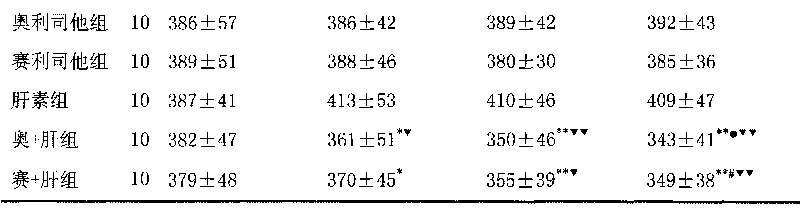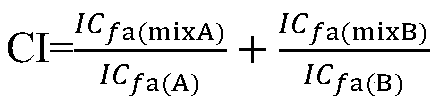Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
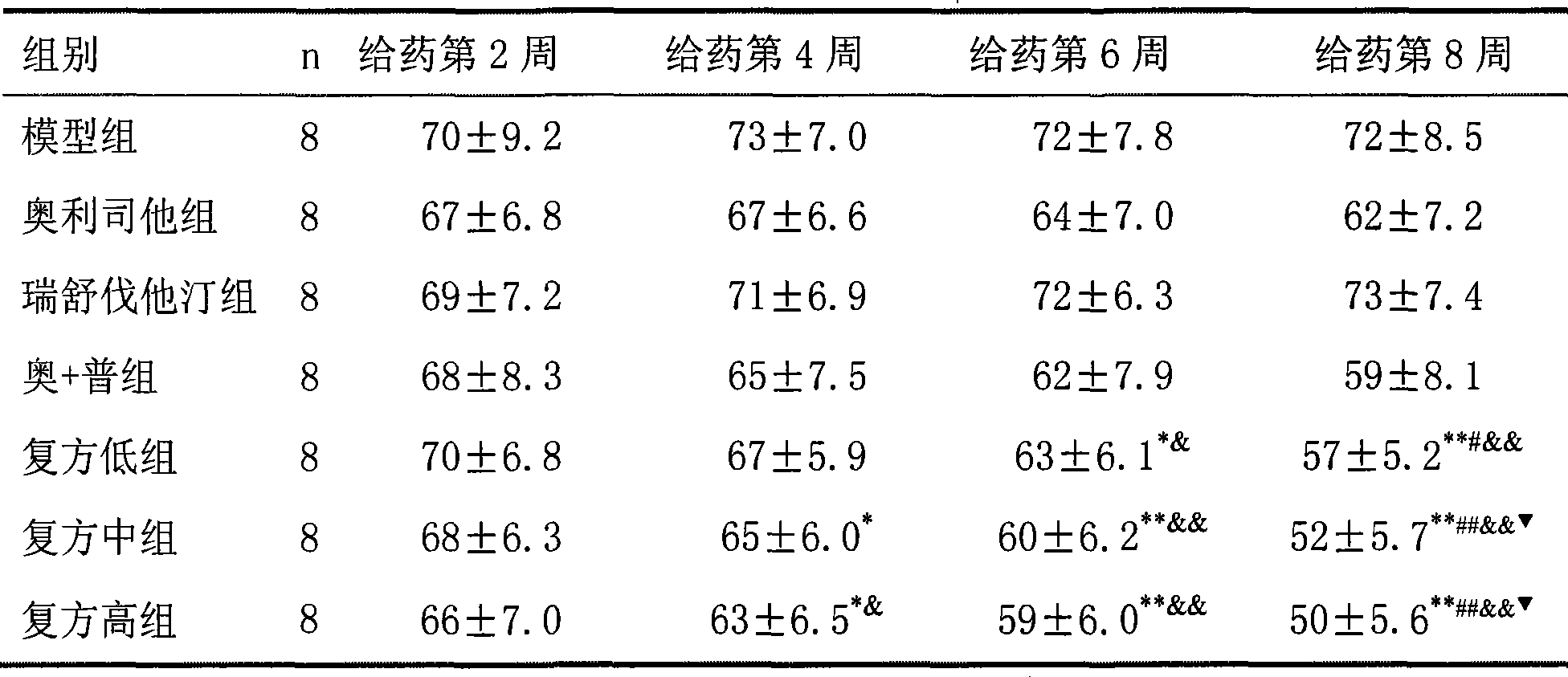
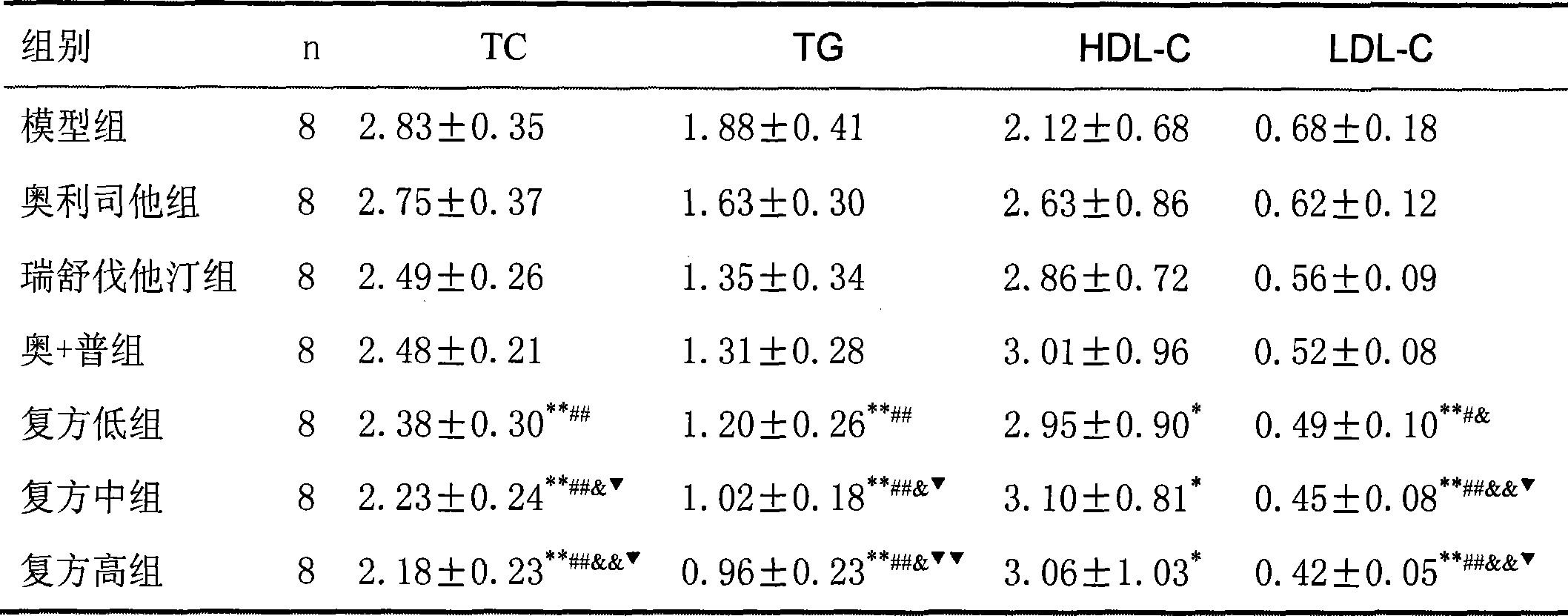
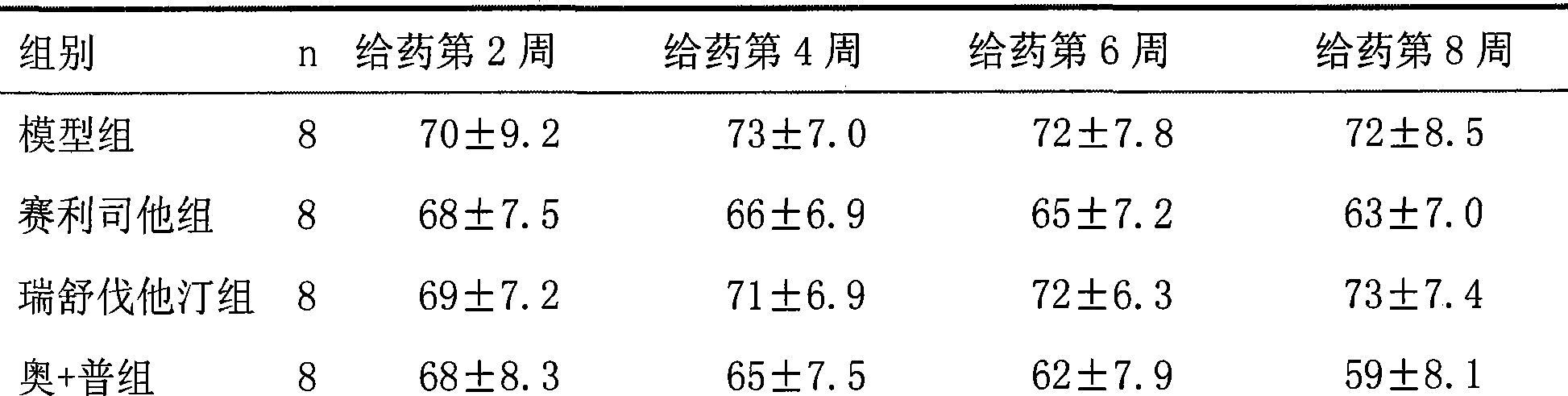
31 results about "Cetilistat" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
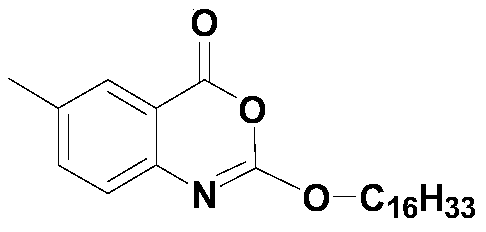
Cetilistat is a drug designed to treat obesity. It acts in the same way as the older drug orlistat (Xenical) by inhibiting pancreatic lipase, an enzyme that breaks down triglycerides in the intestine. Without this enzyme, triglycerides from the diet are prevented from being hydrolyzed into absorbable free fatty acids and are excreted undigested.
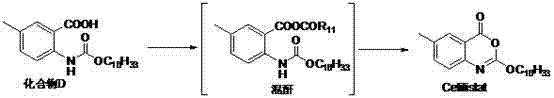
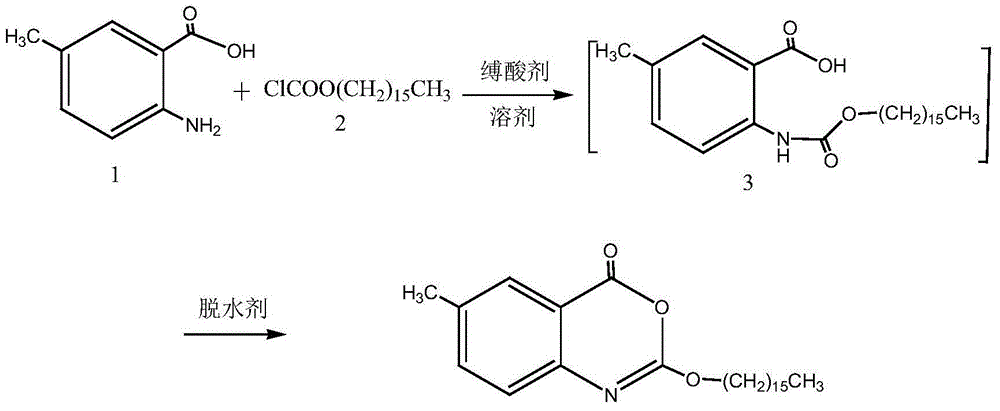
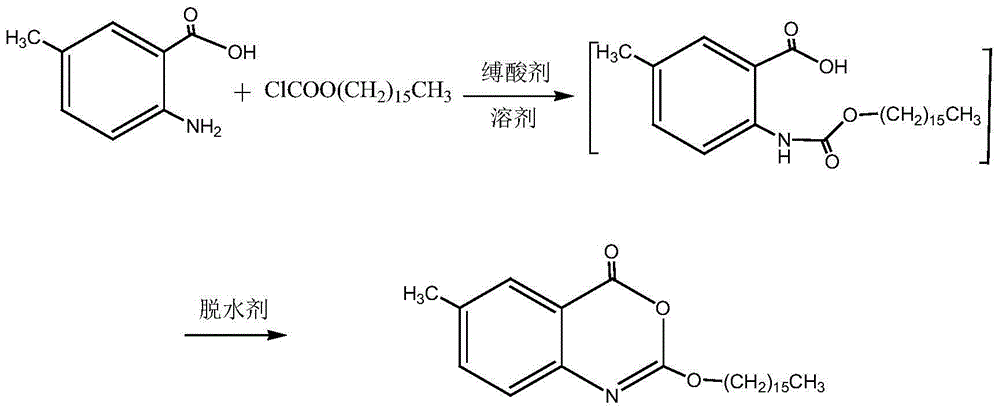
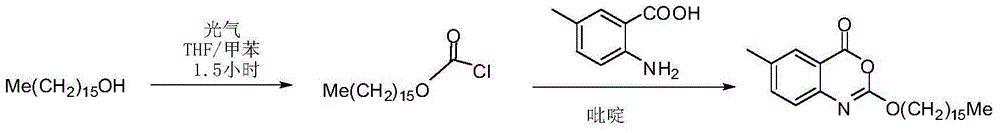
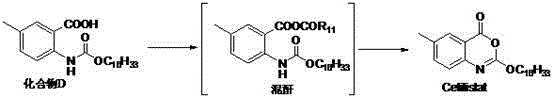
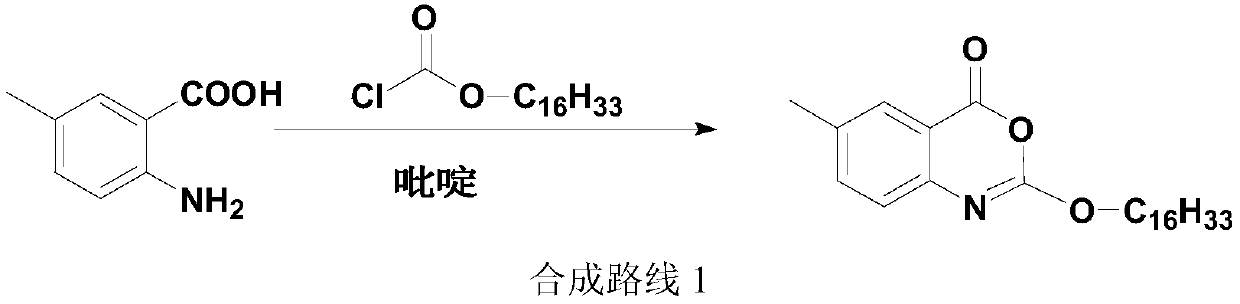
Method for preparing cetilistat
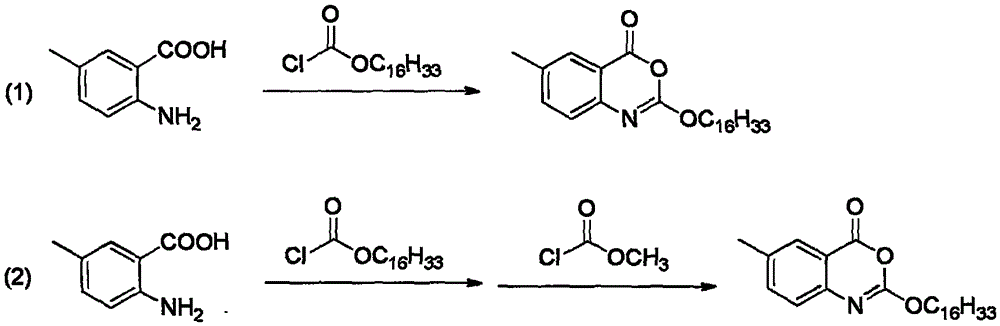
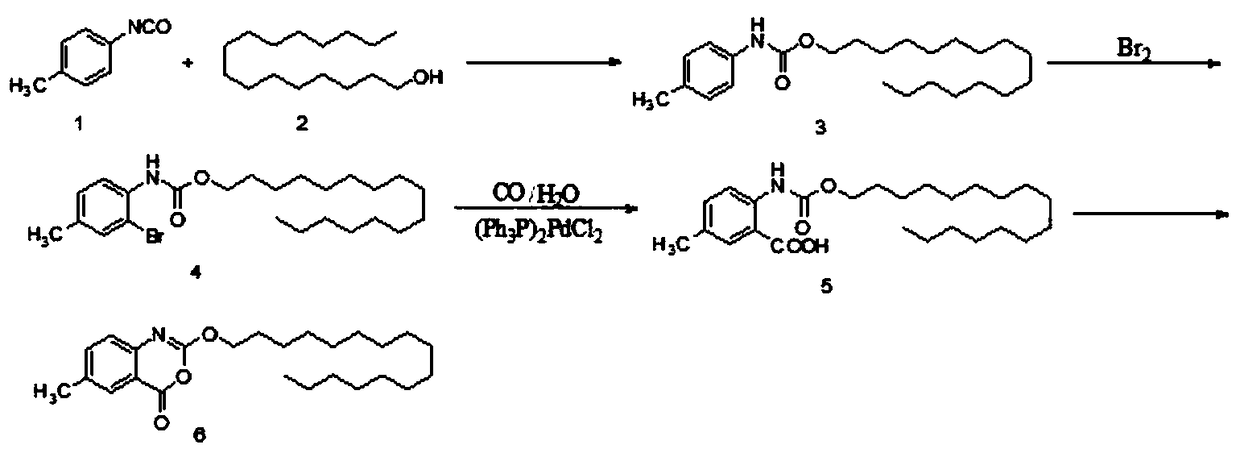
