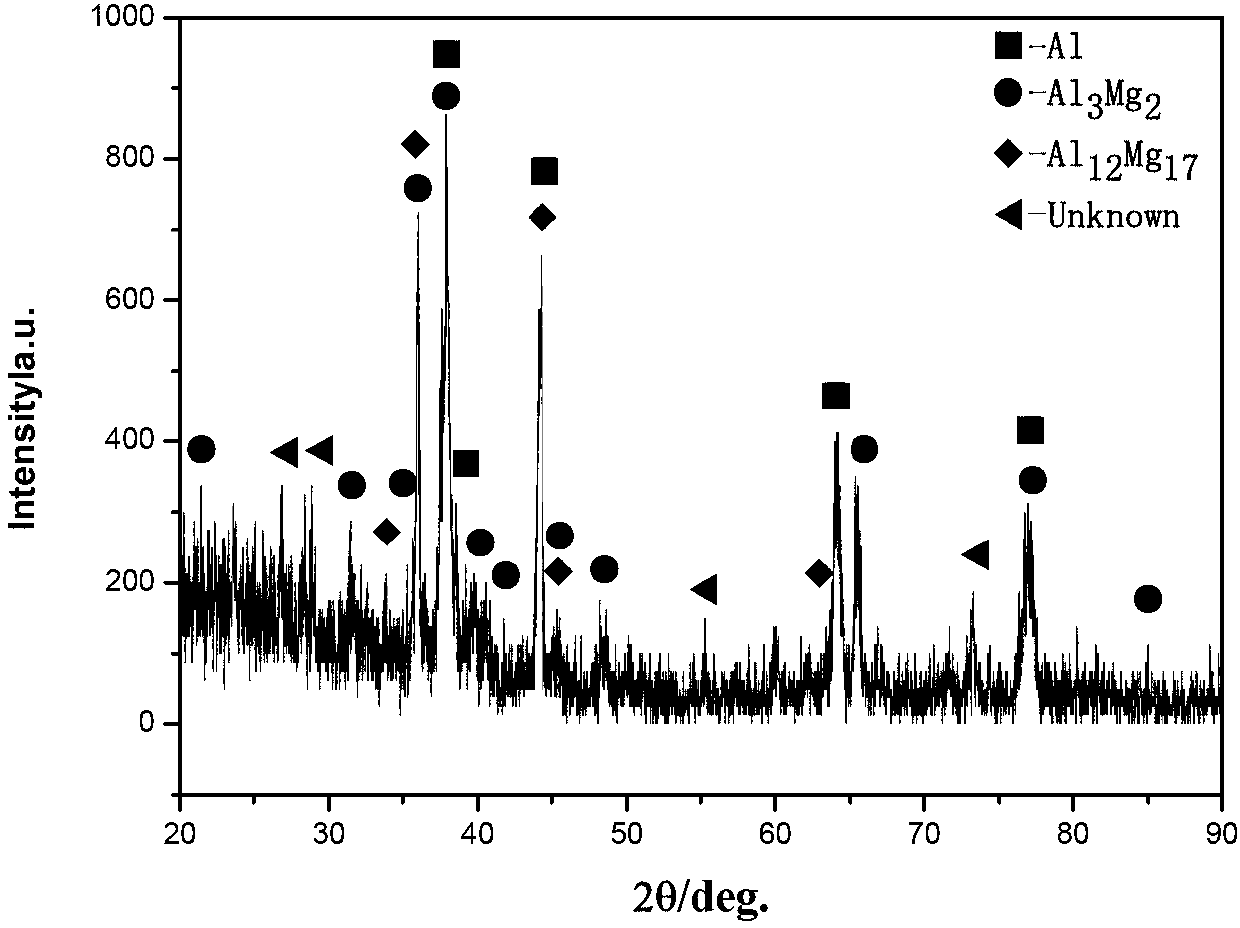
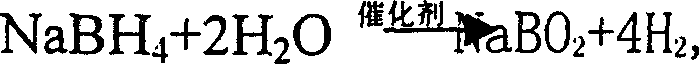Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
91results about How to "Reduced current efficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
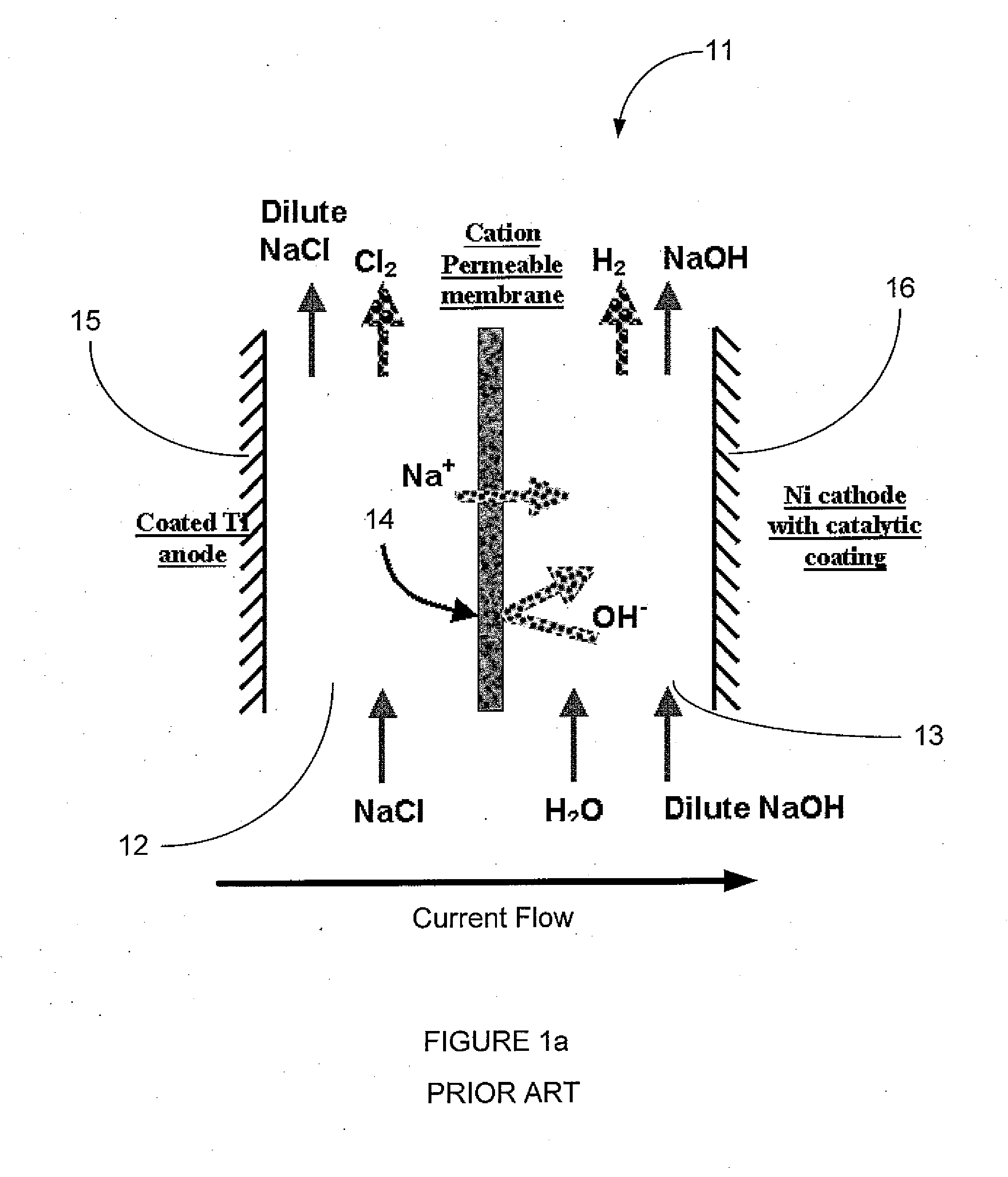
Photoelectrochemical Cell
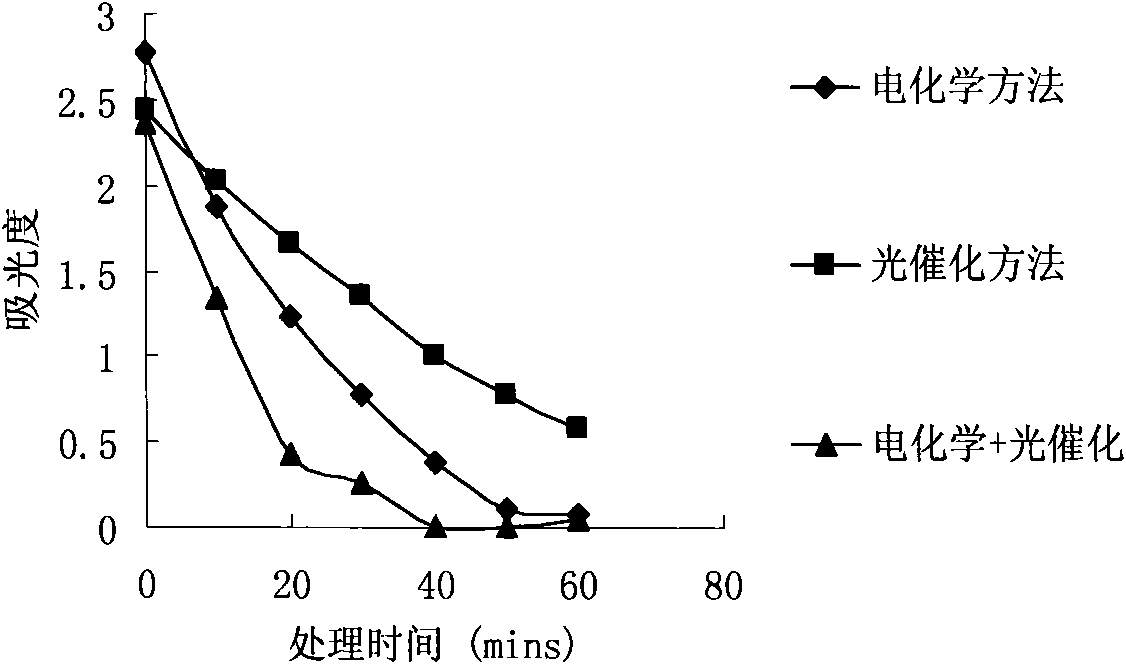
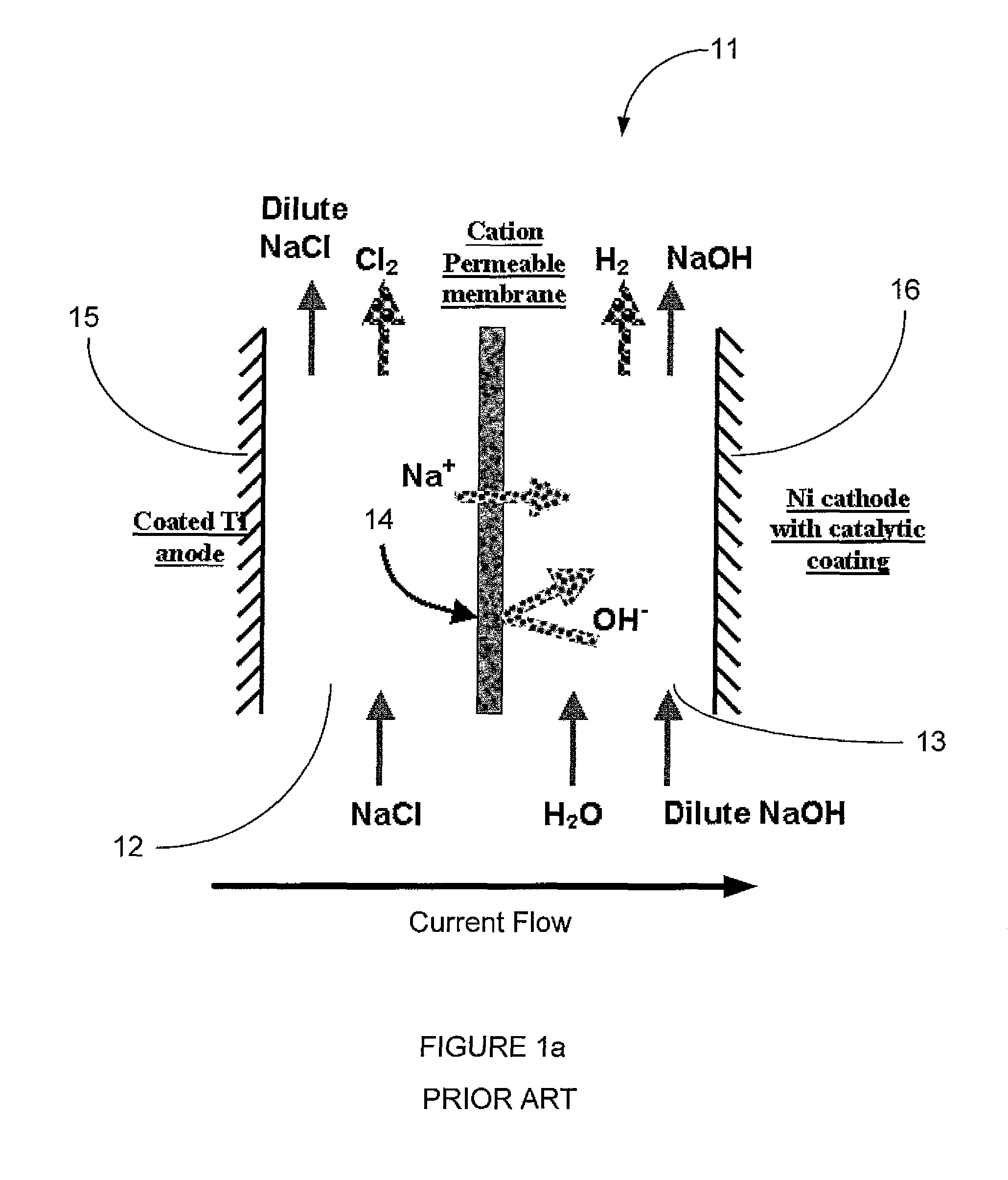
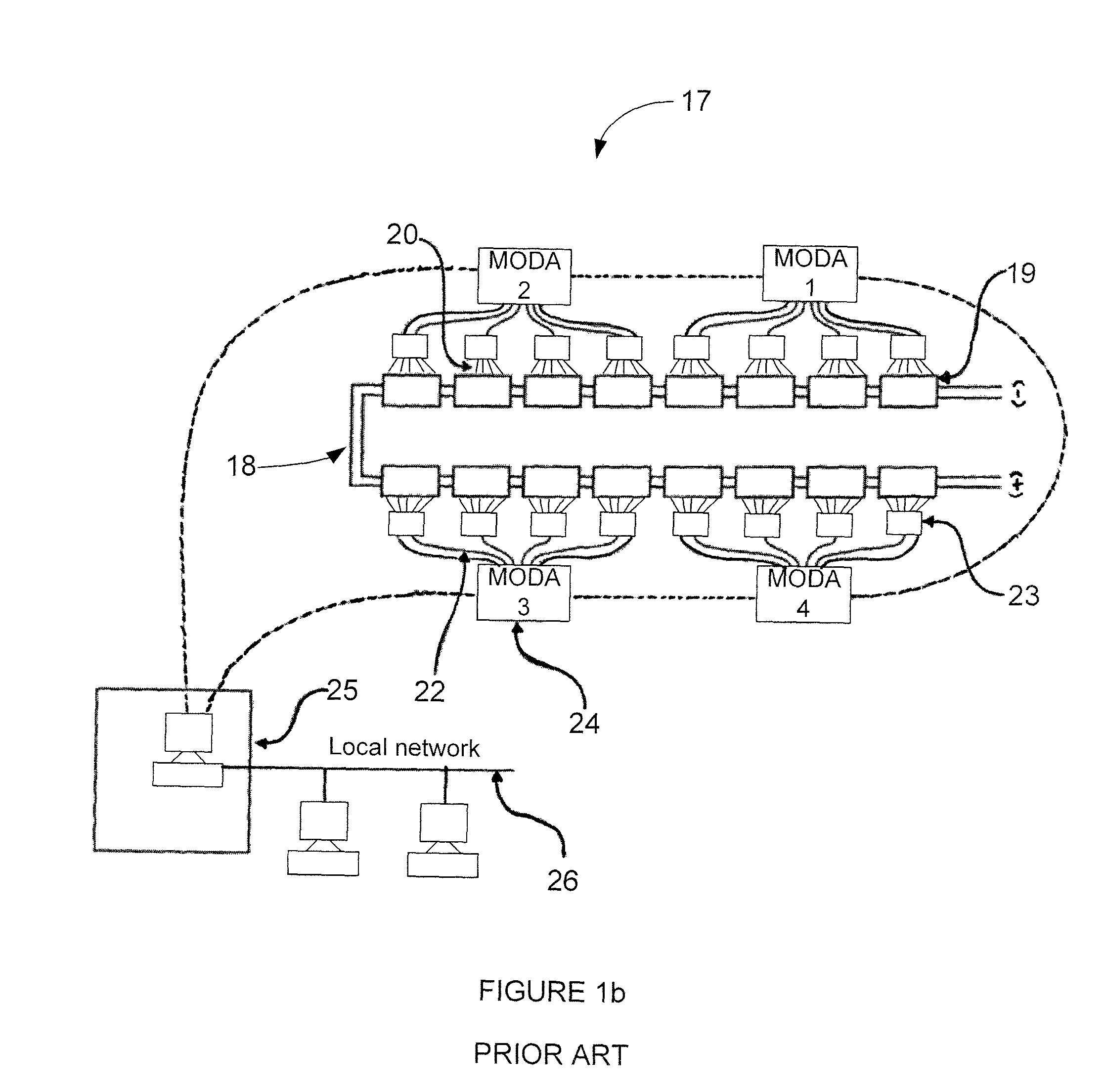
InactiveUS20080286643A1Reduced current efficiencyCurrent efficiency downElectrolysis componentsElectrolytic capacitorsPhotoelectrochemical cellChemical species
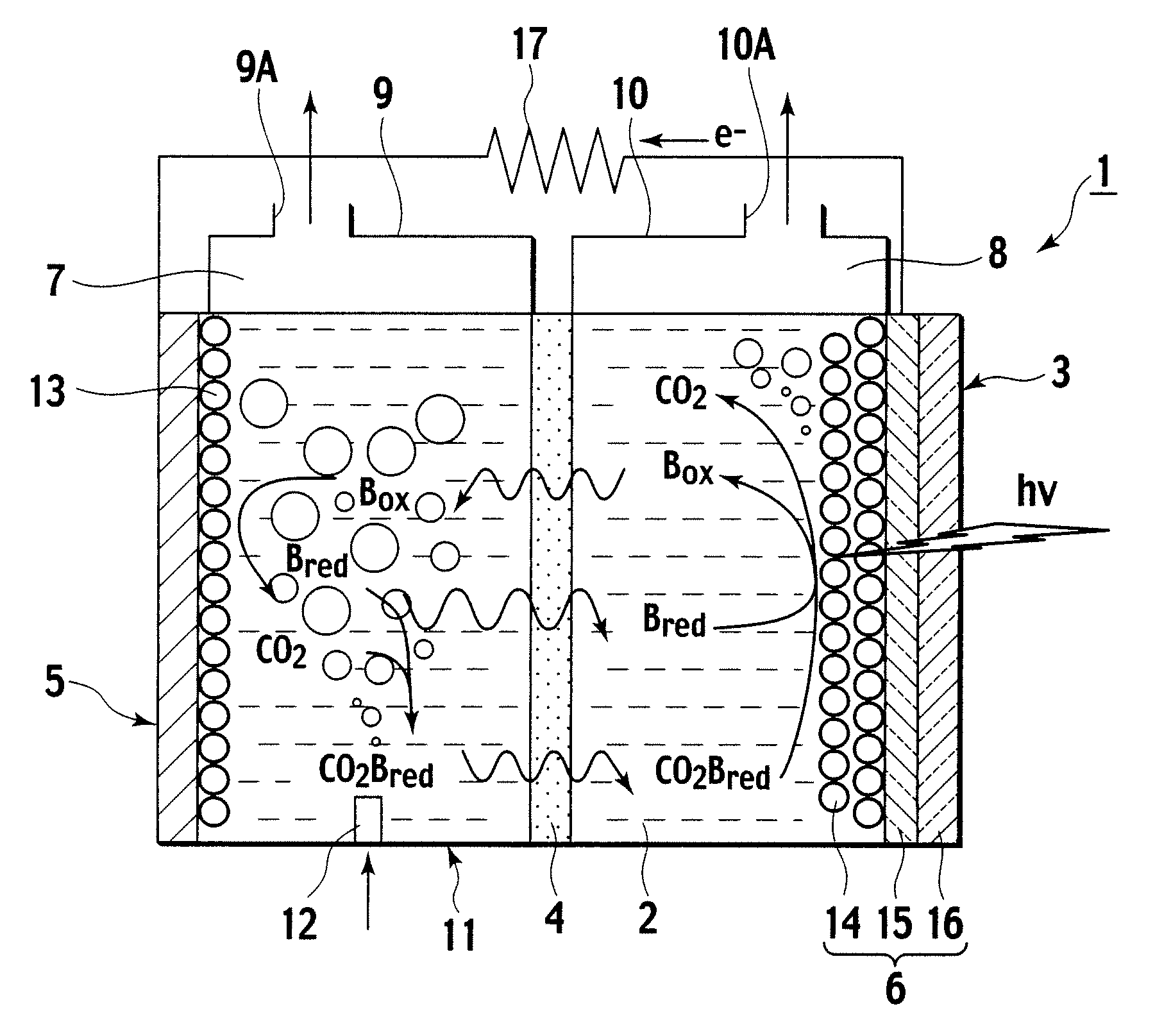
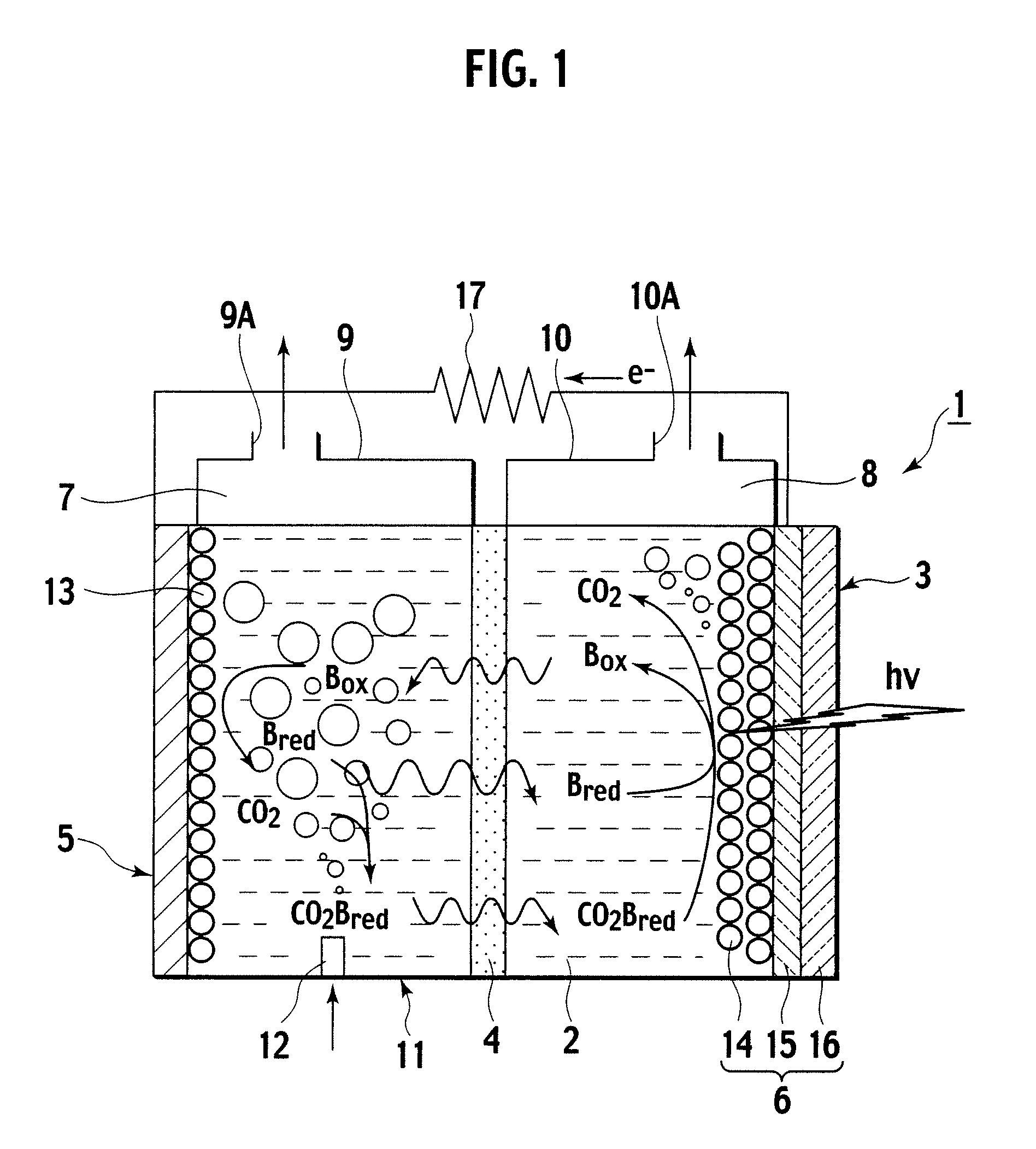
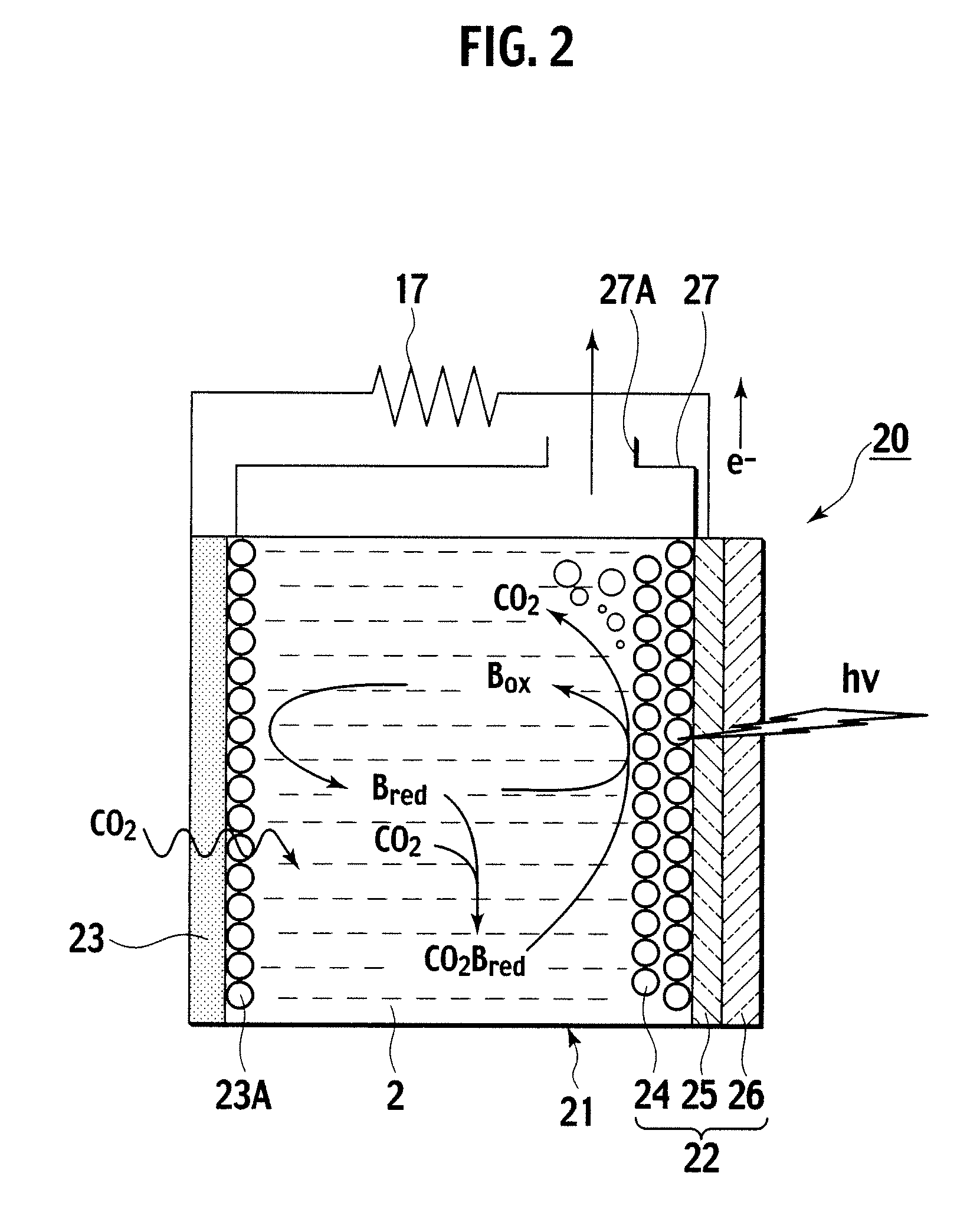
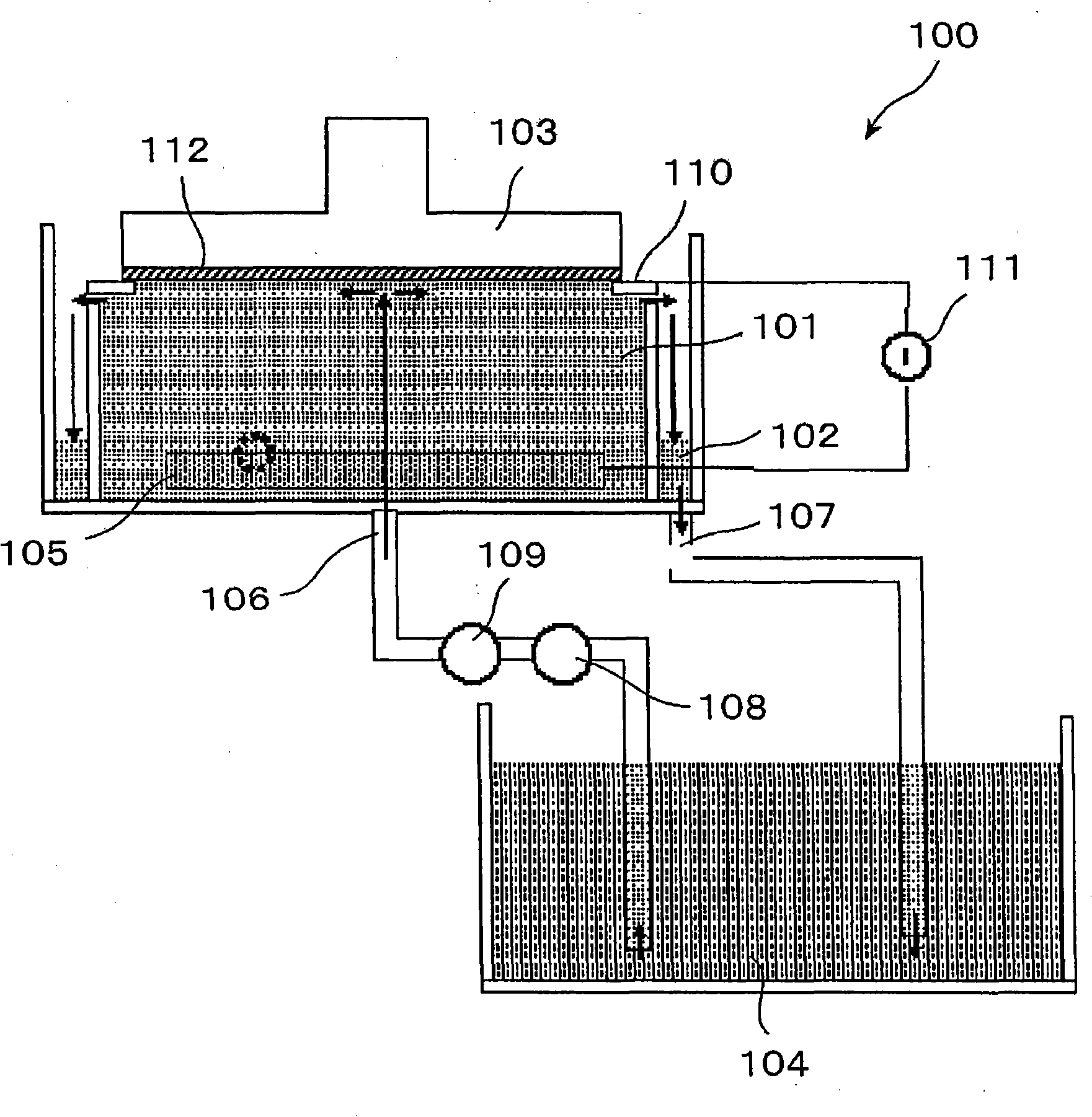
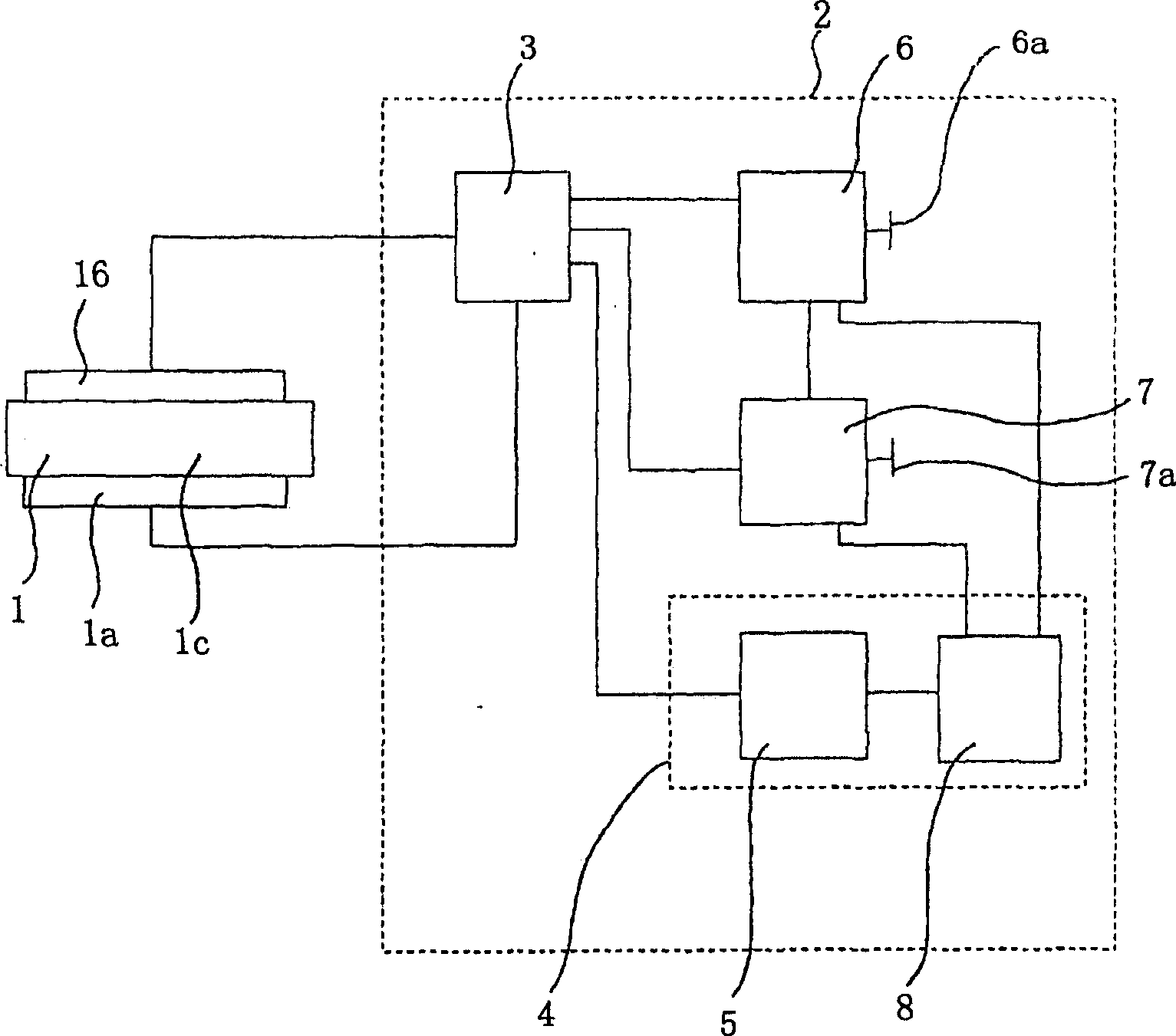
A photoelectrochemical cell (1) includes an electrolyte container (3) containing an ionic liquid (2), and a partitioning membrane (4) dividing an interior of the electrolyte container (3) into two being a CO2 capturing chamber (7) and a CO2 releasing chamber (8), having side walls opposing each other, with the partitioning membrane (4) in between, either as a carbon electrode (5) and the other as a photoelectrode (6). A redox mediator (B) has different bonding forces to carbon dioxide, as it appears as an oxidant Box and a reductant Bred, of which that one which has a greater bonding force serves as an intermediary chemical species carrying carbon dioxide to one of the paired electrodes (5, 6). Over the CO2 releasing chamber (10), an upper wall portion (10) is formed, which has a CO2 take-out port (10A) formed therein, for making use of oxidation and reduction of the redox mediator to achieve separation and concentration of carbon dioxide, converting photo energy of sunlight into electric power.
Owner:NISSAN MOTOR CO LTD
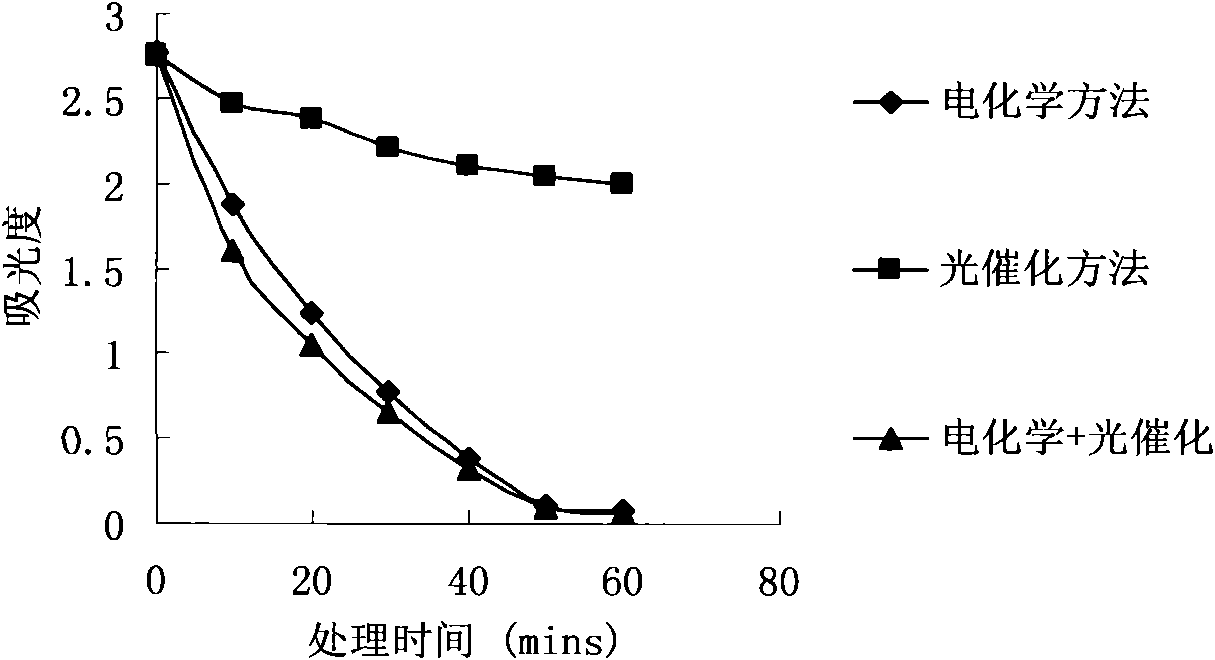
Wastewater treatment method and device combining electrochemical degradation and photocatalysis oxidation technologies
InactiveCN101555082AImprove separation efficiencyImprove current efficiencyWater/sewage treatment by irradiationMultistage water/sewage treatmentEnvironmental chemistryElectrochemical degradation
