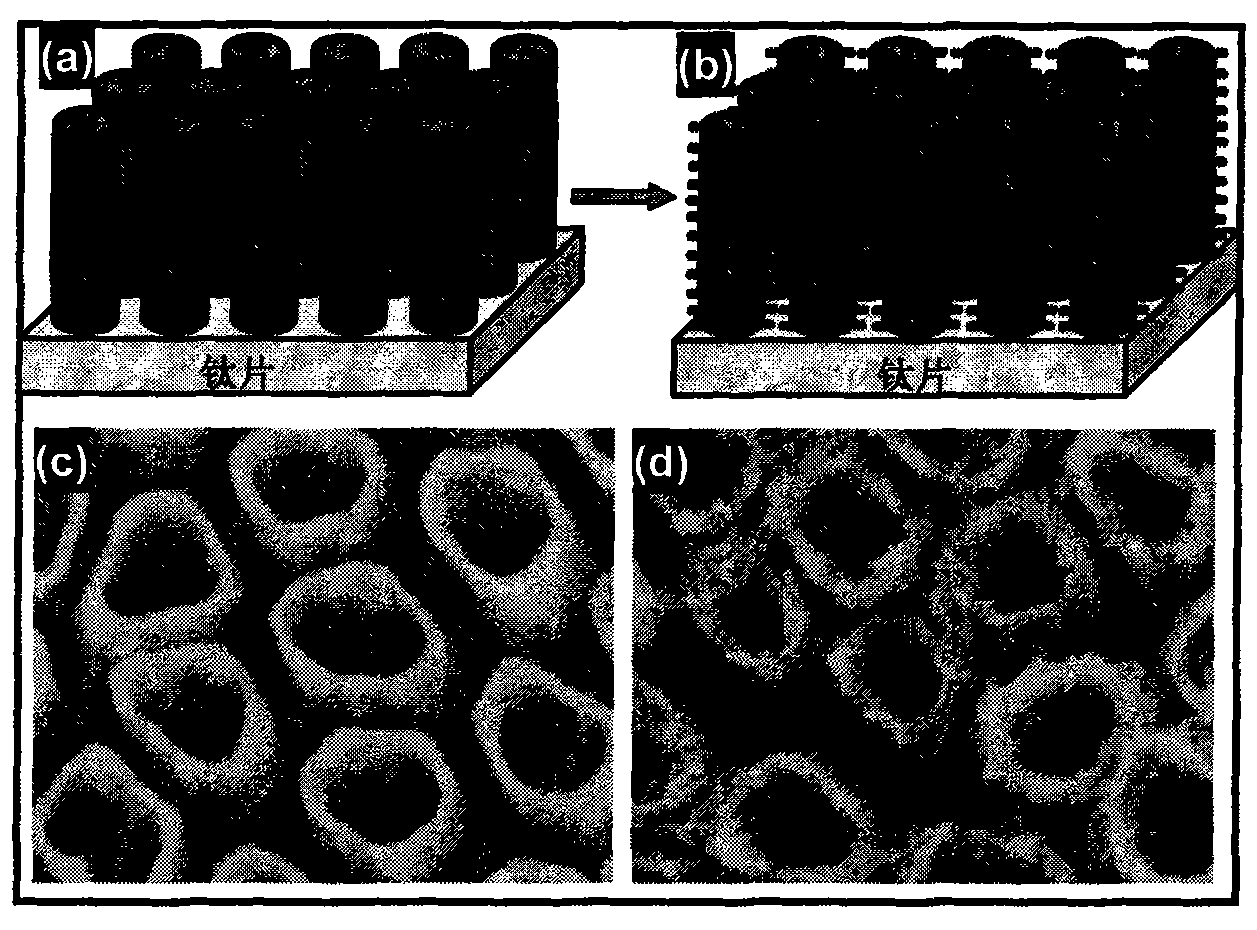
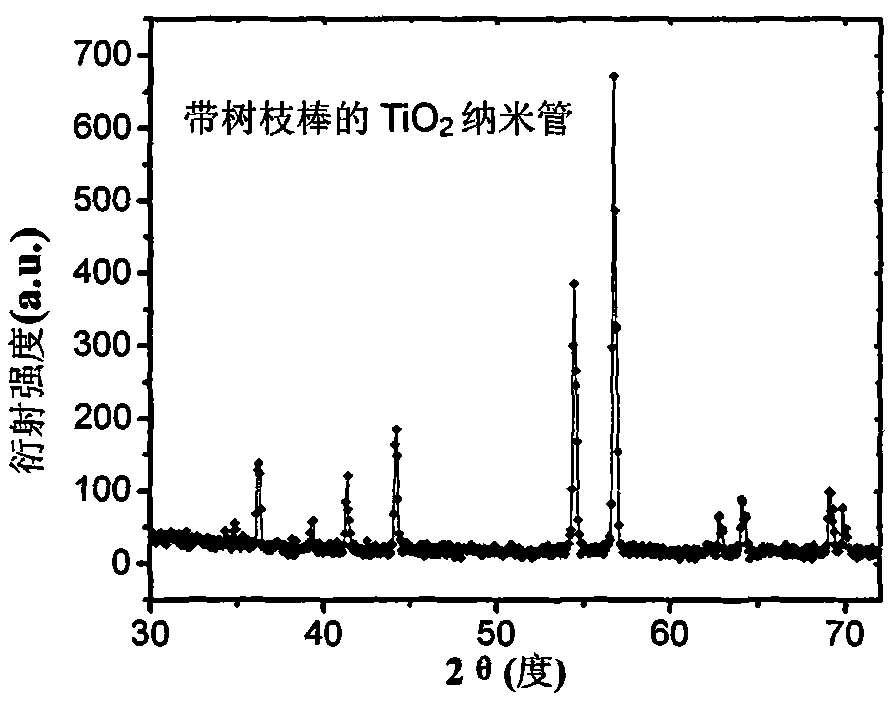
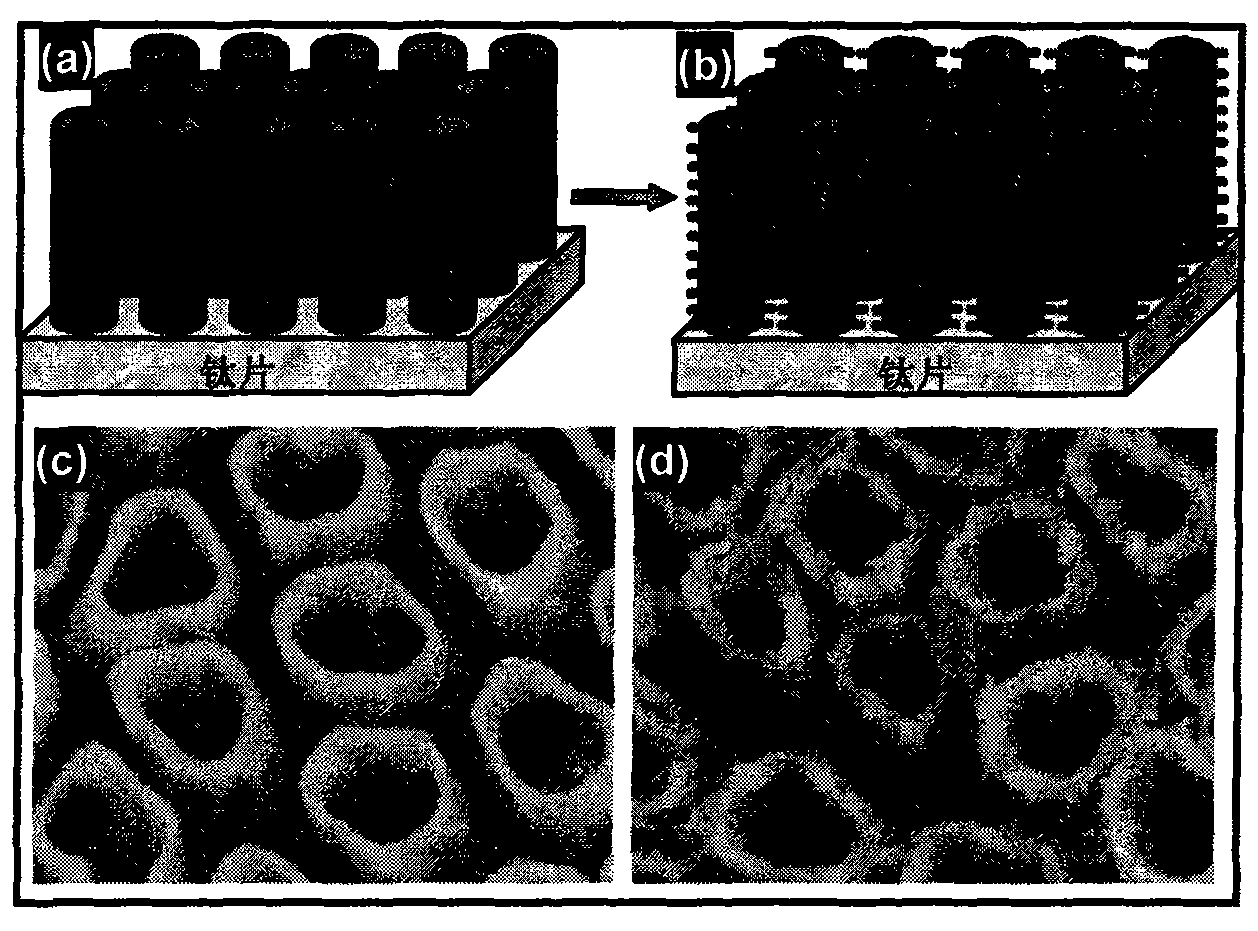
The invention discloses a gold nanoparticle-modified dendritic titanium dioxide nanorod array electrode, as well as a preparation method and an application of hydrogen production by photocatalytic water splitting. The gold nanoparticle-modified dendritic titanium dioxide nanorod array electrode comprises a trunk nanorod densely distributed with dendritic structures on the surface, wherein gold nanoparticles are uniformly loaded on the surfaces of the dendritic structures. The preparation method is composed of three steps, namely, preparation for a TiO2 nanorod array, preparation for a dendritic TiO2 nanorod array, and preparation for gold nanoparticle-sensitized dendritic TiO2 nanorod array. According to the nanorod array electrode, the preparation method, and the application, the compounding of electron-hole pairs is effectively suppressed; the photocatalytic water splitting efficiency of the material is increased; the light absorption range of the material is expanded to a visible region via the surface plasma resonance (SPR) effect of gold nanocrystalline, thus improving the activity of the photocatalytic water splitting of a photoelectrochemical cell, and the material is great in stability simultaneously. The preparation method disclosed by the invention is simple, excellent in the performance of hydrogen production by photocatalytic water splitting, good in chemical stability, and capable of realizing low-cost and large-scale application.
Owner:TIANJIN UNIV
Integrated photoelectrochemical cell and system having a liquid electrolyte
InactiveUS7750234B2Improve efficiencyLow costCellsLight-sensitive devicesPhotoelectrochemical cellIon-exchange membranes
