Preparation method of pitavastatin calcium
A technology of pitavastatin calcium and cyclopropyl, which is applied in the field of preparation of cholesterol-lowering drugs, can solve problems such as unfavorable industrial production, unfriendly environment, complicated operation, etc., and achieves improved safety, friendliness, environment friendliness, and yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
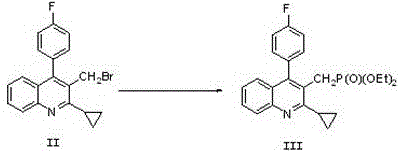
[0043] The preparation of embodiment 1 compound II
[0044]Reaction formula:
[0045]
Embodiment 1-1
[0047] Steps:
[0048] Add 29.1g of compound I, 17.2g of methyldichlorosilane, 8g of ferric chloride, 40.2g of halogenation reagent phosphorus tribromide, and 60ml of acetonitrile into a 100ml three-necked flask, stir and reflux at 75~85°C under nitrogen protection, until TLC Detect that the reaction is complete, add dilute hydrochloric acid to the reaction system to terminate the reaction, let stand for stratification, separate the organic layer, and wash with sodium bicarbonate and saturated brine, separate the washing liquid from the organic layer, add anhydrous magnesium sulfate to the organic layer, After drying, filtering, and concentrating the filtrate under reduced pressure, 30.5 g of compound was obtained, which was confirmed to be compound II by comparison with the melting point data of standard compound II. The melting point of compound II was 138-140°C, and the yield of compound II was 86%.
Embodiment 1-2
[0050] Add 29.1g of compound I, 17.2g of methyldichlorosilane, 8g of ferric chloride, 40.2g of phosphorus tribromide, and 60ml of acetonitrile into a 100ml three-necked flask, stir at 65~75°C under nitrogen protection, and TLC detects that the reaction is complete. Add dilute hydrochloric acid to the reaction system to terminate the reaction, let stand to separate the layers, separate the organic layer, and wash with sodium bicarbonate and saturated brine, separate the washing liquid from the organic layer, add anhydrous magnesium sulfate to the organic layer, dry, filter, The filtrate was concentrated under reduced pressure to obtain 28.4 g of compound, which was confirmed to be compound II by comparison with the melting point data of standard compound II. The melting point of compound II was 138-140 °C, and the yield of compound II was 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com