The invention relates to a wastewater treatment method and device combining electrochemical degradation and photocatalysis oxidation technologies taking a diamond film electrode as the anode. The device comprises a flowing electrolytic bath and a photocatalytic reactor. Contaminants have electrocatalytic oxidation at the anode of the flowing electrolytic bath and are electrolyzed to separate out water and then enter the photocatalytic reactor for further degradation. The combination of two technologies can improve the current efficiency reduction caused by oxygen analyzing reaction in an electrochemistry part because oxygen analyzed by the electrochemistry part is a favorable trapping agent of photoelectrons of a photocatalysis part, can improve the separation efficiency of the photoelectrons and cavities and improve the catalytic degradation of the photocatalysis part. Meanwhile, by opening or closing various parts of the device, proper operational modes can be selected according to the kinds and the concentration of wastewater, such as an independent electrochemistry method, an independent photocatalysis method, an electrochemistry and photocatalysis combined method and other different operational modes.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Method for electroplating zinc-nickel alloy on sintered neodymium-iron-boron material
InactiveCN101724845AImprove corrosion resistanceImprove bindingLiquid/solution decomposition chemical coatingHydrogenNickel alloy
The invention relates to the field of surface treatment corrosion protection for a sintered neodymium-iron-boron material, in particular to a method for electroplating a zinc-nickel alloy on the surface of the sintered neodymium-iron-boron material. The method is mainly characterized by comprising the following steps of: firstly, performing treatment before plating on the sintered neodymium-iron-boron material; secondly, carrying out chemical nickel plating; thirdly, carrying out copper electroplating and zinc-nickel alloy electroplating; and finally, carrying out passivation treatment. The binding force between an adopted chemical nickel-plated layer and the neodymium-iron-boron material is excellent; a copper-plated layer can play a role in hydrogen blocking while electroplating the zinc-nickel alloy so as to reduce the hydrogen corrosion to the neodymium-iron-boron material; and a zinc-nickel alloy layer, as an anode coating, effectively protects the neodymium-iron-boron material. The coating obtained by the method is complete, compact, uniform and bright, and