An integrated photoelectrochemical (PEC) cell generates hydrogen and oxygen from water while being illuminated with radiation. The PEC cell employs a liquid electrolyte, a multi-junction photovoltaic electrode, and a thin ion-exchange membrane. A PEC system and a method of making such PEC cell and PEC system are also disclosed.
Owner:UNIVERSITY OF TOLEDO
Preparation process of dendritic titanium dioxide nanotube array electrode
InactiveCN101969109ALarge specific surface areaLow manufacturing process costNanostructure manufactureLight-sensitive devicesTio2 nanotubeSolar cell
The invention provides a preparation process of a dendritic titanium dioxide nanotube array electrode, which comprises: firstly, pre-preparing a titanium dioxide nanotube array by using an anodizing method and by using pure titanium foil as an anode and mixed solution of ammonium fluoride, lactic acid and dimethyl sulphoxide as electrolyte; secondly, growing nanorods with dendritic titanium dioxide on the pre-prepared titanium dioxide nanotubes serving as a host skeleton by using a low-temperature liquid-phase method and by using aqueous solution of hydrochloric acid and TTIP as growing solution, and thus obtaining the required dendritic titanium dioxide nanotube array; and finally, using the dendritic titanium dioxide nanotube array as a material to assemble the working electrodes of dye-sensitized solar cells, photoelectrochemical cells, photocatalysis devices and the like. The dendritic titanium dioxide nanotube array can improve the conversion efficiency of the cells and the efficiency of the photocatalytic pollutant degradation considerably; and the preparation process is low in cost, simple in process and easy in production.