The invention discloses a method for preparing cetilistat, which belongs to the technical field of the medicine preparation method, and is used for solving the problems of high three wastes, low yield and high cost in the current preparation method. The method comprises the following steps: 1)esterifying a compound A(2-amino-5-methyl benzoic acid) or its salt; 2) acylating an esterification object in the step 1) and chloroformic acid n-cetanol ester; 3)removing ester group of the compound obtained in the step 2) to obtain 2-(cetane carbonyl oxygen)amino-5-methyl benzoic acid; and 4)cyclizing the compound in the step 3) to obtain the target products. By improving the production steps, the invention provides the synthetic method with simple operation, high efficiency, and convenient industrial production for the cetilistat product.
Owner:CHONGQING TOPTECH PHARMA TECH
One-pot high-yielding preparation of cetilistat
The invention relates to a new process for one-pot preparation of cetilistat; the one-pot reaction is adopted, separation steps are sample, the product yield is high, the product quality is good, and the new process is especially suitable for industrialized production.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
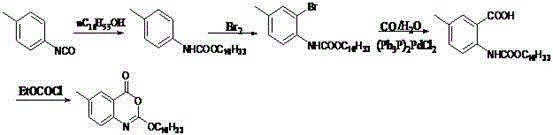
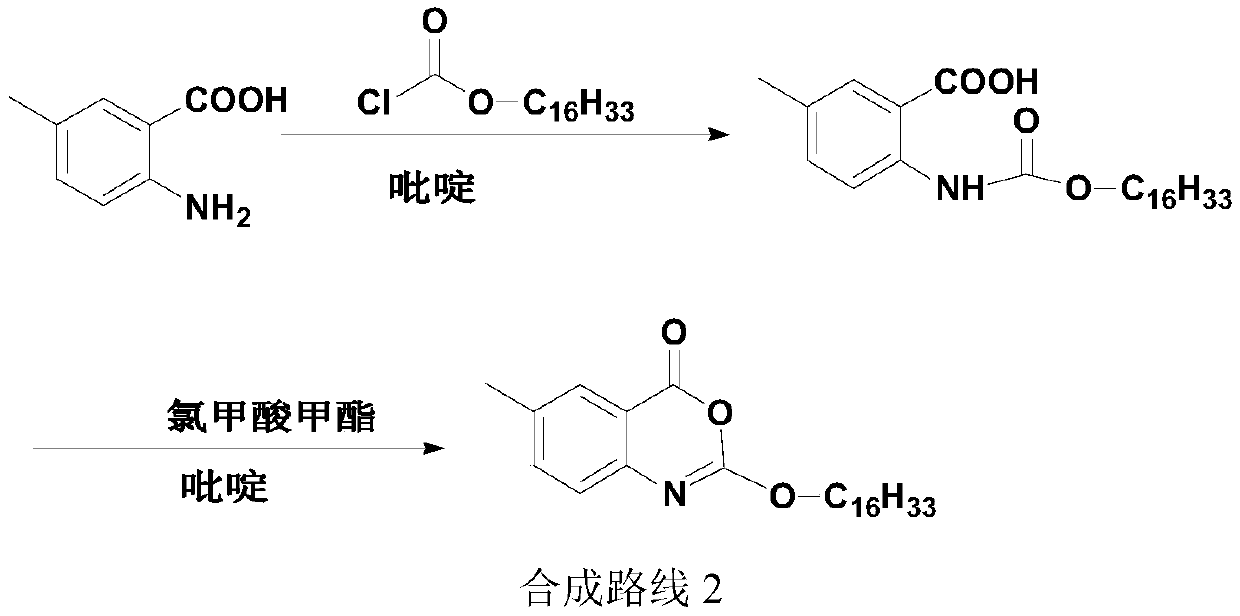
Preparation method of cetilistat
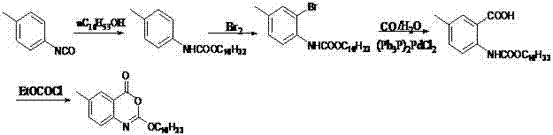