is firmly combined with a substrate; and the corrosion resistance of the coating is further improved after the passivation treatment. The method solves the problem that the neodymium-iron-boron electroplated zinc-nickel alloy has poor binding force and affects the corrosion resistance. The method realizes good binding force between the coating and the substrate, dense coating, uniform component and excellent corrosion resistance.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Method used for preparing fluorine salt taking aluminium fluoride as main component from electrolyte-containing material produced in aluminium electrolysis
ActiveCN110194478AElectrolyte composition is stableEfficient recyclingIron oxides/hydroxidesAluminium fluoridesAluminium electrolysisAluminium fluoride
The invention provides a method used for preparing a fluorine salt taking aluminium fluoride as a main component from electrolyte-containing material produced in aluminium electrolysis. The method comprises following steps: the electrolyte-containing material is mixed with a soluble aluminium salt or an aluminium salt solution, and reaction is carried out at 50 to 100 DEG C; a reaction product issubjected to solid liquid separation, after separation, an obtained solid product is washed and dried so as to obtain the fluorine salt taking aluminium fluoride as a main component, and a liquid solid product obtained through separation and a solid product washing solution obtained in washing of the solid product are mixed so as to obtain a mixture, a strong basicity hydroxide or a solution of the strong basicity hydroxide is added into the mixture to remove calcium and iron impurities in the mixture; evaporation concentration desalting is carried out, and separation is carried out so as to obtain a concentrated mother liquor; a carbonate or a solution of the carbonate is added to remove Lithium-ion impurities in the concentrated mother liquor, after impurity removing, an obtained solution is adopted in diluting or washing step in the method. The method is capable of converting the excess electrolyte-containing material produced in aluminium electrolysis into aluminum fluoride, so that effective circulation of fluoride element in aluminium electrolysis enterprises is realized, and great meaning on aluminium electrolysis industry development of China is achieved.