Owner:XIANGFAN UNIVERSITY
Solar hydrogen charger
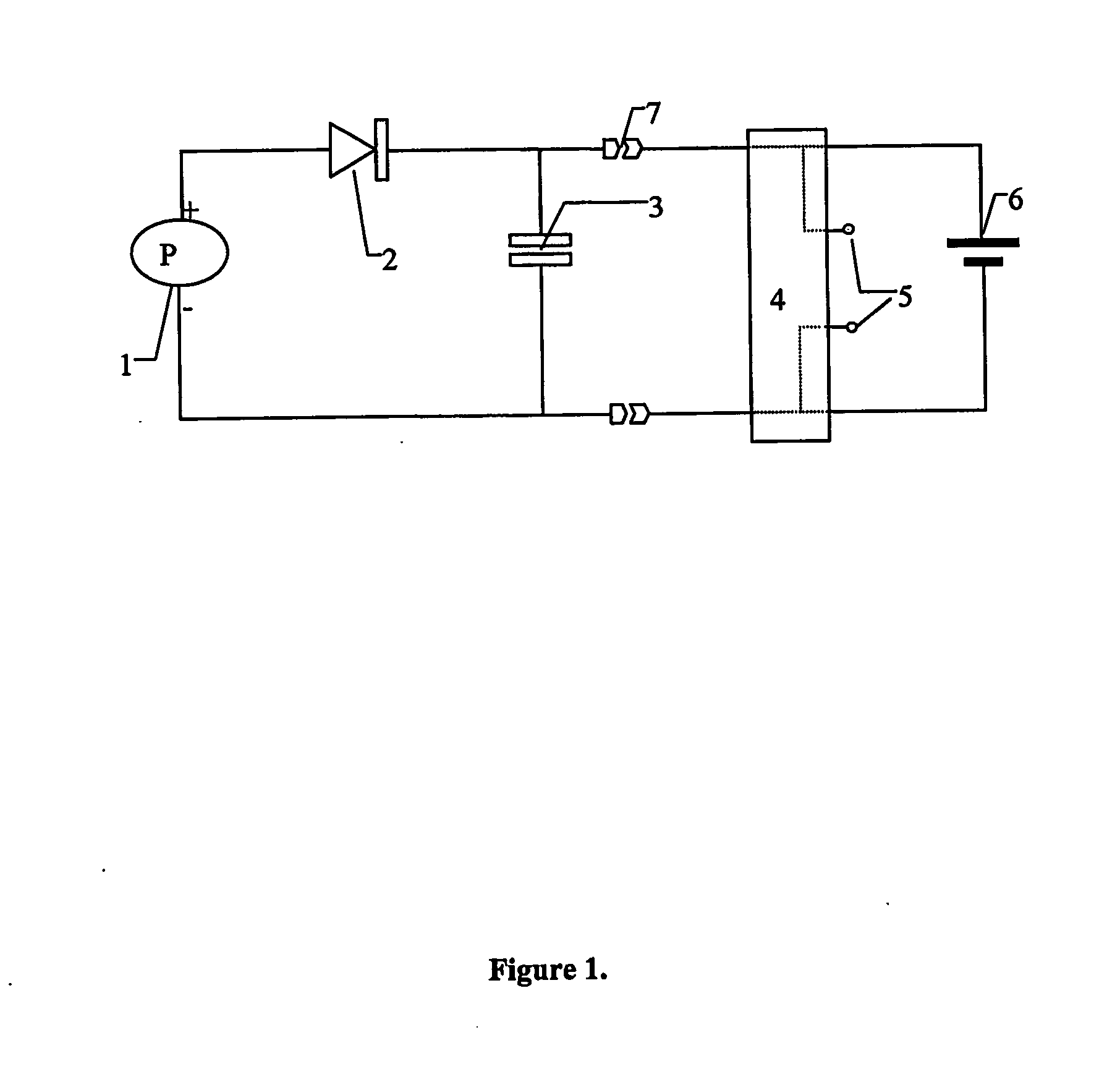
ActiveUS20090026070A1Avoid corrosionPermitting maximum direct solar irradianceCellsDeferred-action cellsPolymer electrolytesHydrogen
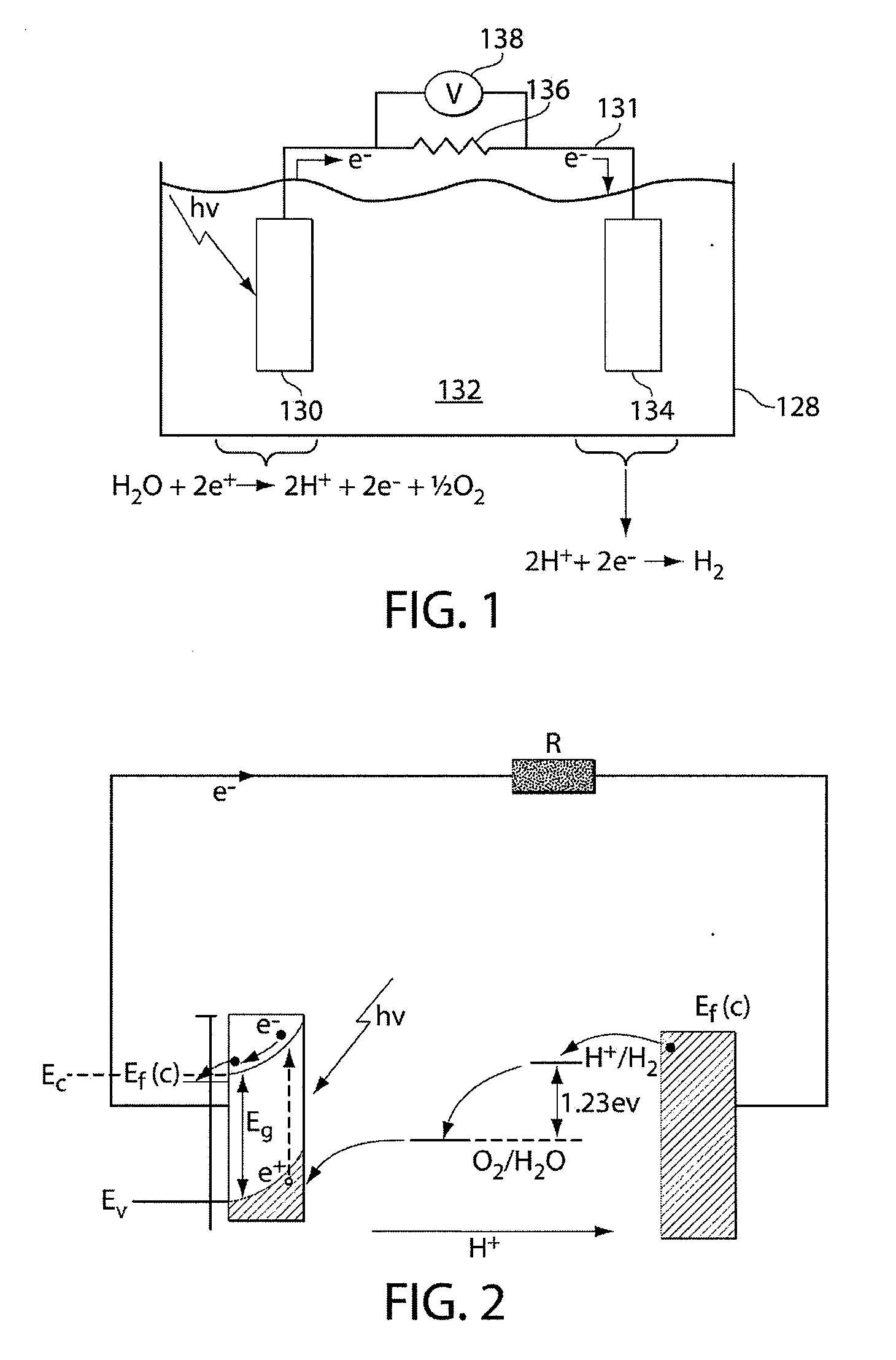
An apparatus for splitting water to produce hydrogen having at least one photoelectrochemical cell. The photoelectrochemical cell includes at least one water permeable photoelectrode having a light sensitive, nano-crystalline catalytic material layer, a polymer electrolyte membrane, a metallic substrate disposed between the light sensitive nano-crystalline catalytic material layer and the polymer electrolyte membrane adjacent to the polymer electrolyte membrane layer, and at least one photovoltaic device connected in series to the light sensitive nano-crystalline material layer and disposed between the light sensitive nano-crystalline catalytic material layer and the metallic substrate layer.