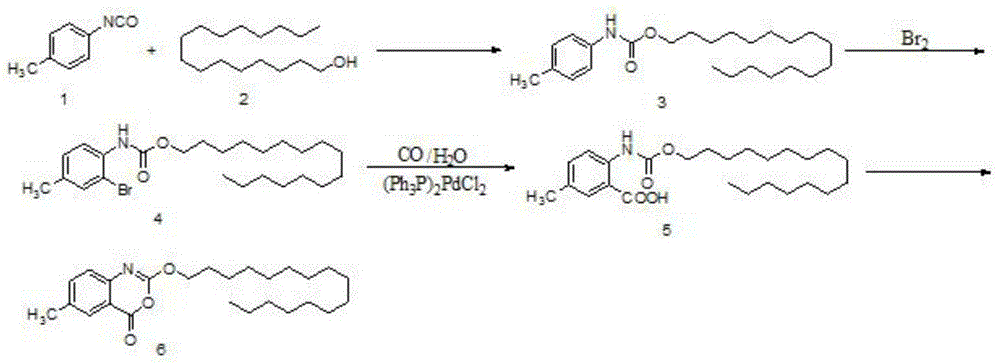
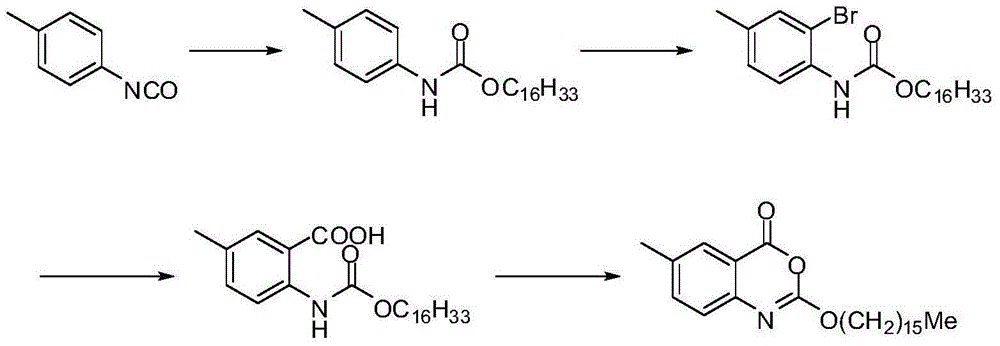
The invention discloses a preparation method of cetilistat, which has the advantages of high yield, mild reaction conditions and the like and can easily implement industrial production. The method comprises the following steps: (1) reacting methyl 2-amino-5-halobenzoate with triphosgene to obtain 2-methoxycarbonyl-4-halophenylisocyanate; adding hexadecanol to generate methyl 2-(hexadecaalkoxycarbonylamino)-5-halobenzoate; (2) adding the methyl 2-(hexadecaalkoxycarbonylamino)-5-halobenzoate, methyl boron dihydroxide and alkali into water and an organic solvent, adding a palladium catalyst, and reacting to obtain methyl 2-(hexadecaalkoxycarbonylamino)-5-methylbenzoate; (3) carrying out esterolysis reaction on the methyl 2-(hexadecaalkoxycarbonylamino)-5-methylbenzoate to obtain 2-(hexadecaalkoxycarbonyl)-5-methylbenzoic acid; (4) suspending the 2-(hexadecaalkoxycarbonyl)-5-methylbenzoic acid in pyridine, and cyclizing under the action of a dehydrating agent; and purifying and carrying out crystal transformation to obtain the cetilistat.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
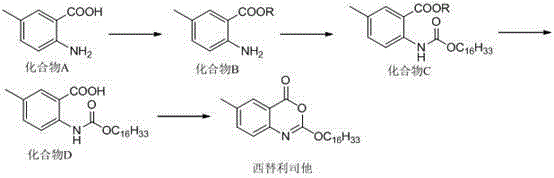
Method for preparing cetilistat
The invention provides a method for preparing cetilistat. According to the method, 2-amidogen-5-methyl benzoic acid is adopted as an initial raw material, under existence of pyridine, the 2-amidogen-5-methyl benzoic acid reacts with chlorine acid cetyl alcohol ester in the first place, a midbody 2-(((hexadecane oxygroup) carbonyl) amidogen)-5-methyl benzoic acid is obtained, then dehydrogenation cyclization reagent is utilized to obtain the cetilistat, and the feeding sequence in step 1 is the 2-amidogen-5-methyl benzoic acid, alkali and the chlorine acid cetyl alcohol ester in sequence. The method has the remarkable advantages that the route is simple, operation is less, atom economy is better than that of other routes, and the cetilistat is suitable for large-scale production; the purity of the cetilistat at the reaction endpoint is larger than 98%, and through simple postprocessing, a final product which meets medical standards can be obtained, wherein the purity is larger than 99.5%, and the single impurity is smaller than 0.1%; the utilized solvent including dichloromethane and pyridine can be recycled for mechanical application, and the method is environmentally friendly.