Owner:郑州于斯新创科技有限公司
Quantum dot electroluminescent device and display
InactiveCN108963087AControl mobilityAchieve balanceSolid-state devicesSemiconductor/solid-state device manufacturingDisplay deviceElectron transfer
The present invention relates to a quantum dot electroluminescent device. The quantum dot electroluminescent device comprises a first electrode layer, a luminescent layer and a second electrode layerwhich are stacked in order; a first electron transfer layer and a second electron transfer layer are arranged between the luminescent layer and the second electrode layer; the materials of the first electron transfer layer comprise inorganic metallic oxide transmission materials, and the materials of the second electron transfer layer comprise organic electron transport materials. The electron transfer layers with two different materials are used in a stack mode to weaken the difference of the hole mobility and the electronic mobility, achieve the carrier balance and greatly improve the current efficiency, and can be applied to the fields of solid-state lighting and panel display.
Owner:GUANGDONG JUHUA PRINTING DISPLAY TECH CO LTD
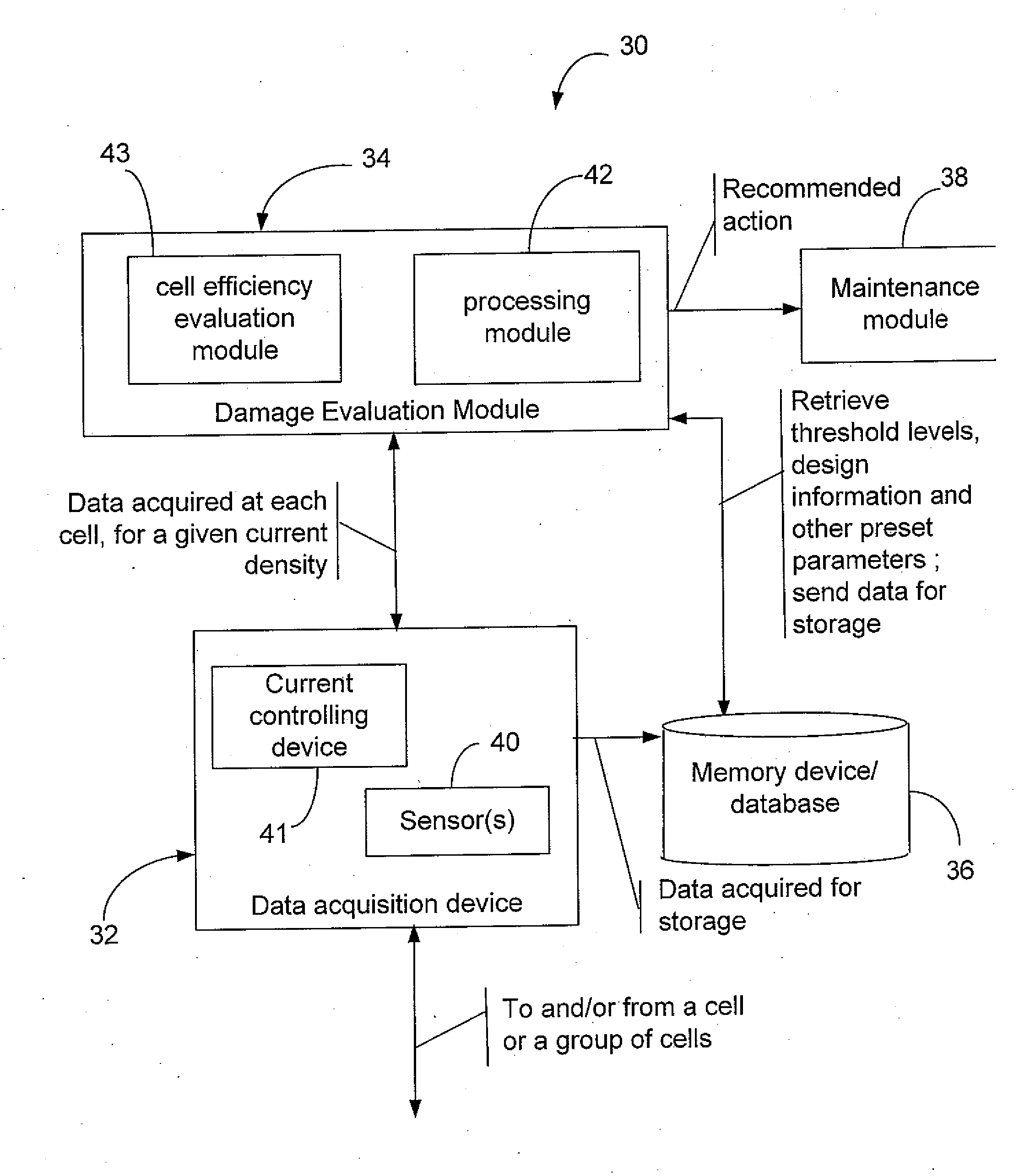
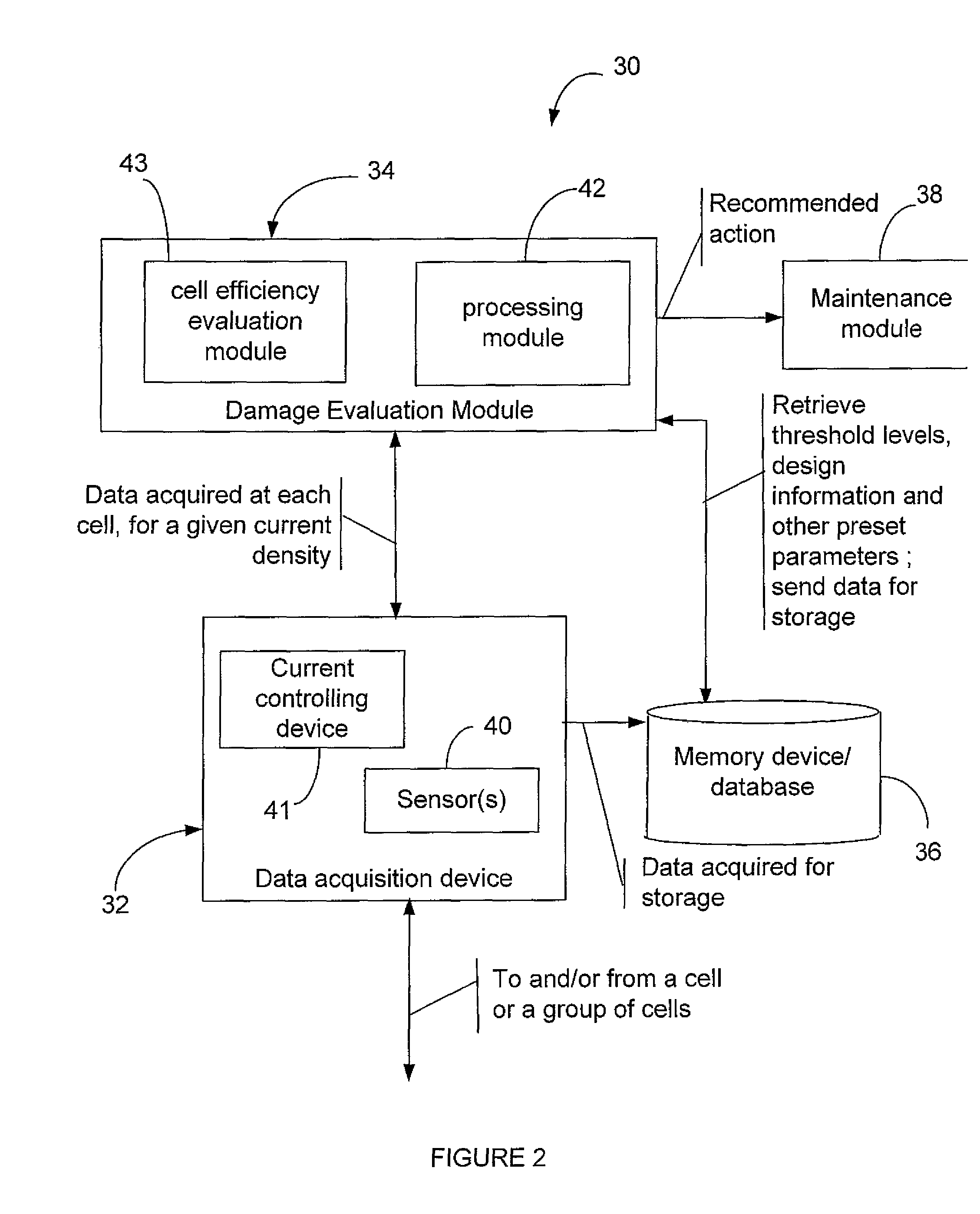
Efficiency optimization and damage detection of electrolysis cells
ActiveUS20090014326A1Reduced current efficiencyImprove efficiencyPhotography auxillary processesElectrolysis componentsElectrolysisSimulation
There is described a method and a system for evaluating damage of a plurality of cells in an electrolyser. The method comprises acquiring a voltage for each one of the cells; comparing the voltage to at least two threshold voltage levels; classifying the cells as one of: severely damaged cells, non-severely damaged cells and undamaged cells, based on the comparison of the voltage with the at least two threshold voltage levels; and deactivating the cells classified as severely damaged cells from the electrolyser.
Owner:RECH 2000 INC
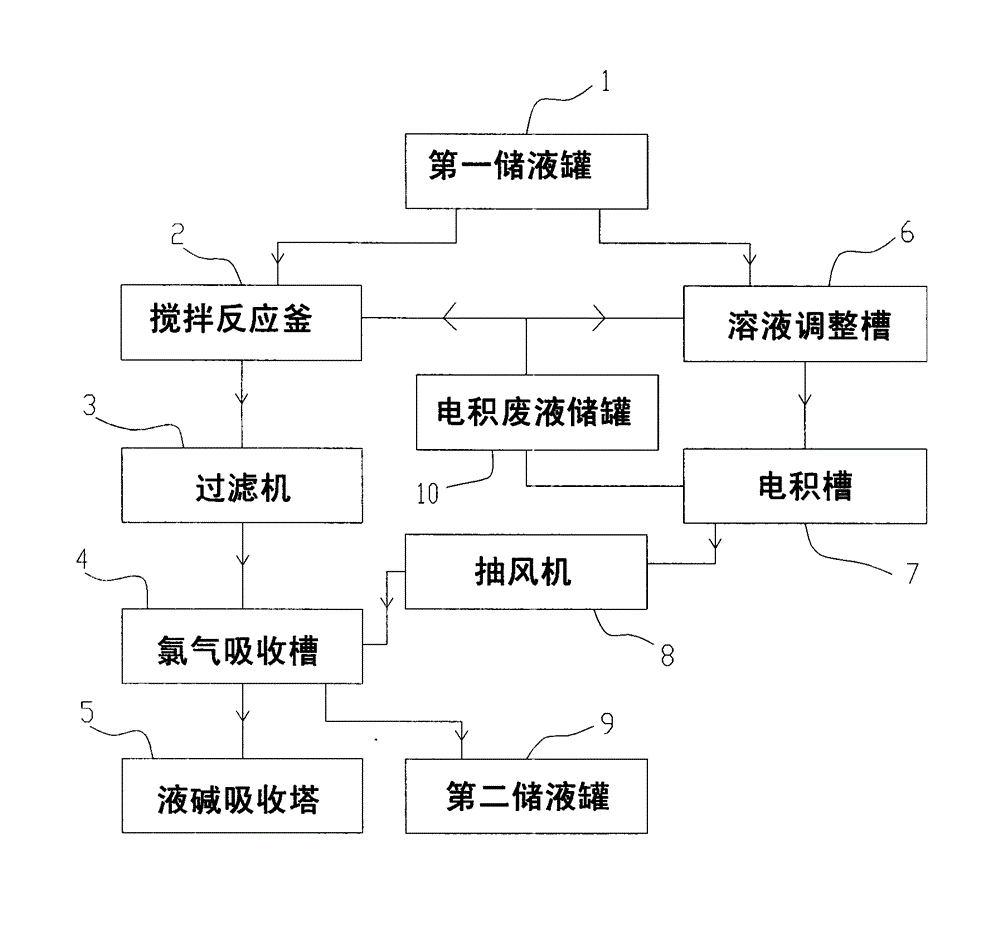
Method and system for preparing ferric chloride, electrodeposited copper and copper powder from copper-containing hydrochloric acid waste liquid
ActiveCN103936081ASolve pollutionHigh pricePhotography auxillary processesIron halidesLiquid storage tankIron(II) chloride
The invention provides a method and a system for preparing ferric chloride, electrodeposited copper and copper powder from copper-containing hydrochloric acid waste liquid. The system comprises a first liquid storage tank, a stirring reactor, a filter, a chlorine absorption tank, a caustic soda solution absorption tower, a solution adjusting tank, an electrodeposition cell and an exhaust fan, wherein the first liquid storage tank is used for storing the copper-containing hydrochloric acid waste liquid; the stirring reactor is used for carrying out replacement reaction between ferrum and copper chloride; the filter is used for filtering a solution obtained through the replacement reaction; the chlorine absorption tank is used for enabling the solution filtered by the filter to absorb chlorine, so that ferrous chloride is oxidized into ferric trichloride; the caustic soda liquid absorption tower is used for treating tail gas emitted from the chlorine adsorption tank; the solution adjusting tank is used for adjusting the concentration of a solution in the electrodeposition cell; the electrodeposition cell is used for an electrodeposition reaction; and the exhaust fan is used for conveying chlorine gas produced by the electrodeposition cell to the chlorine absorption tank. According to the method and the system provided by the invention, replacement of copper ions from ferrous powder is combined with the electrodeposited copper, so that the wastewater discharge problem of the copper-containing hydrochloric acid waste liquid and the pollution problem of the chlorine gas to the environment can be solved, and the phenomenon that the power consumption sharply rises is avoided.