Owner:GAS TECH INST
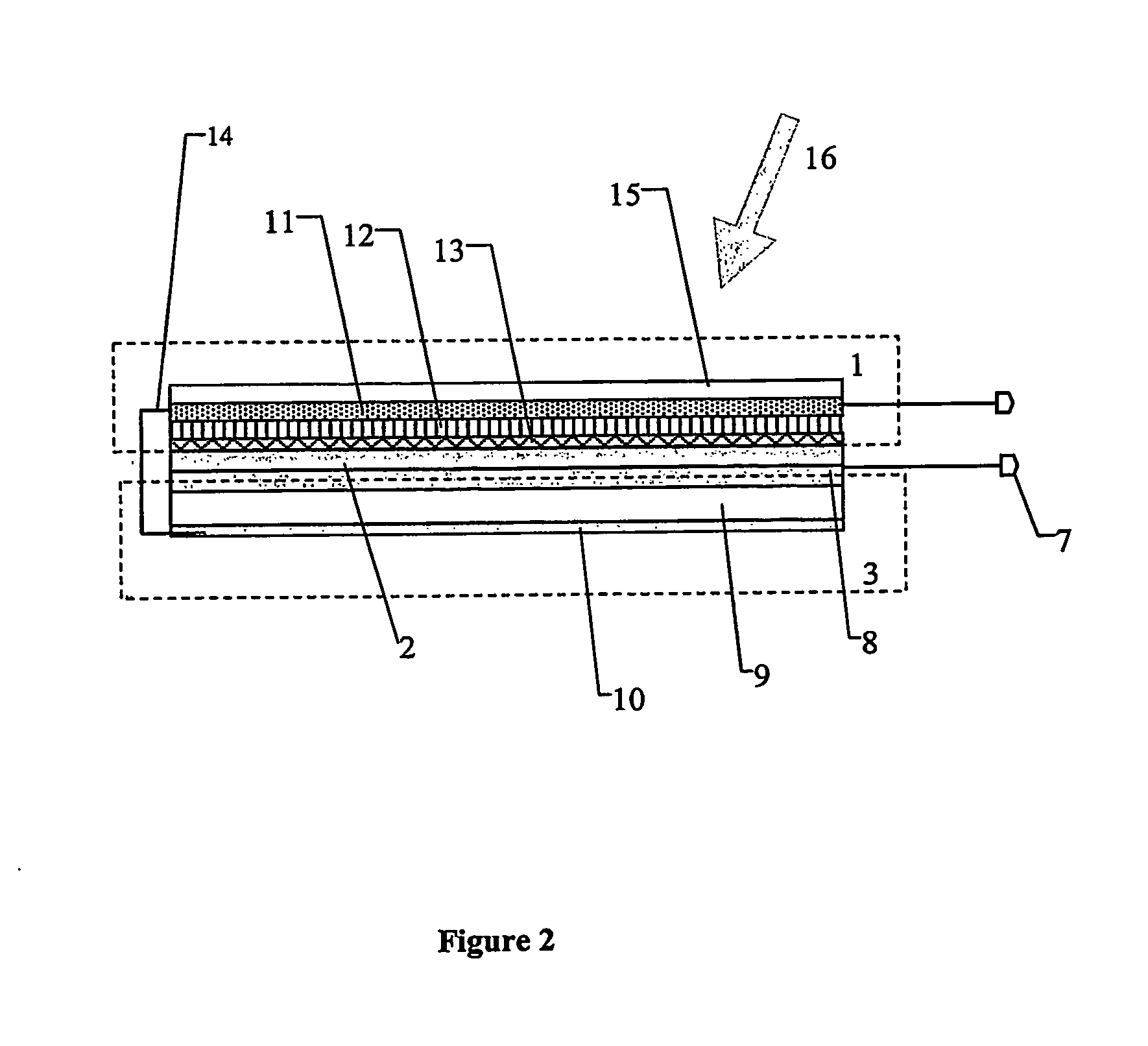
Combined photoelectrochemical cell and capacitor
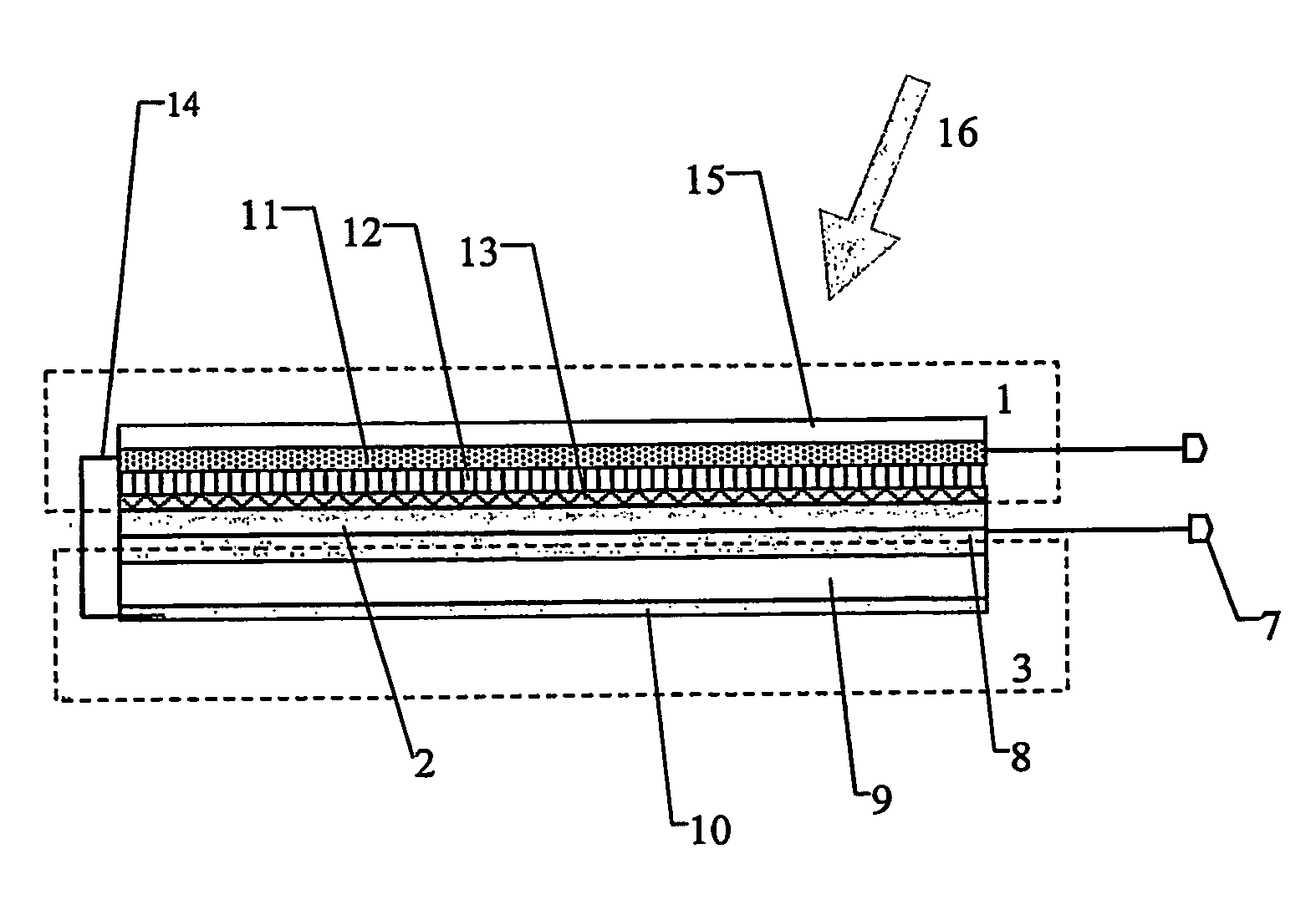
A nanophotocapacitive device wherein: a photovoltaic element (1) extends over substantially the whole area of the device, a charge storage element (3) also extends over substantially the whole area of the device, connection mechanism (14) are provided for interconnecting the photovoltaic and charge storage elements, the photovoltaic element comprises a nanoparticulate dye sensitized layer (11) of wide band gap semiconductor, a catalytic layer (13) and an electrolyte (12) placed between the semiconductor and the catalytic layers. The photovoltaic (photoelectrochemical) element and charge storage element (capacitor) are formed on opposite sides of a titanium foil substrate (8). Light (16) is incident on the transparent electrode (15). A diode (2) may be integral with the device to prevent the charge storage element discharging when there is no solar energy input to the photovoltaic element.