Owner:ZHONGSHAN WANHAN PHARM CO LTD +1
Medical composition for losing weight or treating metabolic syndromes
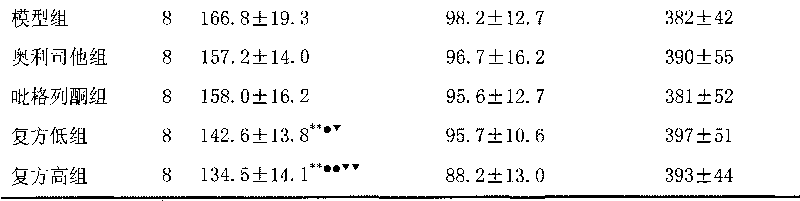
ActiveCN101757019ALose weightIncreased pulse pressureOrganic active ingredientsMetabolism disorderTG - TriglycerideLost Weight
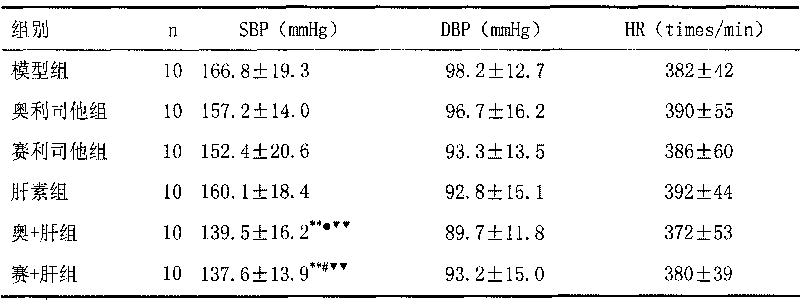
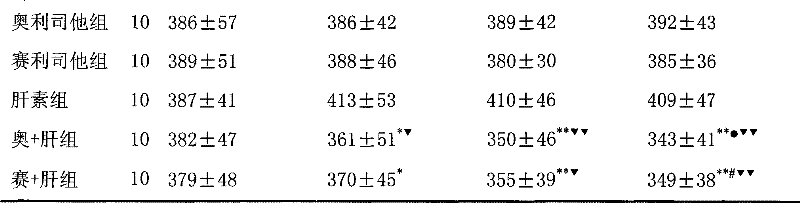
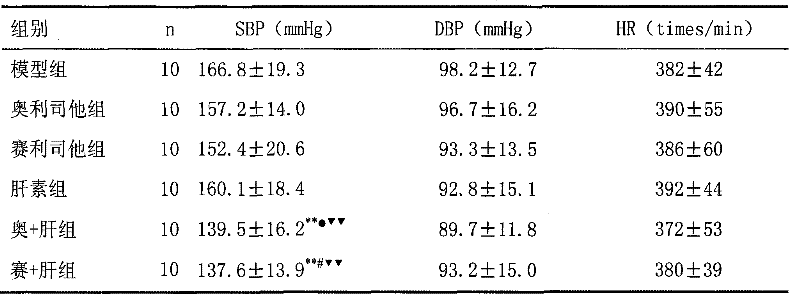
The invention discloses a medical composition for treating metabolic syndromes, belonging to the field of medicines, and in particular relates to a medical composition containing orlistat or cetilistat and heparin, wherein the composition is solid preparations such as compressed tablets, dispersible tablets, sustained release tablets, capsules, granules, and the like. In experiments, the medical composition containing the orlistat or the cetilistat and the heparin is accidentally found to have obvious synergistic effect on the aspects of reducing the blood pressure, the serum total cholesterol, the serum triglyceride and the low density lipoprotein cholesterin and enhancing the carbohydrate tolerance.
Owner:LUNAN PHARMA GROUP CORPORATION
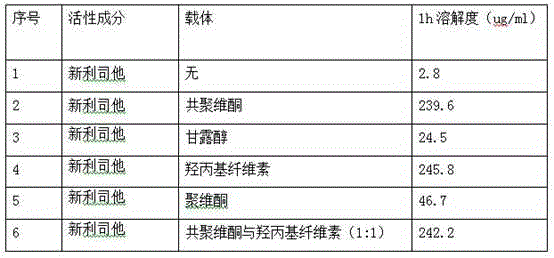
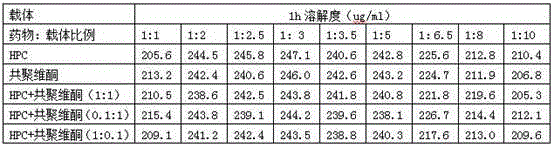
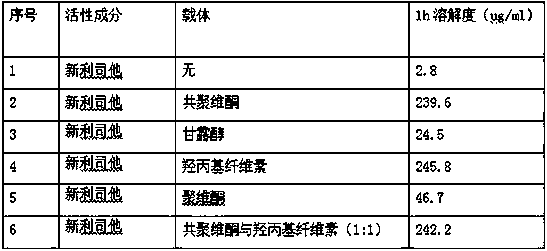
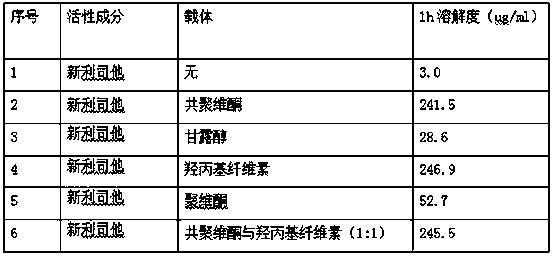
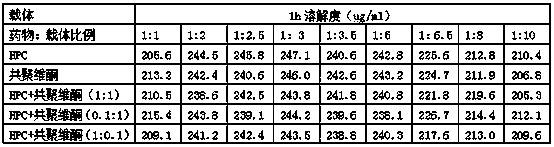
Cetilistat solid dispersion and medicinal preparation prepared from the solid dispersion
The invention relates to cetilistat solid dispersion, and a medicinal preparation prepared from the solid dispersion. The cetilistat and one or both of the carriers hydroxypropyl cellulose and copovidone are prepared into a solid dispersion to significantly improve the solubility of the cetilistat, and overcome low solubility of cetilistat, so that the prepared medicinal preparation achieves good dissolution, and has increased bioavailability.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Medical composition for losing weight or treating metabolic syndromes
ActiveCN101756993ALose weightLower blood pressureOrganic active ingredientsMetabolism disorderTG - TriglycerideLost Weight
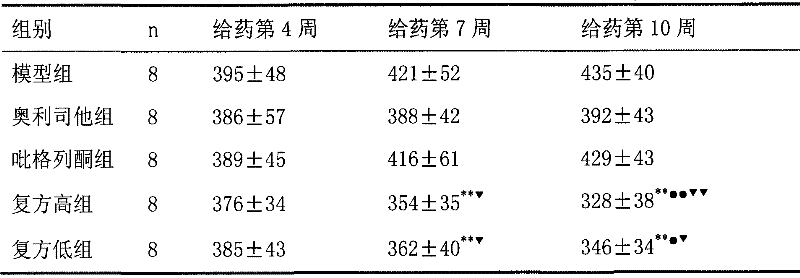
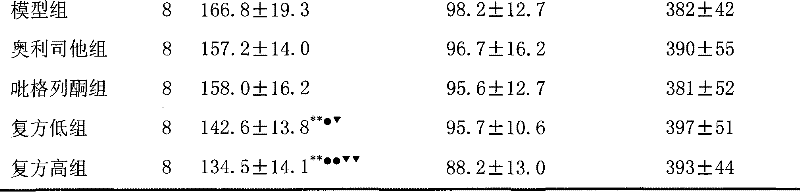
The invention discloses a medical composition for treating metabolic syndromes, belonging to the field of medicines, and in particular relates to a medical composition containing orlistat or cetilistat and actos, wherein the composition is solid preparations such as compressed tablets, dispersible tablets, sustained release tablets, capsules, granules, and the like. In experiments, the medical composition containing the orlistat or the cetilistat and the actos is accidentally found to have obvious synergistic effect on the aspects of reducing the blood pressure, the serum total cholesterol, the serum triglyceride and the low density lipoprotein cholesterin and enhancing the carbohydrate tolerance.