Owner:SHENZHEN YUEPENG ENVIRONMENTAL PROTECTION TECH
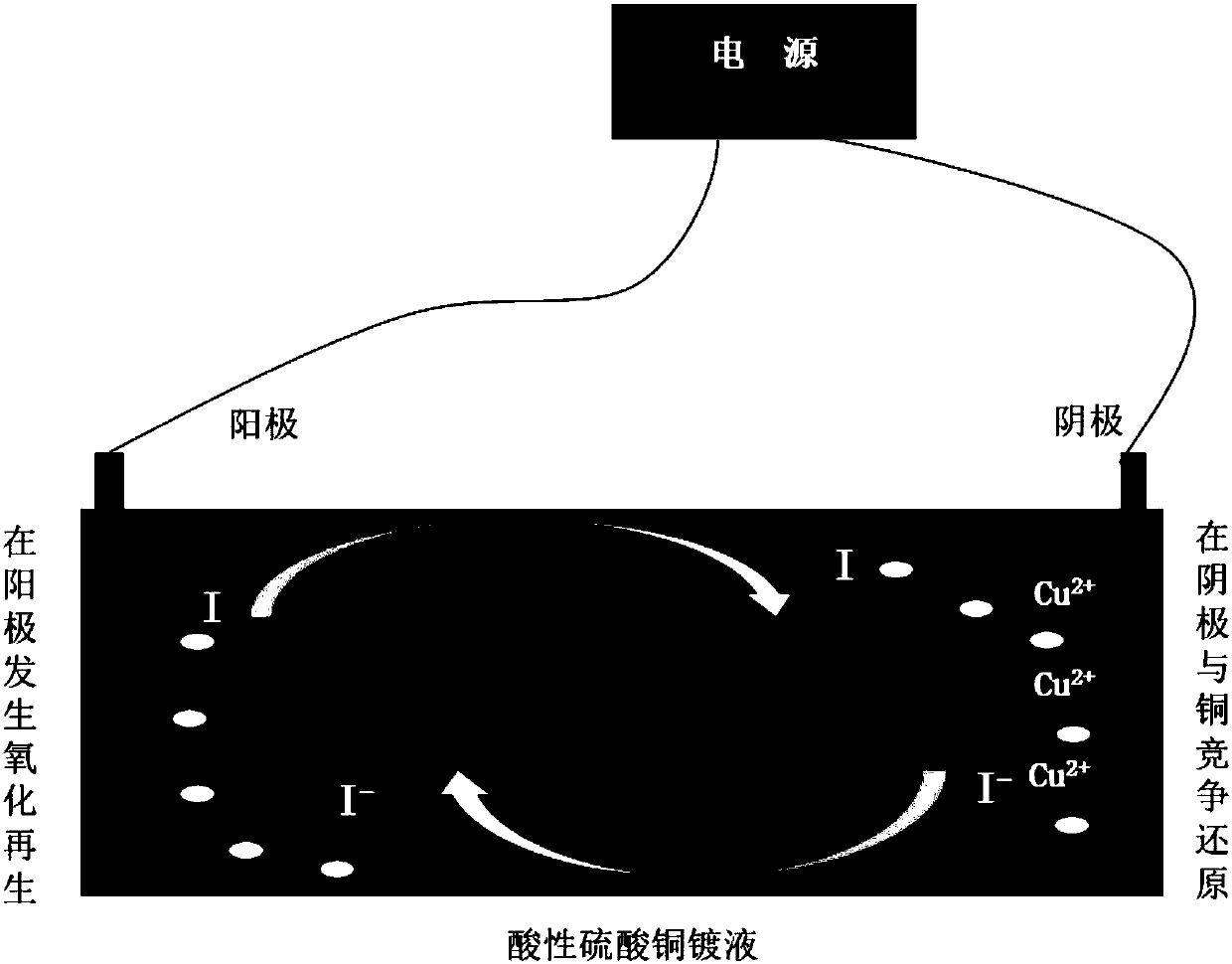
Even-plating agent used for electroplating copper and relevant metal copper electroplating combination agent thereof
The invention discloses an even-plating agent used for electroplating copper and a relevant metal copper electroplating formula, and belongs to the technical field of electroplating and electro-deposition. The even-plating agent is one or more organic matters containing a first kind of active functional group or a second kind of active functional group; the first kind of active functional group and the second kind of active functional group are conjugate; and the reduction potential of the organic matters containing the active functional group under an electrochemical system is not higher thanthe reduction potential of copper electro-deposition so that the active functional group in the even-plating agent can have a competitive reaction with copper ions, the copper deposition current efficiency of a cathode is reduced, reduction of the thickness of surface copper is facilitated, the even-plating agent is especially suitable for improving the throwing power of electroplating and blindhole filling of through holes with high ratios of thickness to diameter, and thus the circuit fineness of existing printed circuit boards is improved.
Owner:UNIV OF ELECTRONIC SCI & TECH OF CHINA
Organic compound and organic electroluminescence device with same
InactiveCN108623545AReduce the driving voltageExtended service lifeOrganic chemistrySolid-state devicesSimple Organic CompoundsOrganic electroluminescence
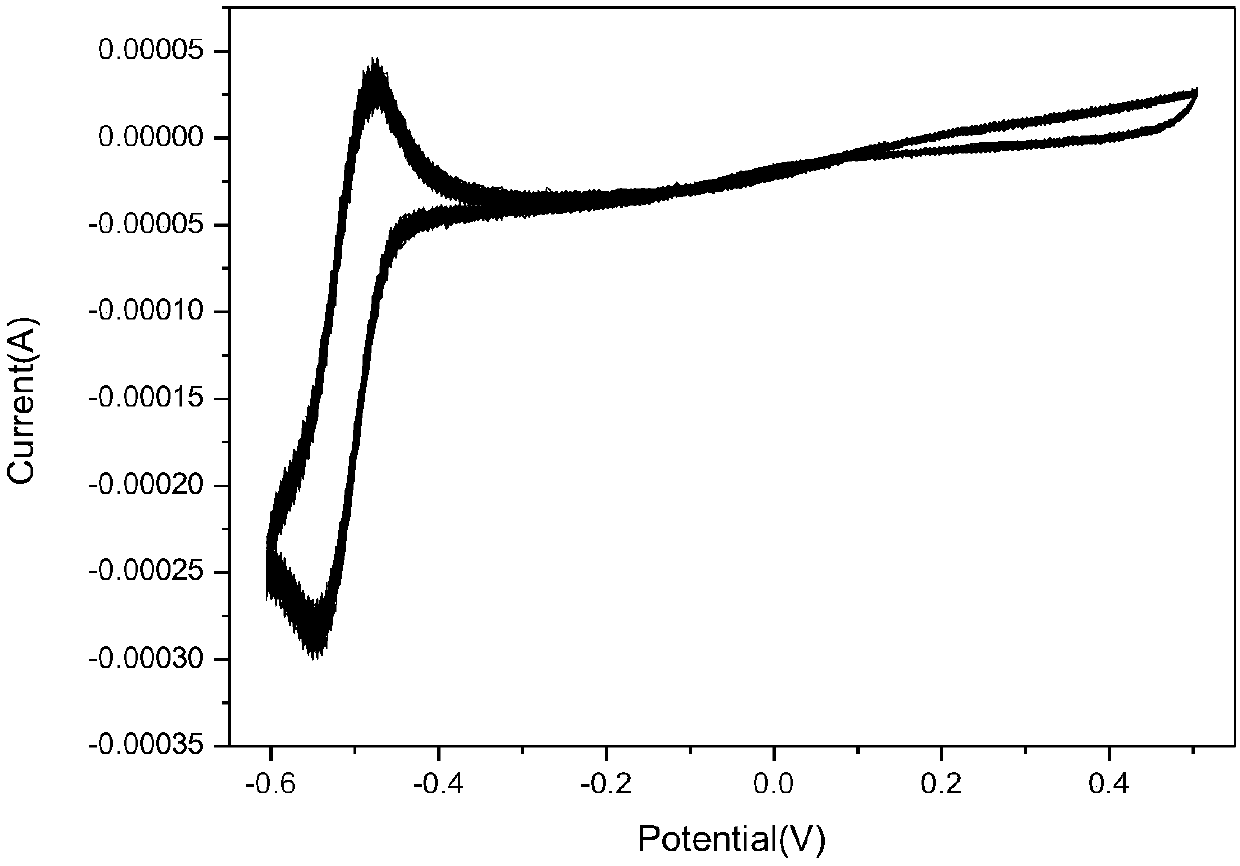
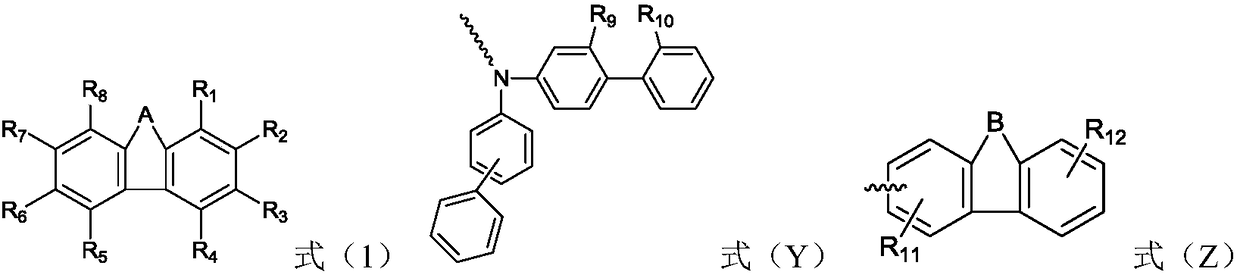
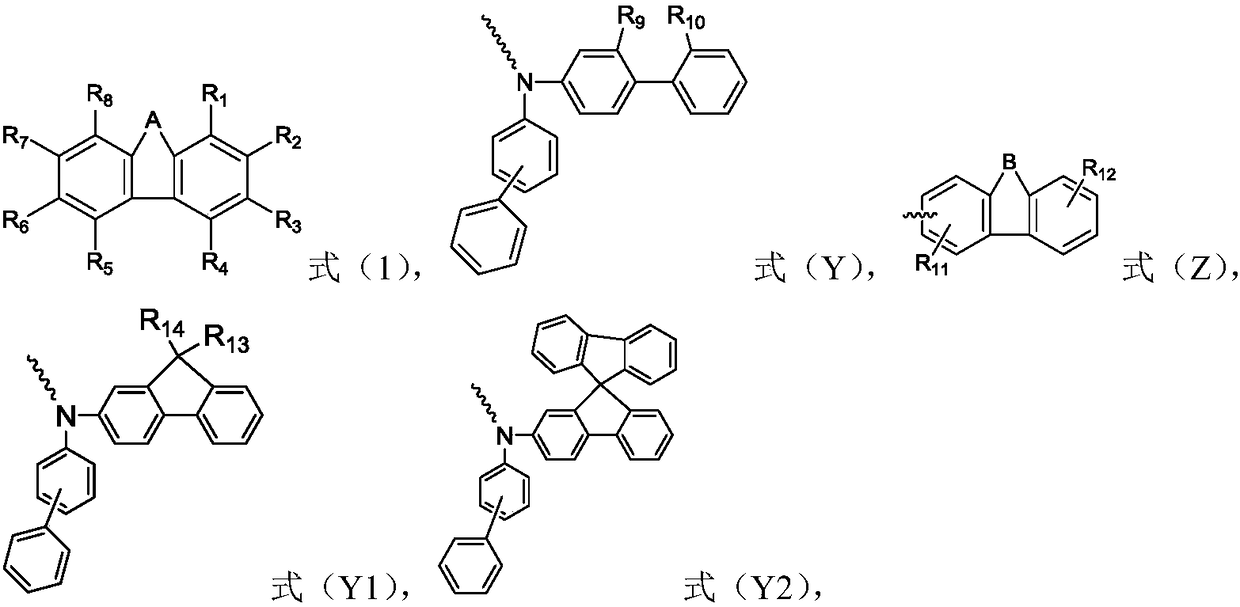
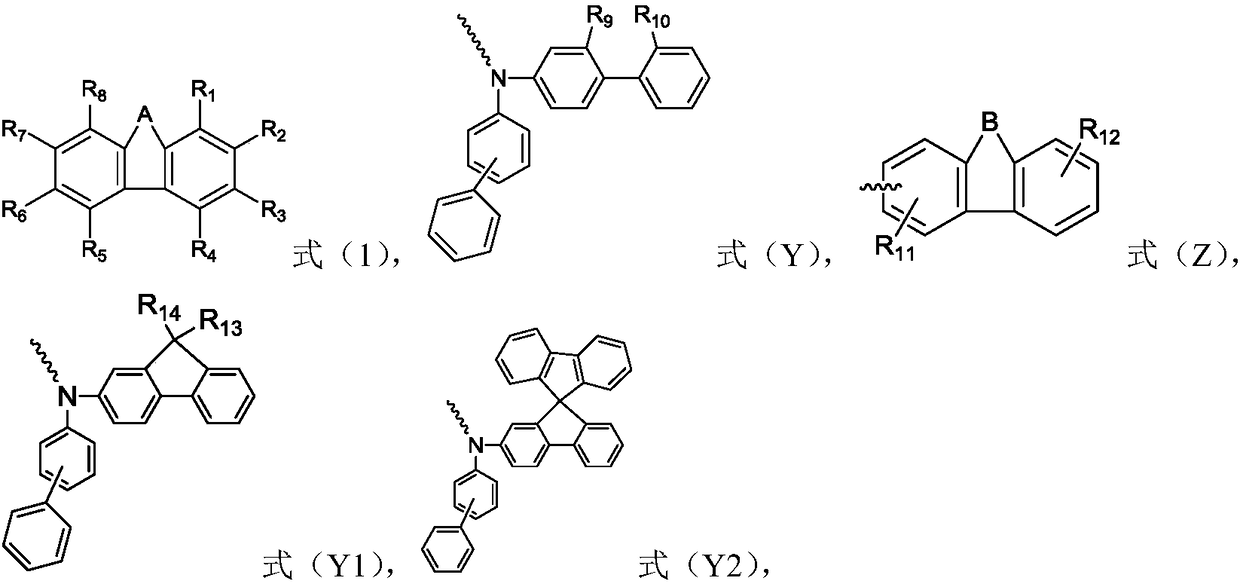
The invention relates to the field of organic electroluminescence devices and discloses an organic compound and an organic electroluminescence device with same. The organic compound is of a structureof formula (I) as shown in the description, in the formula, A and B are O, S, a formula as shown in the description or another a formula as shown in the description; any one of groups consisting of R1, R2, R3 and R4 is a group of a structure of formula (Y) as shown in the description; any one of groups consisting of three rest ones of R1, R2, R3 and R4 and R5, R6, R7 and R8 is group of a structureof formula (Z) as shown in the description; in formula (Y), R9 and R10 form a substituted five-membered ring; because of the substituted five-membered ring, formula (Y) is a group of the structure offormula (Y1) or formula (Y2) as shown in the description. The organic electroluminescence device with the organic compound disclosed by the invention has the advantages of being low in driving voltage, long in service life and high in both current efficiency and brightness.