Owner:DYESOL IND
Device for producing hydrogen through decomposing water
ActiveCN101608316AIncrease profitImprove hydrogen production efficiencyCellsEnergy inputElectricityPhotoelectrochemical cell
The invention relates to a device for producing hydrogen through decomposing water, which comprises a photoelectrochemical cell including an optical anode and an optical cathode, and a solar cell which is used for generating voltage bias and includes an anode and a cathode, wherein the solar cell is electrically connected with the photoelectrochemical cell; the optical anode of the photoelectrochemical cell and the solar cell are arranged in the incident plane of sunshine. The device of producing hydrogen through decomposing water can overcome the problems of lower efficiency and hard hydrogen-oxygen separation during preparing hydrogen through semiconductor photocatalysis, solve the cost problem of photoelectrochemical hydrogen production, and simultaneously improve the operating factors of sunshine.
Owner:ENN SCI & TECH DEV
Charge transfer material, and photoelectric conversion device and photoelectric cell using same, and pyridine compound
InactiveUS20030127129A1Excellent in charge transportabilityIncreased durabilityNon-metal conductorsOrganic chemistryCompound aPhotoelectrochemical cell
A charge transfer material comprising a basic compound having negative charge and represented by the following general formula (I): (A1-L)n1-A2.M (I), wherein A1 represents a group having negative charge; A2 represents a basic group; M represents a cation for neutralizing the negative charge of (A1-L)n1-A2; L represents a divalent linking group or a single bond; and n1 represents an integer of 1 to 3. A photoelectric conversion device and a photo-electrochemical cell comprising the charge transfer material. A new nonvolatile pyridine compound, which is a low-viscosity liquid at room temperature, is preferably used for A2.
Owner:FUJIFILM CORP
Photoelectrolysis of water using proton exchange membranes
ActiveUS20050005963A1Enhanced Efficiency StabilityImprove performance stabilityMachining electrodesCellsWater useElectro catalyst
A photoelectrochemical cell which includes a light transmissive enclosure, a semiconductor photoanode disposed within the light transmissive enclosure, a semiconductor photocathode disposed within the light transmissive enclosure, and an electrolytic solution disposed entirely between the semiconductor photoanode and the semiconductor photocathode. This is achieved by the use of semiconductor photoelectrodes (photoanodes and photocathodes) which include a proton exchange membrane having an electrolyte facing surface in contact with the electrolytic solution and a light transmissive wall facing surface, and having a photo electro-catalyst disposed on the light transmissive wall facing surface.
Owner:GAS TECH INST
Ruthenium or Osmium Complexes and Their Uses as Catalysts for Water Oxidation
InactiveUS20110042227A1Photography auxillary processesGroup 5/15 element organic compoundsPhotoelectrochemical cellElectrochemical cell
The present invention provides ruthenium or osmium complexes and their uses as a catalyst for catalytic water oxidation. Another aspect of the invention provides an electrode and photo-electrochemical cells for electrolysis of water molecules.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Method for preparing nano tungsten trioxide thin film
InactiveCN101734866AImprove photoelectric performanceImprove firmnessPhotoelectrochemical cellTungstate
The invention relates to a method for preparing a nano tungsten trioxide thin film, in particular to a method for preparing a nano tungsten trioxide thin film used for a semiconductor photoelectrode of a photocatalytic or photoelectrochemical cell. The method for synthesizing the nano tungsten trioxide thin film comprises the following steps: dissolving water-soluble poly-tungstate in water, adding dispersant and modifier to the solution to obtain precursor solution which can be saved for more than 3 months, coating the surface of a substrate with the dipping and drawing method or the spin-coating method, and calcinating the substrate at high temperature in the air to decompose organic components to obtain the nano tungsten trioxide thin film. The method for preparing the nano tungsten trioxide thin film has the characteristics of simple steps and convenient operation. The nano tungsten trioxide thin film prepared with the method has high purity and excellent photoelectric performance, can be firmly combined with the substrate and is free from the size limitation.
Owner:CENT SOUTH UNIV
Visible light InGaN based photoelectrochemical cell and preparation
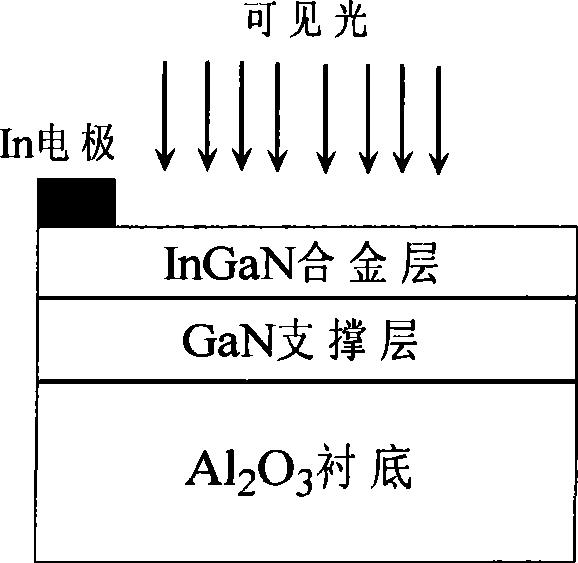
InactiveCN101364482AImprove conversion efficiencyImprove stabilityLight-sensitive devicesFinal product manufacturePhotoelectrochemical cellIndium
A production method of visible light InGaN-based photoelectrochemical cells comprises the steps of performing epitaxial growth of a GaN support layer and an InxGa1-xN alloy layer with single-crystal orientation on an alpha-Al2O3 substrate by using metal organic chemical vapor deposition (MOCVD), and alleviating macro-lattice mismatch between the InGaN layer and the substrate by using the GaN layer; wherein the growth of the GaN layer adopts a two-step method which comprises the following two steps: setting a low temperature buffer layer with thickness of 50-100 nm and growth temperature of 500-550 DEG C, and increasing the growth temperature to 1100 DEG C to allow the GaN layer to grow to obtain the GaN layer with thickens of 1-2 mum; the growth temperature range of the InxGa1-xN alloy layer is 600-850 DEG C, the composition x of indium (In) in the InxGa1-xN alloy layer is higher than or equal to 0 and lower than or equal to 1, the thickness of the InxGa1-xN alloy layer is 50-500 nm, and an ohmic contact electrode is formed on the surface of the InxGa1-xN alloy film when 1-10 mum indium (In) is deposited.