Owner:LUNAN PHARMA GROUP CORPORATION
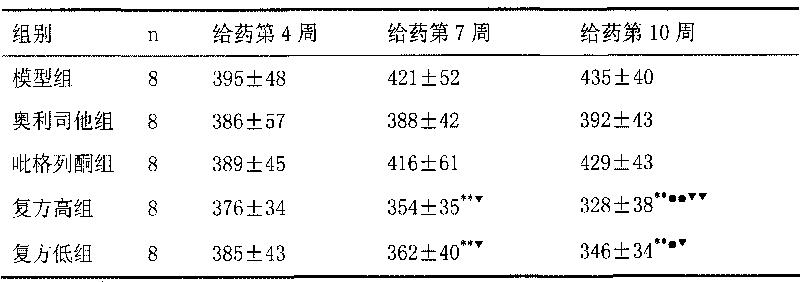
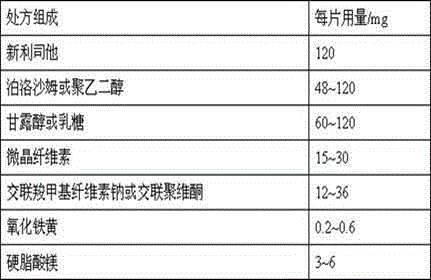
Cetilistat medicinal composition and preparation method thereof
ActiveCN106310287AFacilitated releaseGood dissolution effectOrganic active ingredientsMetabolism disorderDiabetes mellitusCetilistat
The invention belongs to the field of medicine compositions for treating obesity and II-type diabetes, and definitely, relates to a medicine composition of cetilistat and a preparation method thereof. A cetilistat tablet prepared from the medicine composition through the preparation method has excellent dissolving-out effect. The preparation method is simple and is suitable for industrial production.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Preparation method of cetilistat
InactiveCN105669585AMild reaction conditionsEasy to operateOrganic chemistryBenzoic acidChloroformate
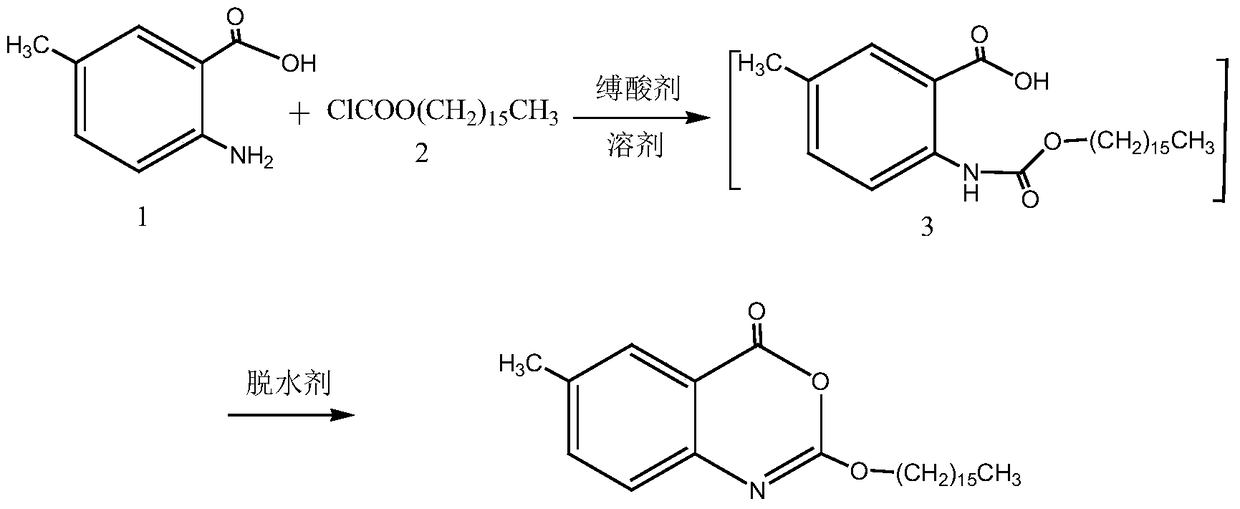
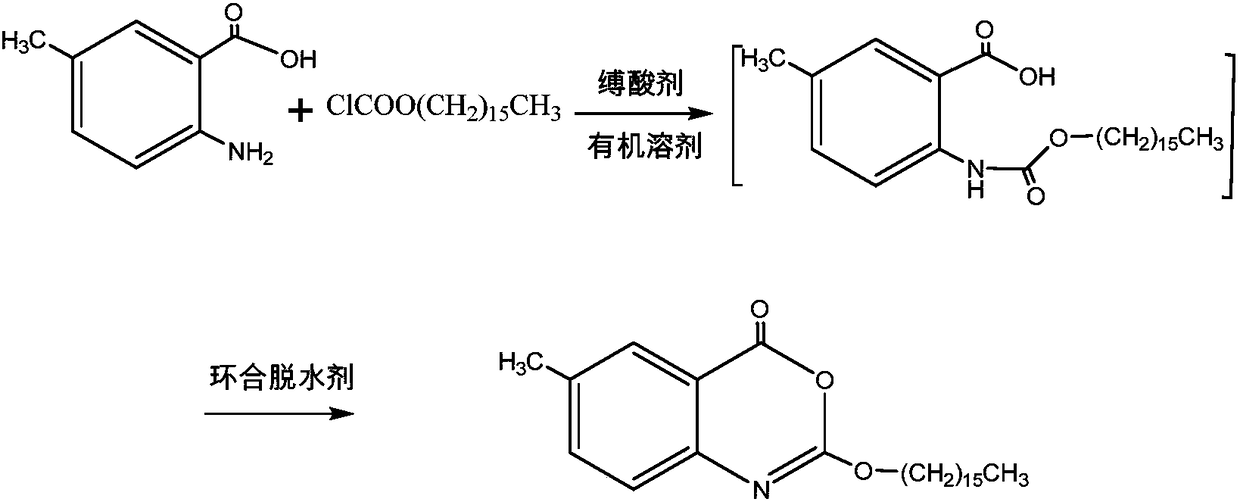
The invention discloses a preparation method of cetilistat; the method has the advantages of concise process, easily obtained raw materials, and mild reaction conditions, and is suitable for industrialized production. According to a reaction route, 2-amino-5-methyl benzoic acid and hexadecyl chloroformate serve as starting raw materials, firstly, amino acylation is carried out, an intermediate is purified, then cyclization is carried out, and the target compound is obtained through a two-step reaction.
Owner:北京修正创新药物研究院有限公司
Composition with lipase inhibitor and hydroxymethyl-glutaryl coenzyme A reductase inhibitor
The invention provides a composition with a lipase inhibitor and a hydroxymethyl-glutaryl coenzyme A reductase inhibitor, and a pharmaceutical preparation with the composition. Preferentially, the lipase inhibitor is one of orlistat and cetilistat, and the hydroxymethyl-glutaryl coenzyme A reductase inhibitor is one of atorvastatin, rosuvastatin, simvastatin, fluvastatin, pravastatin, lovastatin and pitavastatin or pharmaceutically-acceptable salt thereof. The composition can have the synergistic antibacterial effect and the synergistic histamine releasing inhibition effect, and therefore thetreatment effect and economic characteristics of bacterial infection-caused allergic disease patients can be improved.