Owner:BEIJING GREEN GUARDEE TECH
Lanthanum cerium-magnesium intermediate alloy and production method thereof
The invention discloses a lanthanum cerium-magnesium intermediate alloy and a production method thereof, relating to the technical field of preparation of a rare earth-magnesium intermediate alloy product. According to the production method, a coelectrodeposition method is adopted, a graphite crucible is used as an electrolytic tank and an anode, a molybdenum bar is used as a cathode, a magnesium oxide crucible is used as an alloy supporting device, an electrolyte is formed by mixing KCl, anhydrous MgCl2 and RECl3 together. By adopting the preparation method disclosed by the invention, the lanthanum cerium-magnesium intermediate alloy high in quality and low in cost can be produced through one step by adopting magnesium and rare earth compounds through the coelectrodeposition method. The process indicators of the whole production process are relatively high, wherein the average electric efficiency reaches 65-75% and more than 85% at the maximum, the rare earth direct yield is more than 85%, and the magnesium direct yield is more than 95%. The lanthanum cerium-magnesium intermediate alloy prepared by the invention is lowest in price, and resources can be continuously supplied to promote sustainable development of rare earth-magnesium alloys.
Owner:扬州宏福铝业有限公司 +1
Low-energy-consumption electrochemical treatment device and method for degradation-resistant organic wastewater
ActiveCN104176797AReduce processing efficiencyReduced current efficiencyMultistage water/sewage treatmentWater/sewage treatment using germicide/oligodynamic-processSupporting electrolyteChemical treatment
The invention relates to an electrochemical treatment device and method for degradation-resistant organic wastewater, and designs a'zero polar distance' SPE (Solid Polymer Electrolysis) electrooxidation sewage treatment electrolytic bath like a solid polymer electrolyte fuel cell technology. According to the device, an anode chamber and a cathode chamber are separated by utilizing an ion exchange membrane, the anode with stable titanium-based size, the ion exchange membrane and the nickel cathode are pressed by utilizing an end plate, and a'zero polar distance' SPE electrooxidation sewage treatment electrolytic bath is formed. When the device is subjected to electrolytic operation, the wastewater is electrically oxidized at the anode, so that organic matters and ammonia nitrogen in the water are mineralized and degraded; and running water or the wastewater is introduced into the cathode chamber, and hydrogen separated out due to electrolysis at the cathode is recovered. The device has the advantages that extra supporting electrolyte is not needed to be added, the bath pressure and energy consumption of electrooxidation can be greatly reduced due to the'zero polar distance', and the problem that oxygen (chlorine) and hydrogen separated out in the electrolysis process is solved. In addition, a carbon material is not used, reduction of current efficiency caused by carbon corrosion is avoided, and the reliability and stability of the sewage treatment device are improved. Moreover, continuous water flow is utilized at the cathode, and the scaling and plugging possibility on the cathode side of the ion exchange membrane can be greatly reduced.
Owner:BEIJING JINDAYU ENVIRONMENT TECH CO LTD
Efficiency optimization and damage detection of electrolysis cells
ActiveUS8114265B2Reduced current efficiencyImprove efficiencyCellsPhotography auxillary processesElectrolysisSimulation
There is described a method and a system for evaluating damage of a plurality of cells in an electrolyser. The method comprises acquiring a voltage for each one of the cells; comparing the voltage to at least two threshold voltage levels; classifying the cells as one of: severely damaged cells, non-severely damaged cells and undamaged cells, based on the comparison of the voltage with the at least two threshold voltage levels; and deactivating the cells classified as severely damaged cells from the electrolyser.
Owner:RECH 2000 INC
Method for recycling rhenium from tungsten-rhenium alloy waste
ActiveCN106430320AHigh energy consumptionReduced current efficiencyProcess efficiency improvementRhenium compoundsRheniumPotassium
The invention provides a method for recycling rhenium from tungsten-rhenium alloy waste. The method comprises the following steps that 1, the tungsten-rhenium alloy waste is subjected to electrochemical dissolution, and a rhenium-containing solution is obtained; 2, excessive potassium chloride is added into the rhenium-containing solution, rhenium in the rhenium-containing solution is made to perform a precipitation reaction with potassium chloride to generate KReO4 precipitations, then, the KReO4 precipitations are subjected to recrystallization, and high-purity KReO4 is obtained. The technology is simple, the problem of collecting rhenium in a comparison oxidation volatilization method does not exist, and the rhenium recovery rate is high. Due to the fact that both a traditional oxidation volatilization method and a niter fusion-ion exchange method are implemented under the high-temperature condition, comparatively, the technology is low in energy consumption.
Owner:NORTHWEST INSTITUTE FOR NON-FERROUS METAL RESEARCH
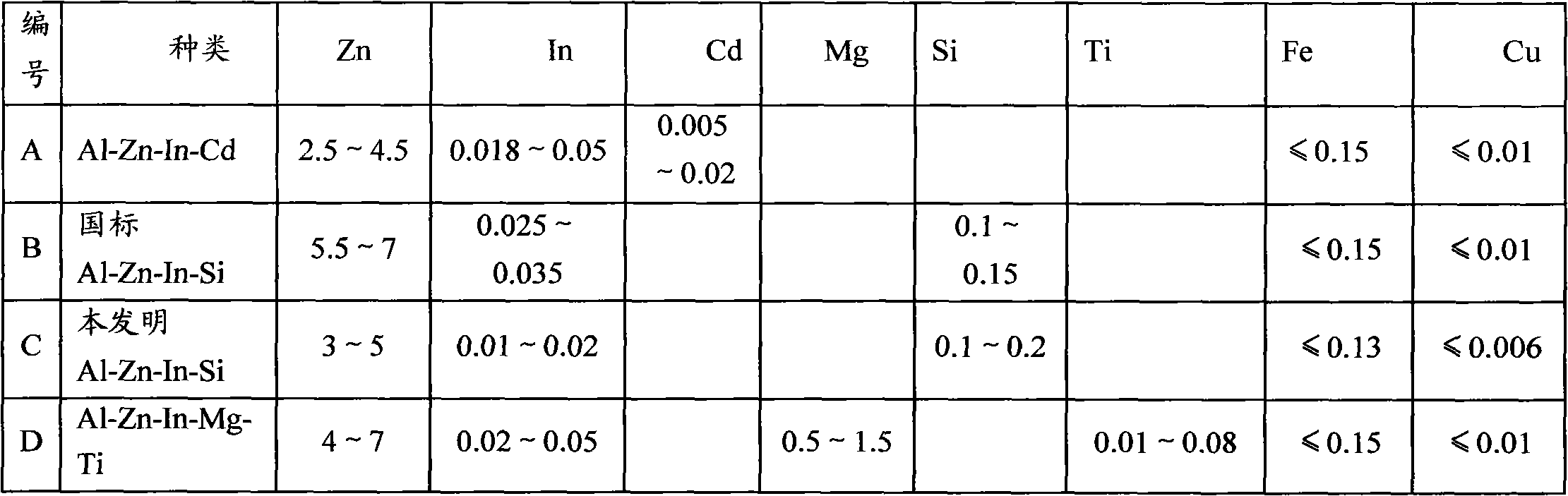
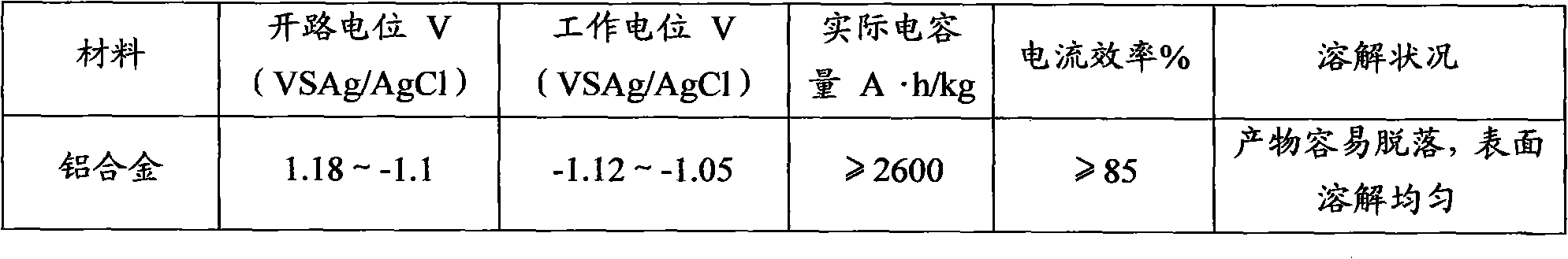
Sacrificial anode for deep sea environment and manufacturing method thereof
The invention discloses a sacrificial anode for deep sea environment. The sacrificial anode comprises main components by weight percent: 3.0-5.0% of zinc, 0.01-0.02% of indium, 0.10-0.20% of silicon and less than or equal to 0.16% of impurities, wherein the impurities comprise less than or equal to 0.13% of iron, less than or equal to 0.006% of copper and the balance of aluminum. The sacrificial anode has the working potential of -1.12 to -1.05V, the current efficiency of more than or equal to 93% and the capacitance of more than or equal to 2600 in the deep sea environment, and is even in corrosion, and corrosion products can easily fall off. The sacrificial anode has higher actual capacitance and current efficiency in the environment of low temperature and low dissolved oxygen, has the working potential of -1.12 to -1.05V, the current efficiency of more than or equal to 93% and the capacitance of more than or equal to 2600, and is even in corrosion. Furthermore, the sacrificial anode for the deep sea environment is low in indium content, effectively reduces the anode cost, and is especially suitable for cathode protection of ocean structure in the deep sea environment, thus the sacrificial anode has high performance.
Owner:李振国
Process for preparing boron hydride by electrolytic method
InactiveCN1396307AThe electrochemical reaction rate is lowReduced current efficiencyElectrolysis componentsElectrochemical responseHydrogen fuel
A process for preparing boron hydride from the metaborate by electrolytic method features that catalytically hdyrolzying alkali-metal boron hydride to generate hydrogen and metaborate as by-product, and electrolyzing the metaborate to generate boron hydride again, forming a cycle. The boron hydride used as hydrogen source of fuel battery features high electrochemical speed, long charge-discharge life, and low cost.
Owner:TAIYUAN UNIV OF TECH
Electrolytic ni plating apparatus and method of manufacturing semiconductor device
InactiveCN101307482AAvoid passivationReduced current efficiencySolid-state devicesSemiconductor/solid-state device manufacturingElectrolysisSemiconductor
The invention relates to an electrolyzed nickel plating device and method for producing a semiconductor device. The invention aims at providing an electrolyzed nickel plating device which can restrain passivation on the surface of nickel anode, prevent reduction of current efficiency and film forming speed, provide stable nickel plating for improving quality and maintain stable output. The electrolyzed nickel plating device is provided with the nickel (Ni) anode with the average grain size of 10 um or less.
Owner:NEC ELECTRONICS CORP
Preparation method of multicomponent microalloying aluminium alloy containing titanium, zirconium and scandium
This invention provides a making method for the multicomponent microalloy aluminium alloy such as titanium, zirconium, the scandium, the main steps as follows: under the steadiness of the electrolyzation aluminium production establishment condition, adding the titanium compound, the zirconium dioxide, the scandium trioxide with a certain proportion, using the electrolytic method of the alumina compound and cryolite to produce, the relative mass as follows: Tiíœ0.2úÑú¼Zríœ0.5úÑú¼Scíœ0.5úÑú¼ the rest is aluminium, wherein there are at least two is not 0 in the TiíóZríóSc. This invention decreases the cost for adding the TiíóZríóSc, decreases the oxide lard and hydrogen content in the alloy, thins and strengthens the aluminium.
Owner:ZHENGZHOU UNIV
Electrochemical water scale removal device
ActiveCN104291450AIncrease the chance of crystallizationIncrease resistanceSpecific water treatment objectivesScale removal and water softeningEngineeringAuxiliary electrode
The invention provides an electrochemical water scale removal device. The device comprises a water scale crystal nucleus generating unit used for electrochemical water treatment, wherein the water scale crystal nucleus generating unit comprises a tank body, diaphragms, an anode, cathodes and auxiliary electrodes; a water inlet and a drain outlet are formed in the bottom of the tank body; the diaphragms are arranged inside the tank body and divide the tank body into cathode chambers and an anode chamber; the anode is arranged inside the tank body; the cathodes and the auxiliary electrodes are arranged inside the cathode chambers; a catholyte water outlet and an anolyte water outlet are formed in the top end of the tank body. The device has the beneficial effects that with electrochemical operation, water scales can be deposited on the surfaces of the diaphragms and hinder ionic migration through the diaphragms, so that the resistance is increased, the current efficiency is reduced and the diaphragms need to be regenerated; the cathode chambers are emptied, the anolyte is led into the cathode chambers, then the auxiliary electrodes serving as anodes are connected and the anolyte generates chlorine and hydrochloric acid near the auxiliary electrodes, so that the water scales on the surfaces of the diaphragms are completely washed and the diaphragms are regenerated in situ.
Owner:章明歅 +1
Method of electrolytically synthesizing nitrogen trifluoride
InactiveUS20100006449A1Reduced current efficiencyImprove current efficiencyElectrolysis componentsNitrogen and non-metal compoundsElectrolysisSynthesis methods
The present invention provides an electrolytic synthesis method of nitrogen trifluoride, comprising electrolytically synthesizing nitrogen trifluoride gas from ammonium fluoride in an ammonium fluoride-containing molten salt mixture using a carbonaceous electrode as an anode, wherein the method comprises: a step of dissolving, in the molten salt mixture, metal ions capable of electrolytically yielding a highly oxidized metal fluoride through reaction with fluorine radicals (F.) that are generated upon the discharge of fluoride ions which are a component of the ammonium fluoride, thereby reacting the metal ions with the fluorine radicals (F.) to yield the highly oxidized metal fluoride, and reacting the highly oxidized metal fluoride with ammonium ions on a surface of the electrode and in a solution to synthesize nitrogen trifluoride gas.