Owner:NANJING UNIV
Hybrid polymer light-emitting devices
InactiveUS20080084158A1Maintain good propertiesShort response timeDischarge tube luminescnet screensLamp detailsPhotoelectrochemical cellLuminescent polymers
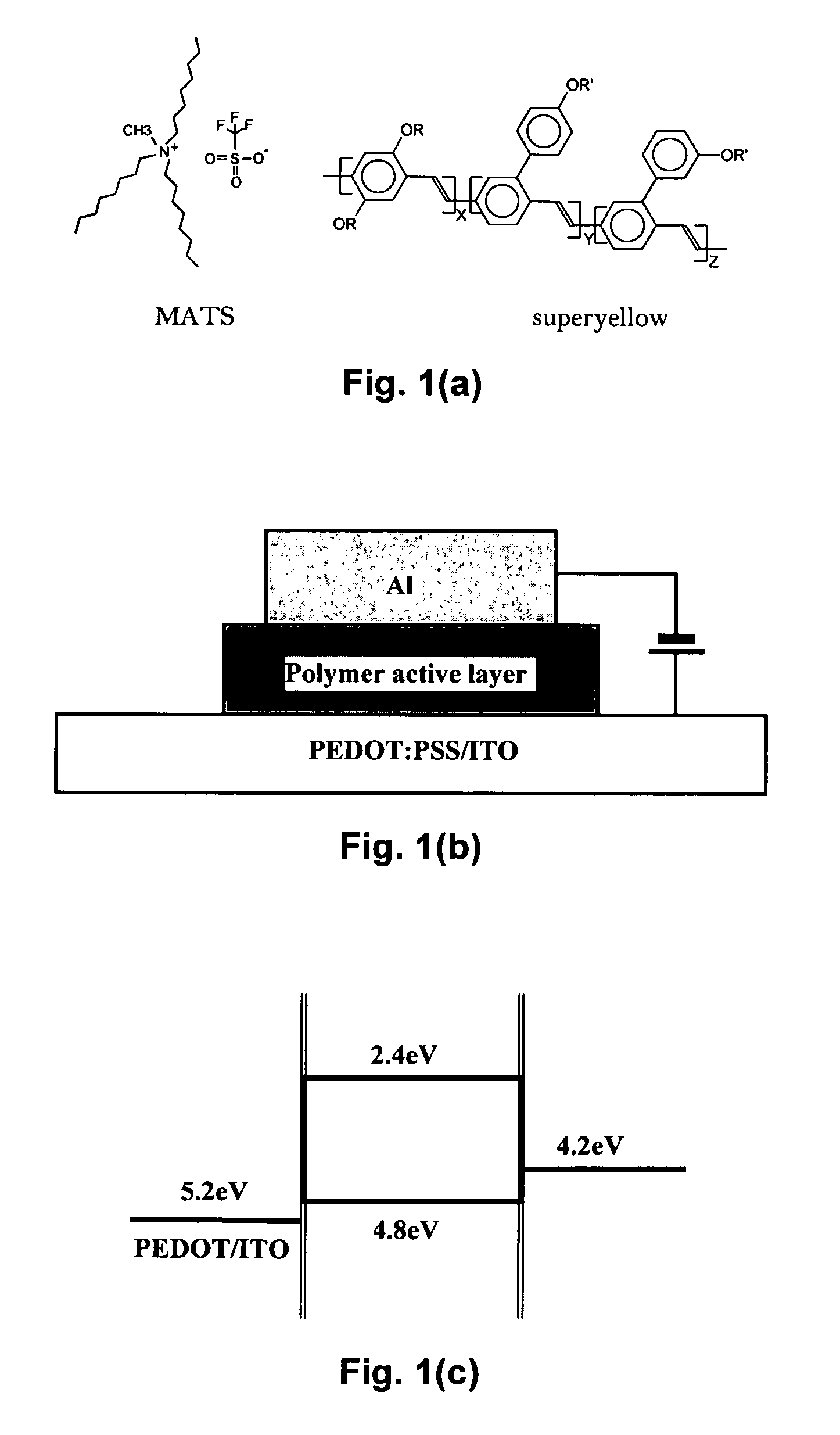
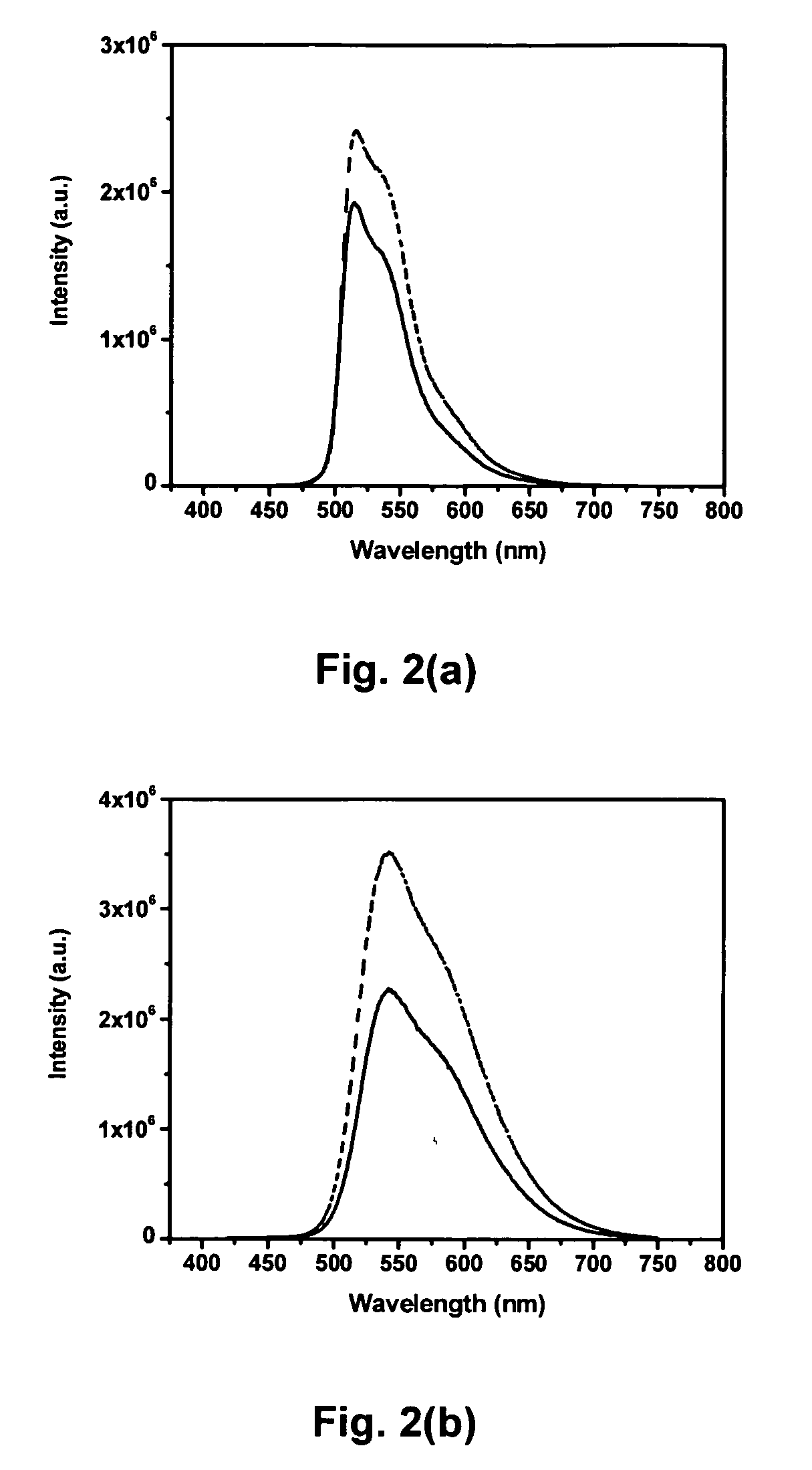
Mixtures and light-emitting devices that incorporate such mixtures are disclosed in which a soluble phenyl-substituted poly(para-phenylene vinylene) (PPV) copolymer (“superyellow”) is used as the host light-emitting polymer and methyltrioctylammonium trifluoromethanesulfonate, an ionic liquid, is used to introduce a dilute concentration of mobile ions into the emitting polymer layer. These mixtures and devices incorporating them are able to combine some of the characteristics of polymer light emitting diodes (PLEDs) and polymer light-emitting electrochemical cells (PLECs).
Owner:RGT UNIV OF CALIFORNIA
Functionalized Graphene-Pt composites for fuel cells and photoelectrochemical cells
ActiveUS20150247258A1Polycrystalline material growthCell electrodesPhotoelectrochemical cellFuel cells
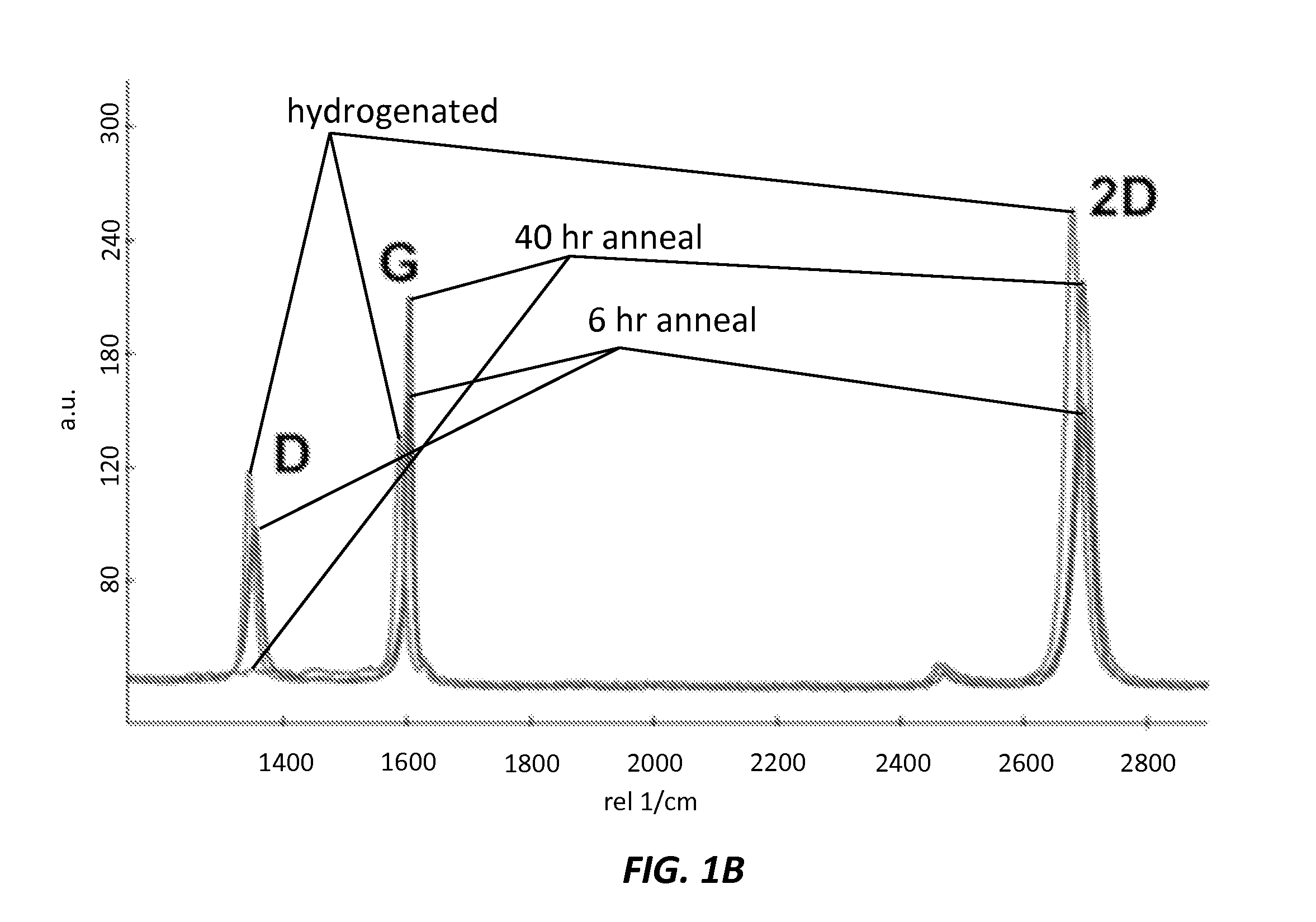