Owner:黄泳华
Drug combination for treating or preventing fatty hyperlipidemia
InactiveCN105232554AFunction and effect enhancementLose weightOrganic active ingredientsMetabolism disorderLow density lipoprotein cholesterolObesity prevention
The invention relates to a drug combination for treating or preventing fatty hyperlipidemia, in particular to a drug combination containing orlistat or cetilistat and acipimox, and belongs to the field of pharmaceuticals. The drug combination can be solid preparations such as conventional tablets, dispersible tablets, sustained-release tablets, capsules and granules. Experiments accidentally show that a drug combination containing orlistat or cetilistat and rosuvastatin calcium has an obvious synergistic effect in the aspects of lowering serum total cholesterol, serum triglyceride and low-density lipoprotein cholesterol. A preparation method is simple, convenient to operate and suitable for industrialized production.
Owner:QINGDAO YUNTIAN BIOTECH
Cetilistat crystal
InactiveCN106032364AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryPhysical chemistryCetilistat
The present invention relates to a cetilistat crystal, which has good solubility and good stability, and is not easily subjected to crystal transition. The invention further provides a preparation method of the cetilistat crystal, wherein the method has characteristics of good reproducibility, easy condition control, high crystal yield, high crystal purity, and industrialized large-scale production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Cetilistat efficient synthesizing method
ActiveCN105884706AReduce manufacturing costHigh yieldCarbamic acid derivatives preparationOrganic compound preparationCetilistatKetone
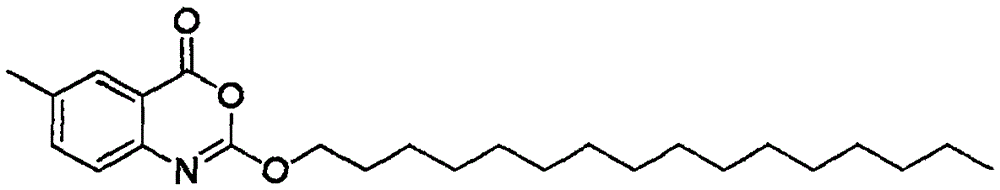
The invention provides a method for efficiently synthesizing 2-hexadecyloxy-6-methyl-4H-3,1-benzoxazine-4-ketone. A reaction is thorough and complete, the reaction time is short, the yield is high, industrial production is easy to achieve, and industrial production cost is reduced.
Owner:NANJING HEALTHNICE MEDICAL TECH +2
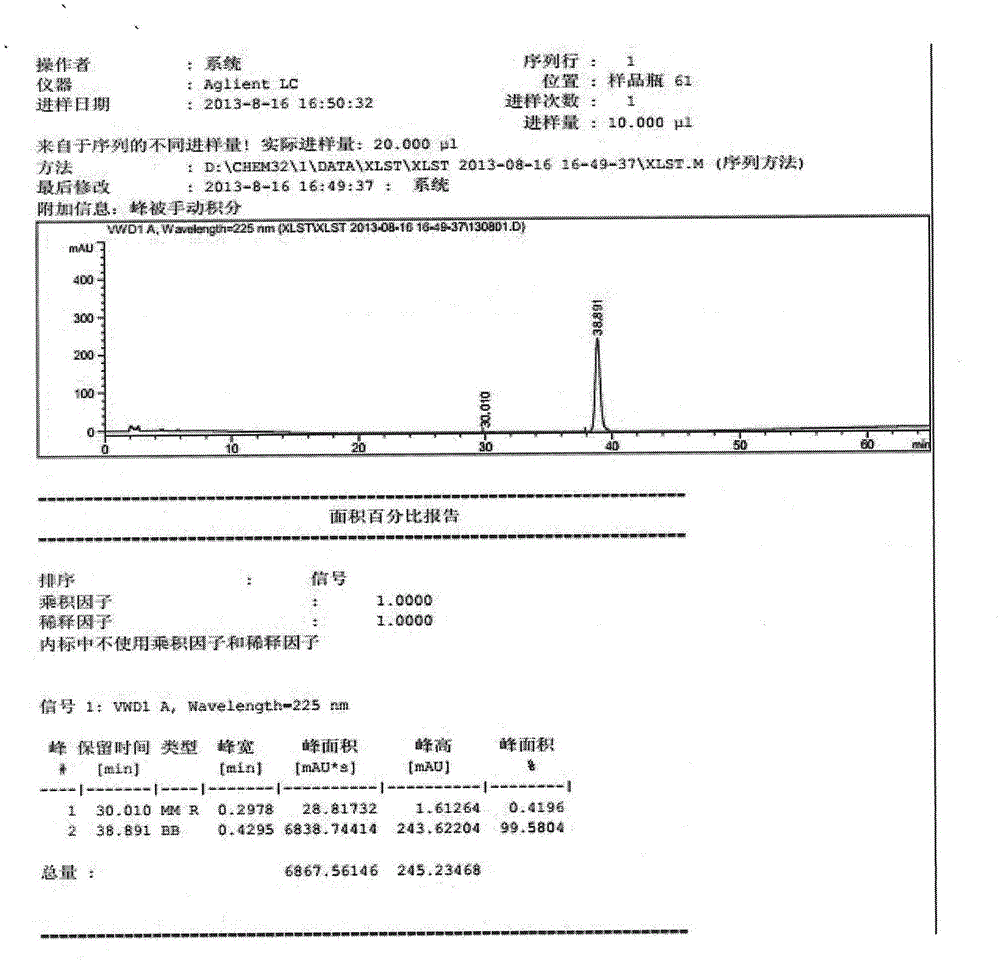
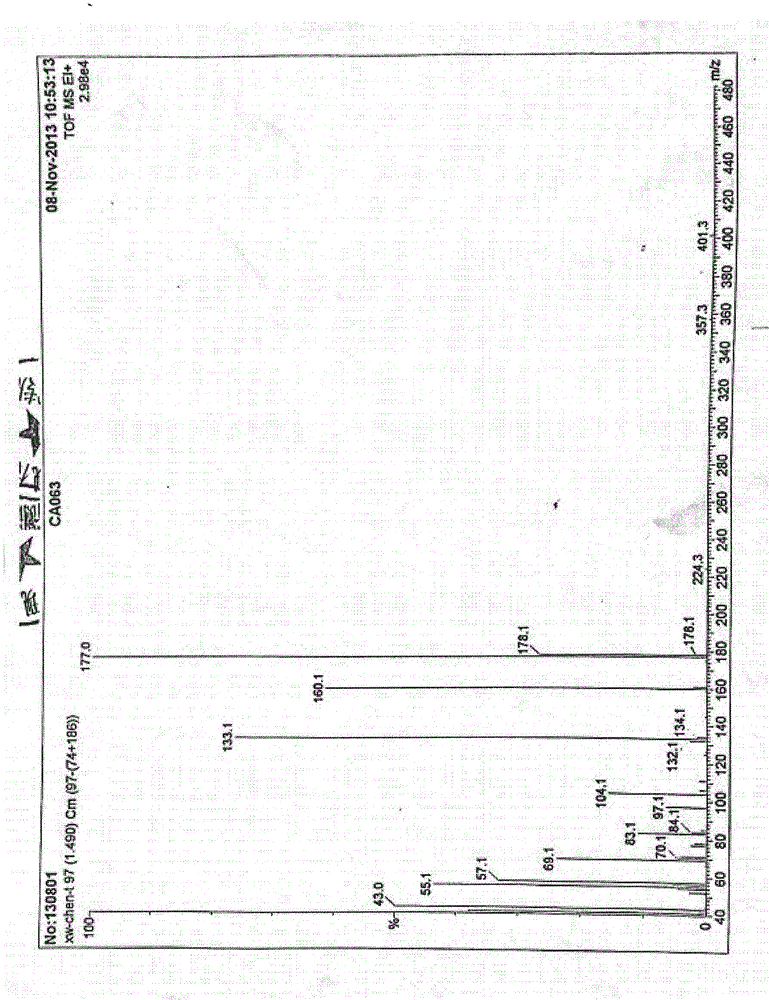
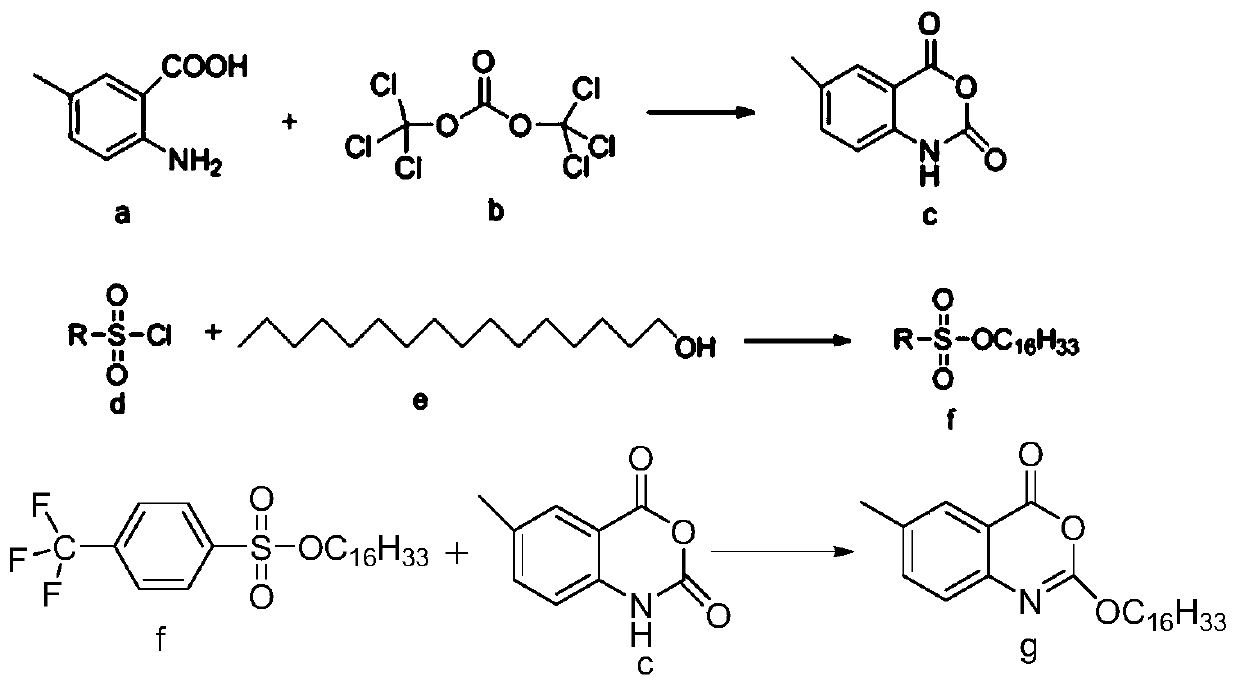
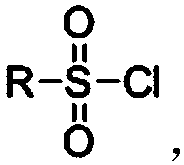
Process for efficiently synthesizing Cetilistat by taking 2-amino-5-methyl benzoic acid as raw material