Owner:DE NORA PERMELEC LTD
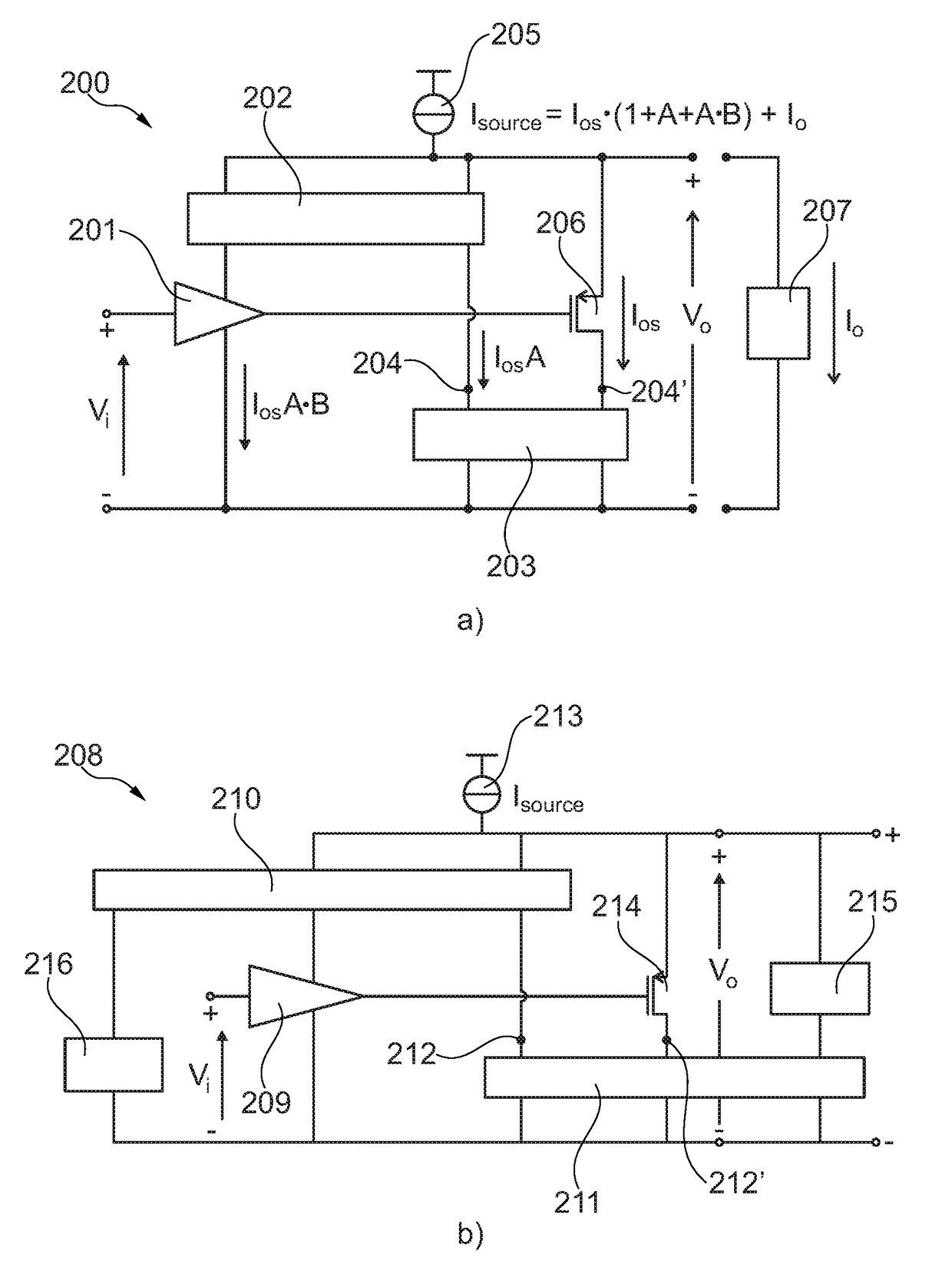
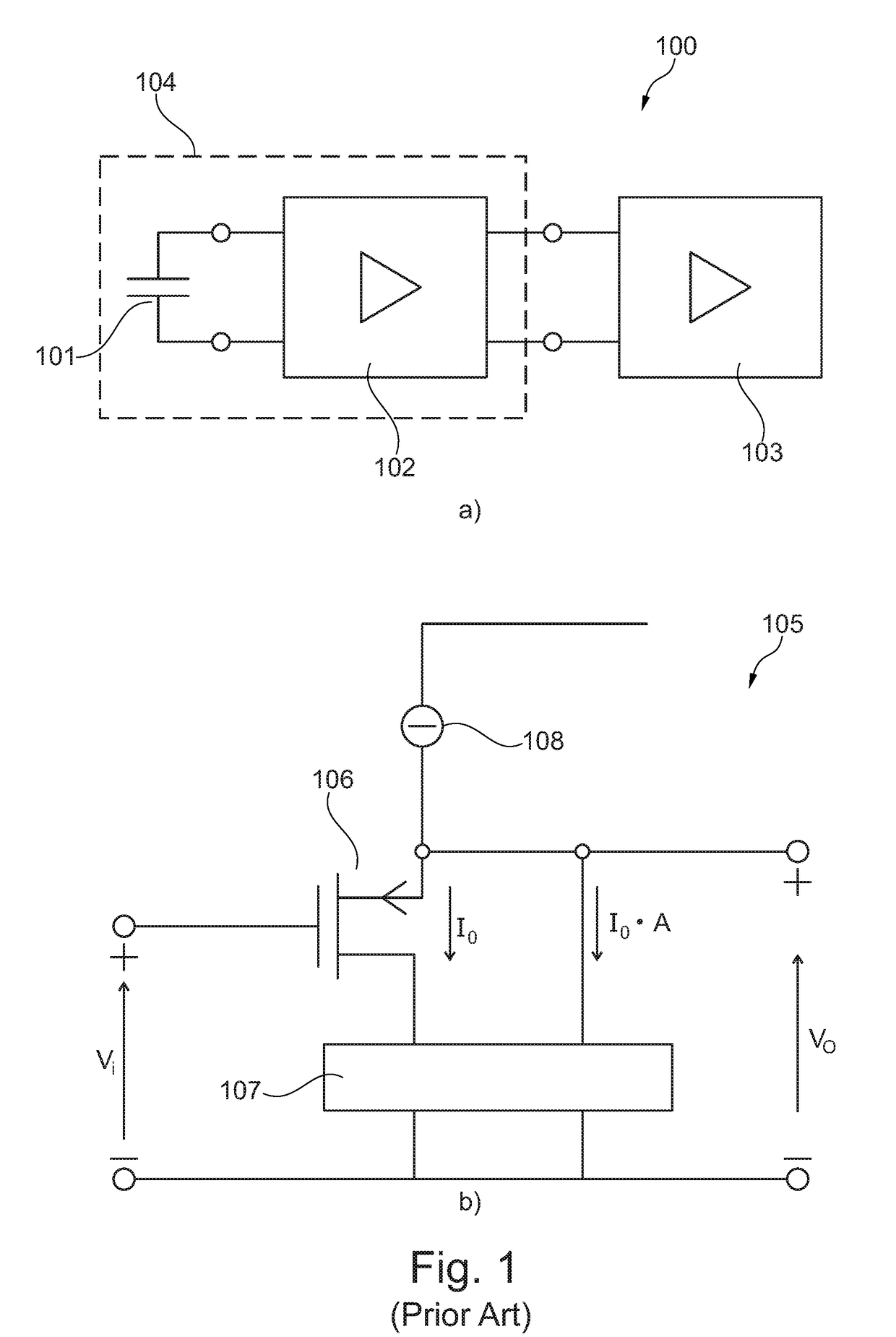
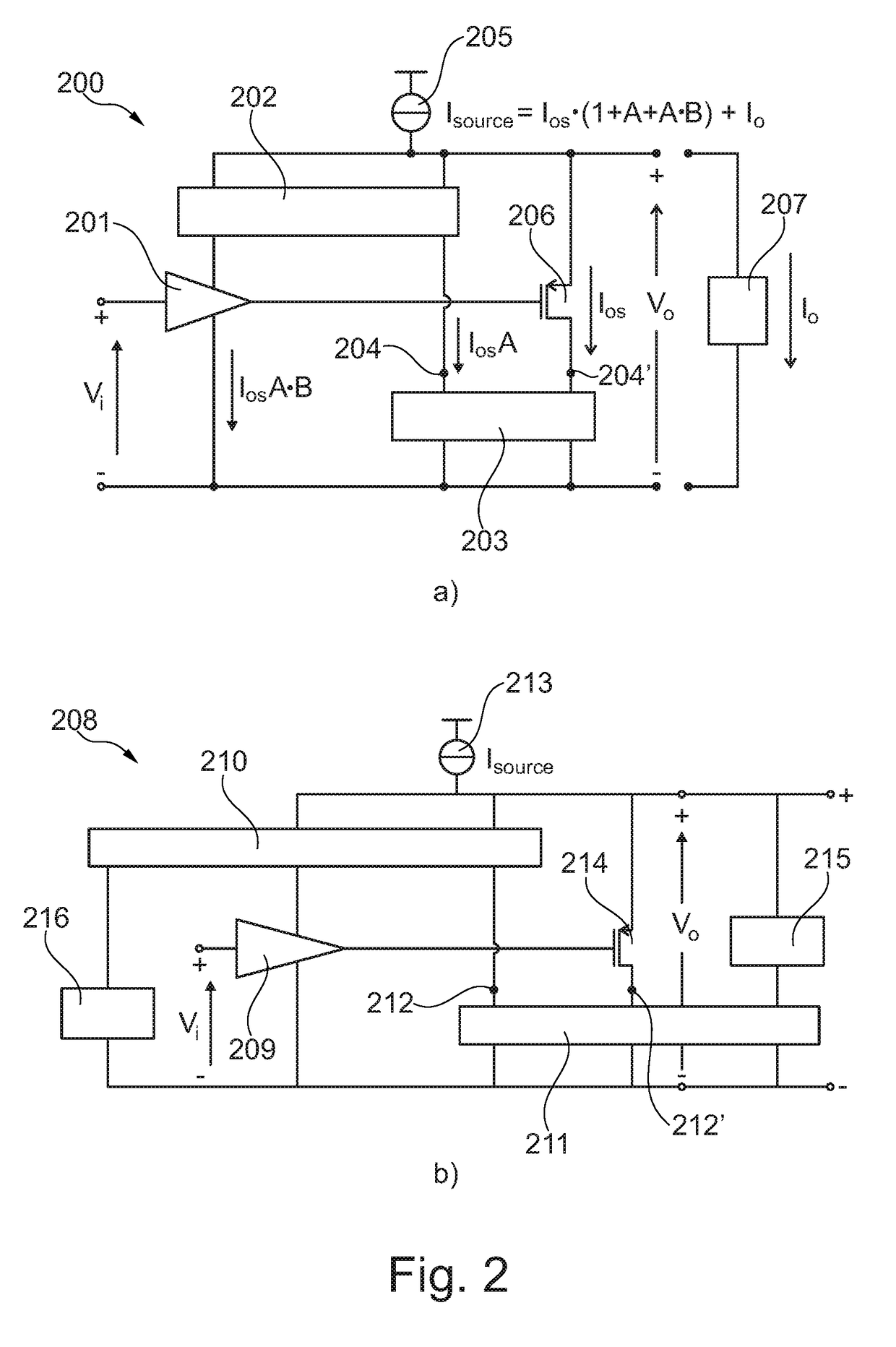
Self-biasing output booster amplifier and use thereof
ActiveUS20170215006A1Promote useFeed less oftenGated amplifiersLow frequency amplifiersAudio power amplifierEngineering
A self-biasing output booster amplifier having an input amplifier stage, an output amplifier stage being operatively connected to an output of the input amplifier stage, and first and second current copying circuits. The second current copying circuit is biased from an output of the self-biasing output booster amplifier. The first and second current copying circuits are configured to copy at least a portion of the current through the output amplifier stage. The sum of the output of the second current copying circuit and the output of the output amplifier stage provides the output current of the self-biasing output booster amplifier, Finally, the input amplifier stage is biased from the output of the second current copying.
Owner:SONION NEDERLAND
Tin plating solution as well as preparation method and application thereof
The invention relates to a tin plating solution as well as a preparation method and application thereof. The tin plating solution comprises the following components of 0.01-1 mol / L of a stannous ion source, 0.1-2 mol / L of methanesulfonic acid, 0-1.3 mol / L of boric acid, 0.2-5 mol / L of carboxylate, 0.02-2 mol / L of complexing agents and 0. 0001-0.01 mol / L of surfactant, and the pH value range of thetin plating solution is 3.5-5.5. The tin plating solution has the advantages that excellent current density bearing capacity is achieved, and the situation of 'sticking' and 'scorching' can be avoided under the condition of relatively high current density.
Owner:GUANGDONG GUANGHUA SCI TECH
Method for environmentally friendly and efficiently producing electrolytic manganese metal
ActiveCN103173786AHigh temperature toleranceReduced current efficiencyPhotography auxillary processesSolubilityEnvironmental resistance
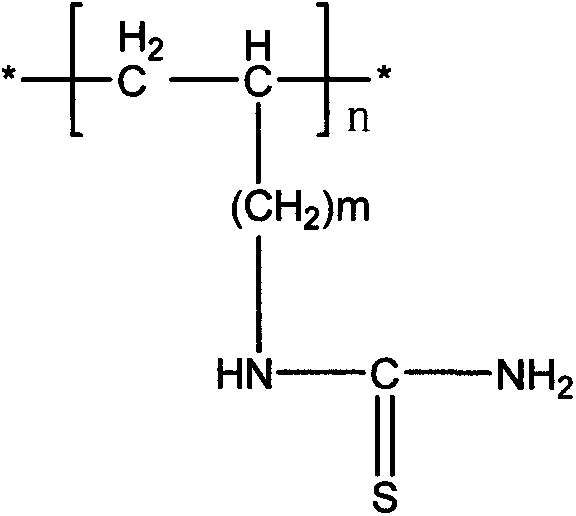
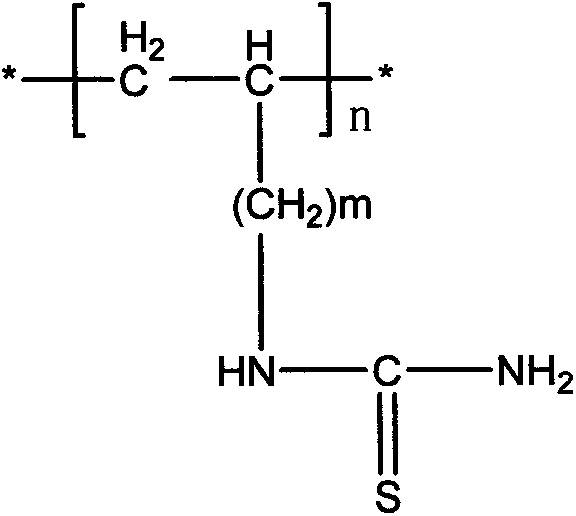
The invention belongs to the technical field of chemical production and provides a method for efficiently producing electrolytic manganese metal in an environment-friendly manner. The method provided by the invention takes a vinyl polymer hooked with a thiourea side group as an electrolysis additive, and the electrolysis additive is added into electrolyte containing manganese sulphate and ammonium sulphate for the production of electrolytic manganese, wherein the content of the electrolysis additive in the electrolyte is controlled to be 10-120mg / L. The electrolysis additive is an environment-friendly and non-toxic polymer and can be used for replacing the existing widely applied poisonous or pollutional selenium dioxide and sulphur dioxide; the electrolysis additive is low in solubility and cannot be accumulated in the electrolyte, a production process operation is simple, manganese metal electrolysis efficiency is high, the product purity is high, an electrolysis process is stable, purity requirement to the electrolyte is lower, and electrolytic manganese metal production difficulty and cost are reduced, so that the method provided by the invention has higher popularization and application values.