A method of growing crystals on two-dimensional layered material is provided that includes reversibly hydrogenating a two-dimensional layered material, using a controlled radio-frequency hydrogen plasma, depositing Pt atoms on the reversibly hydrogenated two-dimensional layered material, using Atomic Layer Deposition (ALD), where the reversibly hydrogenated two-dimensional layered material promotes loss of methyl groups in an ALD Pt precursor, and forming Pt—O on the reversibly hydrogenated two-dimensional layered material, using combustion by O2, where the Pt—O is used for subsequent Pt half-cycles of the ALD process, where growth of Pt crystals occurs.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
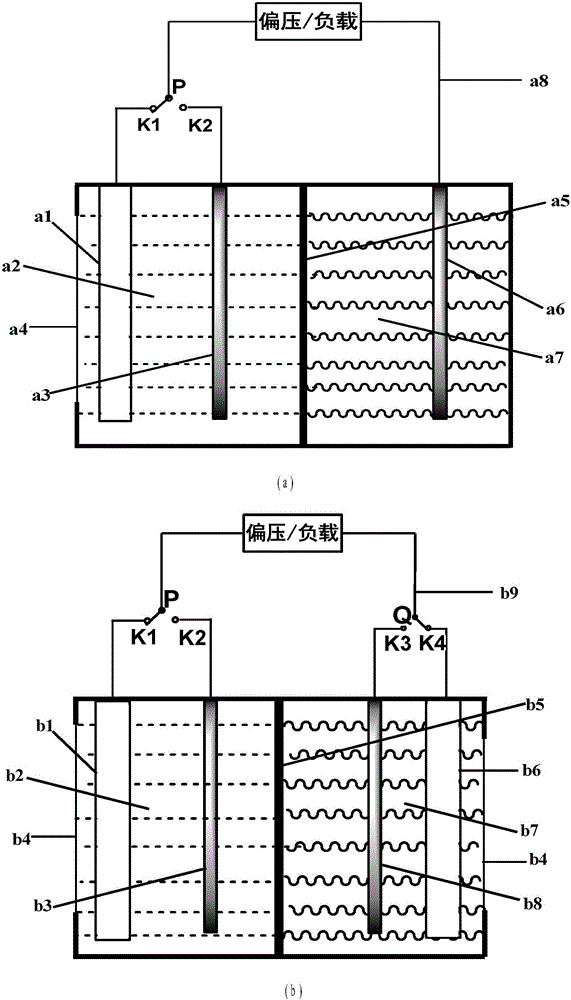
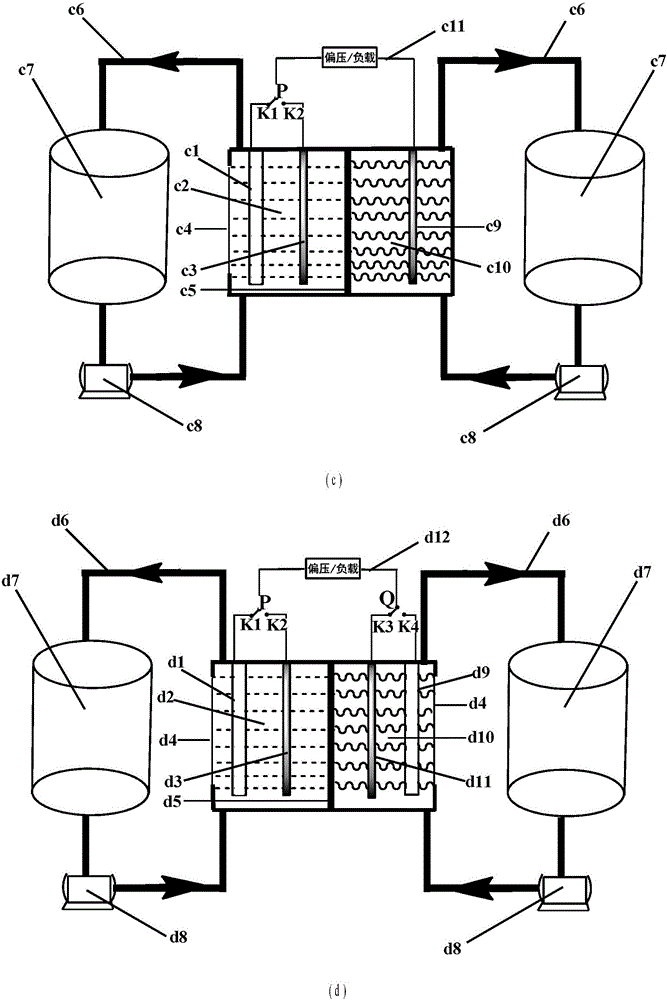
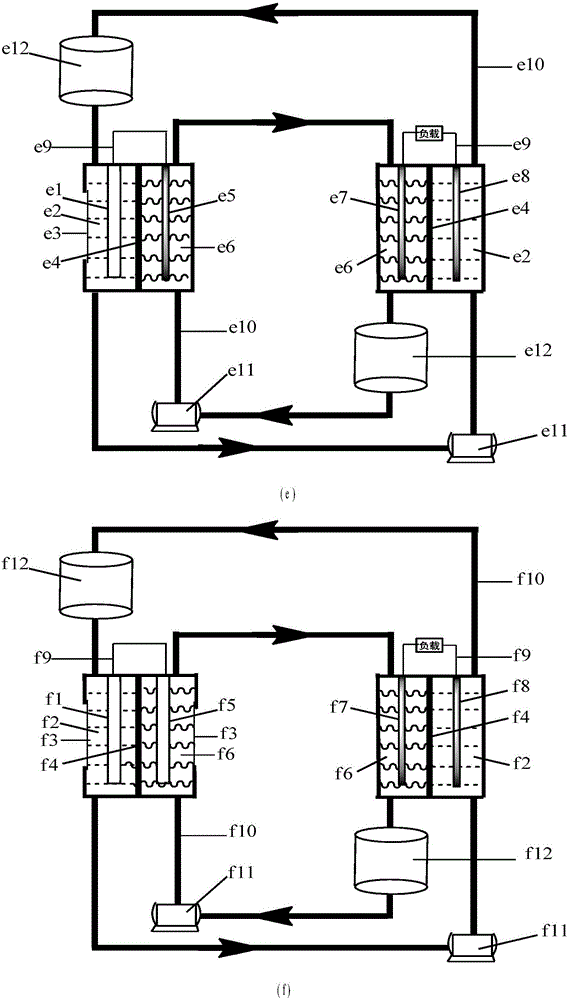
Water-soluble fast reaction kinetics couple-based photoelectrochemical energy storage battery
ActiveCN106329033AEasy in situ transformationAdjustable capacityPhotoelectrochemical storage cellsElectrochemical responseExternal bias
The invention provides a water-soluble fast reaction kinetics couple-based photoelectrochemical energy storage battery. When the battery is charged, in-situ transformation of light energy into chemical energy is achieved by using photoelectrochemical reaction driven by self-bias of narrow band gap photoelectrodes and the chemical energy is stored into an active material of a battery electrolyte; and when the battery is discharged, electrochemical reaction is carried out, thereby achieving transformation of the chemical energy into electric energy. The photoelectrochemical energy storage battery integrates a photoelectrochemical battery and a flow battery; the disadvantage that a solar cell cannot achieve electric energy storage is overcome; meanwhile, a single charging mode of the energy storage battery is also expanded; and solar energy in-situ transformation, storage and controllable utilization without assistance of external bias are achieved. Water-soluble fast reaction kinetics redox couples are adopted as the active material; the utilization rate of photo-induced carriers on the surfaces of photoelectrodes is close to 100%; meanwhile, the discharge power density of the battery can reach 0.5W / cm<2>; large-scale amplification can be achieved; and the photoelectrochemical energy storage battery is suitable for different scales of solar energy-energy storage-power generation processes.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com