Preparation method of tofacitinib intermediate
A compound, benzyl technology, applied in the field of preparation of -1-benzyl-N,4-dimethylpiperidin-3-amine dihydrochloride, can solve the problems of high price and increased operation difficulty, and achieve The effect of improving synthesis yield, avoiding the use of dangerous reagents, and shortening process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
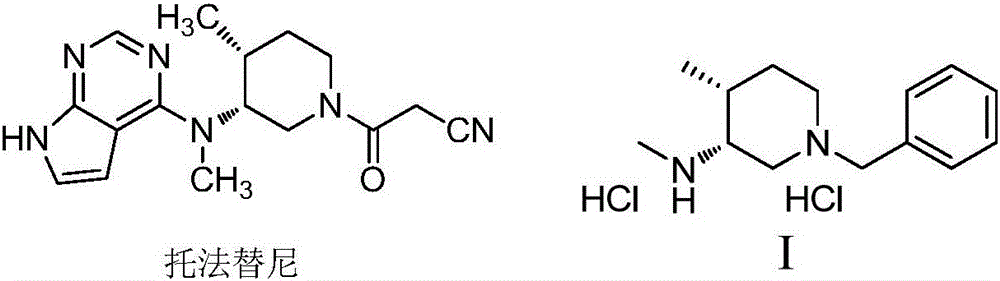
[0029] A preparation method of a drug intermediate I of tofacitinib, the chemical structural formula is as follows:
[0030]
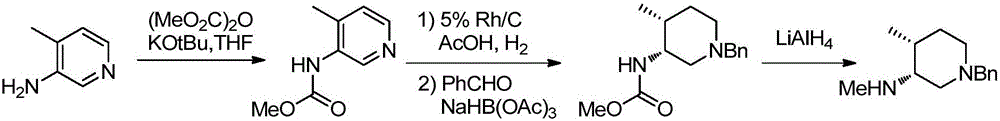
[0031] The preparation method is as follows: using 1-benzyl-4-methyl-1,2,3,6-tetrahydropyridine as the starting material, oxidizing alkenes to ketones by one-step method to obtain ketone compound II, and then forming ketone compound II with amines After amine compound III, use asymmetric reduction of imine to form amine, remove the trans isomer by recrystallization to obtain cis structure IV, and finally use chiral resolution to obtain the final product (3R,4R)-1-benzyl-N , 4-dimethylpiperidin-3-amine dihydrochloride I, to obtain;
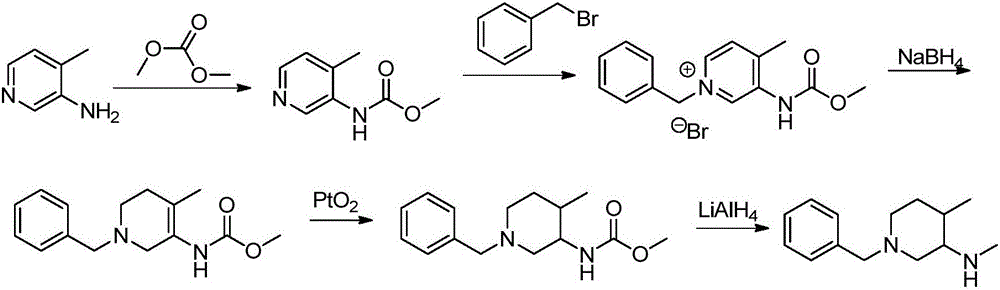
[0032] The synthetic route is as follows:
[0033]
Embodiment 1
[0034] Example 1: The raw material 1-benzyl-4-methyl-1,2,3,6-tetrahydropyridine (187 mg) was dissolved in dichloromethane (10 mL), and trimethyl was added under nitrogen protection at -20°C A dichloromethane solution of silyl trifluorosulfonate (1.1 g) was added, and a catalytic amount of cyclohexanone was added, the reaction was stirred and controlled until the reaction was complete, and compound II was obtained by column chromatography. (Yield 89%).
Embodiment 2
[0035] Example 2: Add compound II (17.6g) and 50ml tetrahydrofuran to a three-necked flask in turn, protect with nitrogen, cool down to 15°C, add methylamine hydrochloride (7g) dropwise, control the temperature at 15-20°C for reaction, and control it until the reaction completely. Jump straight into the next step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com