Owner:云南江南锰业股份有限公司
Anode changing method of electrolyzer
The invention discloses an anode changing method of an electrolyzer. The method comprises the steps of: removing a residual electrode ; and arranging a heated new anode at a temperature of 50-500 DEG C into the electrolyzer. Compared with direct usage of a cold wet anode in the electrolyzer, the heated anode can reduce frequency of ''needle vibration'' of the electrolyzer, improve stability of the electrolyzer, reduce Fe-C pressure drop of the anode and improve anode conductivity, so as to realize the electrolyzer production with high efficiency and low consumption.
Owner:SNTO TECH GRP
Method of electrolytically synthesizing nitrogen trifluoride
ActiveCN101624708AReduced current efficiencyNitrogen and non-metal compoundsElectrodesElectrolysisSynthesis methods
The present invention provides an electrolytic synthesis method of nitrogen trifluoride, comprising electrolytically synthesizing nitrogen trifluoride gas from ammonium fluoride in an ammonium fluoride-containing molten salt mixture using a carbonaceous electrode as an anode, wherein the method comprises: a step of dissolving, in the molten salt mixture, metal ions capable of electrolytically yielding a highly oxidized metal fluoride through reaction with fluorine radicals (F.) that are generated upon the discharge of fluoride ions which are a component of the ammonium fluoride, thereby reacting the metal ions with the fluorine radicals (F.) to yield the highly oxidized metal fluoride, and reacting the highly oxidized metal fluoride with ammonium ions on a surface of the electrode and in a solution to synthesize nitrogen trifluoride gas.
Owner:DE NORA PERMELEC LTD
Tube type waste water treatment device and waste water treatment method
ActiveCN105417811AAvoid easy passivationAvoid consuming too quicklyWater/sewage treatment by irradiationWater treatment compoundsPhoto catalyticMicrofiltration membrane
The invention discloses a tube type waste water treatment device and a waste water treatment method. The tube type waste water treatment device comprises a regulating pond, a first water pump, an ultrasonic generator, a tube type ultrasonic reactor, a tube type electric tractor, a middle water tank, a second water tank, an ejector and a tube type micro filter membrane. Due to the fact that power ultrasonic treatment, ultraviolet light catalysis, induced electricity fenton, heterogeneous catalytic oxidation and other advanced oxidization technologies are effectively integrated, a superimposed effect is formed, the defects that conventional reaction current is low in efficiency, electrode plates are prone to passivation, consumption of the electrode plates is too fast, and the electrode plates cannot be easily replaced are avoided at the same time, all units are designed into a tube type structure as mush as possible in whole scheme design, and therefore the whole device can be small, modularized and intelligent.
Owner:SHENZHEN WATER TECH SERVICE CO LTD
Electroanalysis eutectrol process for producing alloy of aluminum and titanium
This invention relates to a method for producing Al-Ti alloy by electrolytic coprecipitation. The method comprises: feeding TiO2 into an electrolytic bath from the feeding mouth 3-5 times each day, samplying and analyzing after 2-3 h, discharging Al, terminating TiO2 feeding to lower the content of Ti in the electrolytic bath when electrolytic coprecipitation is performed for 4-6 months, feeding TiO2 into another electrolytic bath, and controlling the content increase of TiO2 in the electrolytic bath match the content decrease of TiO2 in the first electrolytic bath to guarantee the supply of Al-Ti alloy. By the method, the electrolytic baths have stable running and no obvious decrease of current efficiency; the furnace bottom is protected. The method can be applied to 300 kA electrolytic bath stably and permanently.
Owner:SHANDONG NANSHAN ALUMINUM
Process for preparing boron hydride by electrolytic method
InactiveCN1239748CThe electrochemical reaction rate is lowReduced current efficiencyElectrolysis componentsElectrochemical responseHydrogen fuel
A process for preparing boron hydride from the metaborate by electrolytic method features that catalytically hdyrolzying alkali-metal boron hydride to generate hydrogen and metaborate as by-product, and electrolyzing the metaborate to generate boron hydride again, forming a cycle. The boron hydride used as hydrogen source of fuel battery features high electrochemical speed, long charge-discharge life, and low cost.
Owner:TAIYUAN UNIV OF TECH
Method and device for operating an OLED device
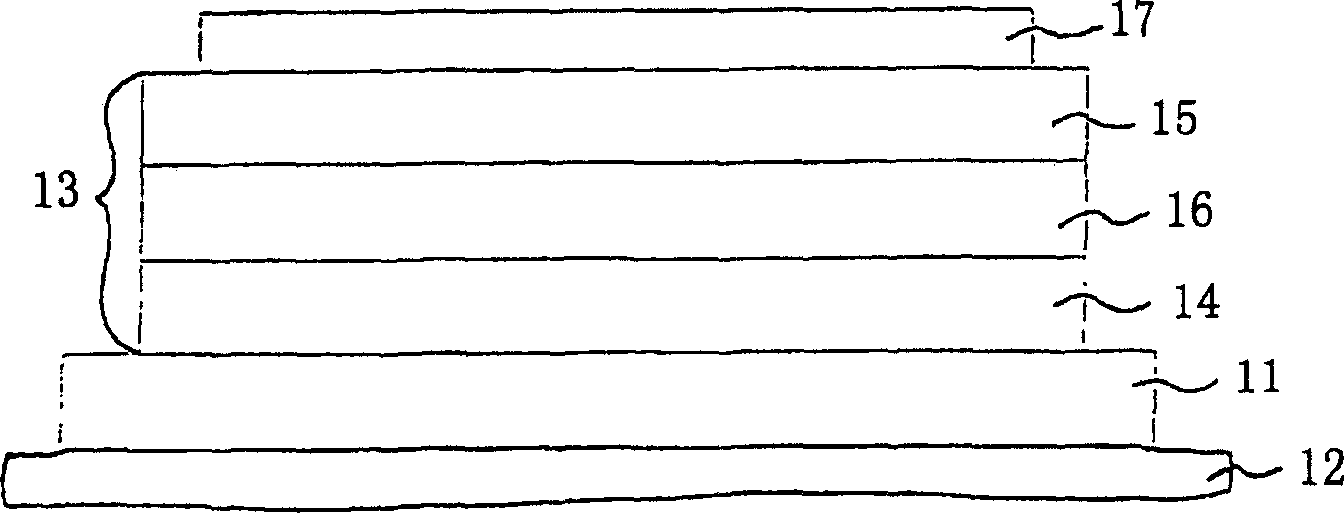
InactiveCN1893744ADifferential Color Aging CompensationReduced current efficiencyElectrical apparatusStatic indicating devicesLuminophorePulse height
The invention relates to a method for operating an organic light emitting component ( 1 ), in particular organic light emitting diode (OLED), and an organic light emitting component ( 1 ) with an organic layer ( 1 c) comprising a plurality of different emitter materials, which emit light of different colours, and an electrode arrangement ( 1 a , 1 b) for applying electric control pulses to the organic layer ( 1 c), the method having the following steps: operating the organic light emitting component ( 1 ) with the help of the electric control pulses in a pulsed operating mode with an operating frequency of at least about 25 Hz; at least partially compensating an ageing induced varying colour change of the light emitted from the organic layer ( 1 c) during the course of the pulsed operation by recording at least one operating parameter characterizing the running pulsed operation of the organic light emitting component ( 1 ) and for which a dependence relationship to the ageing induced varying colour change is known at least approximately, and by adjusting a pulse height of the electrical control pulses according to the at least one recorded operating parameter and the ageing induced varying colour change; and regulating a predetermined luminance of the light emitted from the organic layer ( 1 c) during the course of the pulsed operation by adjusting a pulse length of the electric control pulses for regulating the predetermined luminance of the light emitted from the organic layer ( 1 c).
Owner:NOVALED GMBH
Electrolytic method, apparatus and product
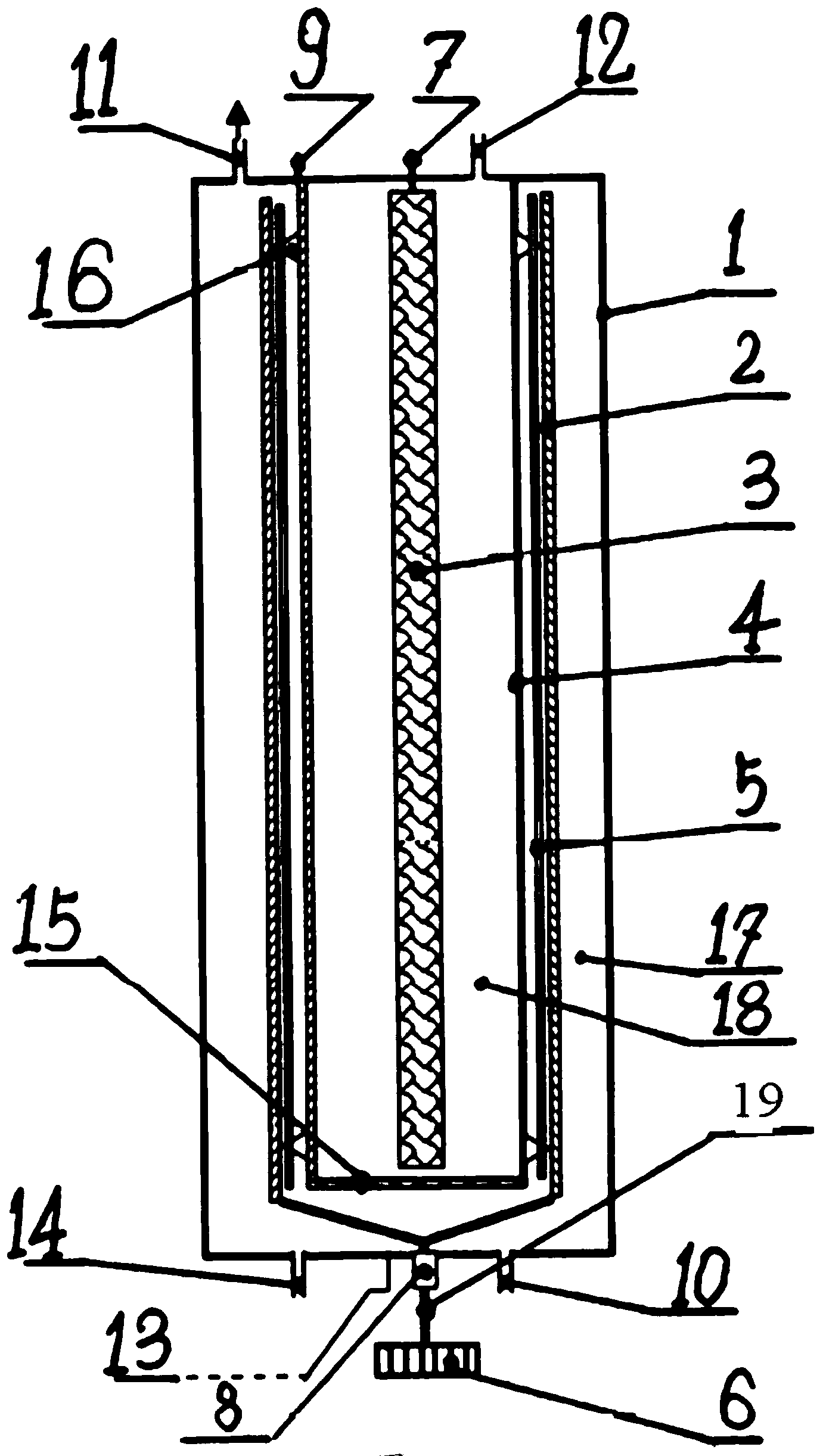
ActiveUS20150129432A1Shorten the timeEliminate currentPolycrystalline material growthElectrolysis componentsElectrolysisMolten salt
In a method for removing a substance from a feedstock comprising a solid metal or a solid metal compound, the feedstock is contacted with a fused-salt melt. The fused-salt melt contains a fused salt, a reactive-metal compound, and a reactive metal. The fused salt comprises an anion species which is different from the substance, the reactive-metal compound comprises the reactive metal and the substance, and the reactive metal is capable of reaction to remove at least some of the substance from the feedstock. A cathode and an anode contact the melt, and the feedstock contacts the cathode. An electrical current is applied between the cathode and the anode such that at least a portion of the substance is removed from the feedstock. During the application of the current, a quantity of the reactive metal in the melt is maintained sufficient to prevent oxidation of the anion species of the fused salt at the anode. The method may advantageously be usable for removing the substance from successive batches of the feedstock, where the applied current is controlled such that the fused-salt melt after processing a batch contains the quantity of the reactive metal sufficient to prevent oxidation of the anion species at the anode.
Owner:METALYSIS
Electrolytic method for manufacturing aluminum-manganese alloy in fluoride-chloride molten salt system
The invention aims to provide an electrolytic method for manufacturing aluminum-manganese alloy in a fluoride-chloride molten salt system. The electrolytic method includes that in an electrolytic bath, inert metal molybdenum is used as a cathode and is placed at a lower portion of the electrolytic bath, and graphite is used as an anode; an electrolyte comprises 39% of NaCl, 50% of KCl and 11% of AlF3, is prepared according to the mass ratio and is heated, so that the temperature of the electrolyte ranges from 680 DEG C to 730 DEG C; MgCl2 is added into the electrolyte after the electrolyte is melted, and the quantity of the added MgCl2 accounts for 40-75% of the mass of the AlF3; direct-current power is switched on for electrolysis, the electrolytic temperature ranges from 680 DEG C to 730 DEG C, the current density of the cathode ranges from 5.2A / cm<2> to 8.7A / cm<2>, the current density of the anode ranges from 0.6A / cm<2> to 1.1A / cm<2>, and the voltage of the bath ranges from 4.7V to 6.1V; and liquid Al-Mg alloy is deposited at a position close to the cathode of the electrolytic bath after electrolysis is carried out for 1.5 to 4 hours, and is cooled down to obtain the solid Al-Mg alloy. The electrolytic method has the advantages that a process of a production technology can be greatly shortened, the technology is simple, energy is saved, and the purity of the product is high.
Owner:HARBIN ENG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com