The invention discloses a process for efficiently synthesizing Cetilistat by taking 2-amino-5-methyl benzoic acid as a raw material. The process comprises the steps: taking 2-amino-5-methyl benzoic acid as a raw material, and carrying out transesterification on 2-amino-5-methyl benzoic acid and triphosgene under the action of organic base catalysis to generate an intermediate c, namely 6-methyl-2,4-dihydro-1H-3,1-benzoxazine-2,4-dione; carrying out a reaction on sulfonyl chloride (d) with a specific structure with n-hexadecanol under the condition of base catalysis to generate an intermediatef; and finally, carrying out an alkylation reaction on oxygen atoms of the intermediate c and the intermediate f in the presence of organic base to generate the target product Cetilistat. According tothe method, the reaction steps for synthesizing the Cetilistat are few, the process route is short, side reactions are few, the yield of the final product is high, and the purity is more than 99%.
Owner:HEFEI UNIV OF TECH
Cetilistat crystal
InactiveCN106032363AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryPhysical chemistryCetilistat
The present invention relates to a cetilistat crystal, which has good solubility and good stability, and is not easily subjected to crystal transition. The invention further provides a preparation method of the cetilistat crystal, wherein the method has characteristics of good reproducibility, easy condition control, high crystal yield, high crystal purity, and industrialized large-scale production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Eutectic compound formed by lipase inhibitor and hydroxy methylglutaryl coenzyme A reductase inhibitor
The invention provides a eutectic compound formed by a lipase inhibitor and a hydroxy methylglutaryl coenzyme A reductase inhibitor and composition containing the eutectic compound. The eutectic compound is characterized in that the lipase inhibitor is selected from one of orlistat and cetilistat; the hydroxy methylglutaryl coenzyme A reductase inhibitor is selected from one of atorvastatin, rosuvastatin, simvastatin, fluvastatin, pravastatin, lovastatin and pitavastatin or pharmacologically acceptable salts thereof. The eutectic compound can simultaneously have synergistic antibacterial action and synergistic histamine release inhibition function, so that the treatment effect on patients with allergic diseases caused by bacterial infection and the economic characteristics can be improved.
Owner:黄泳华
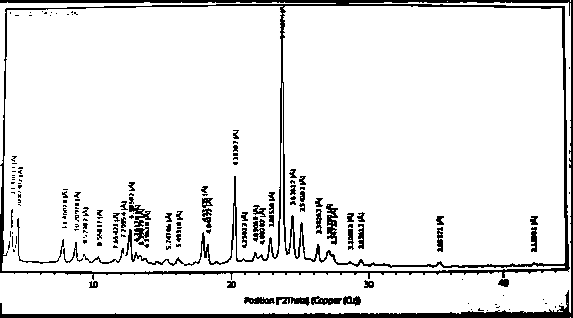
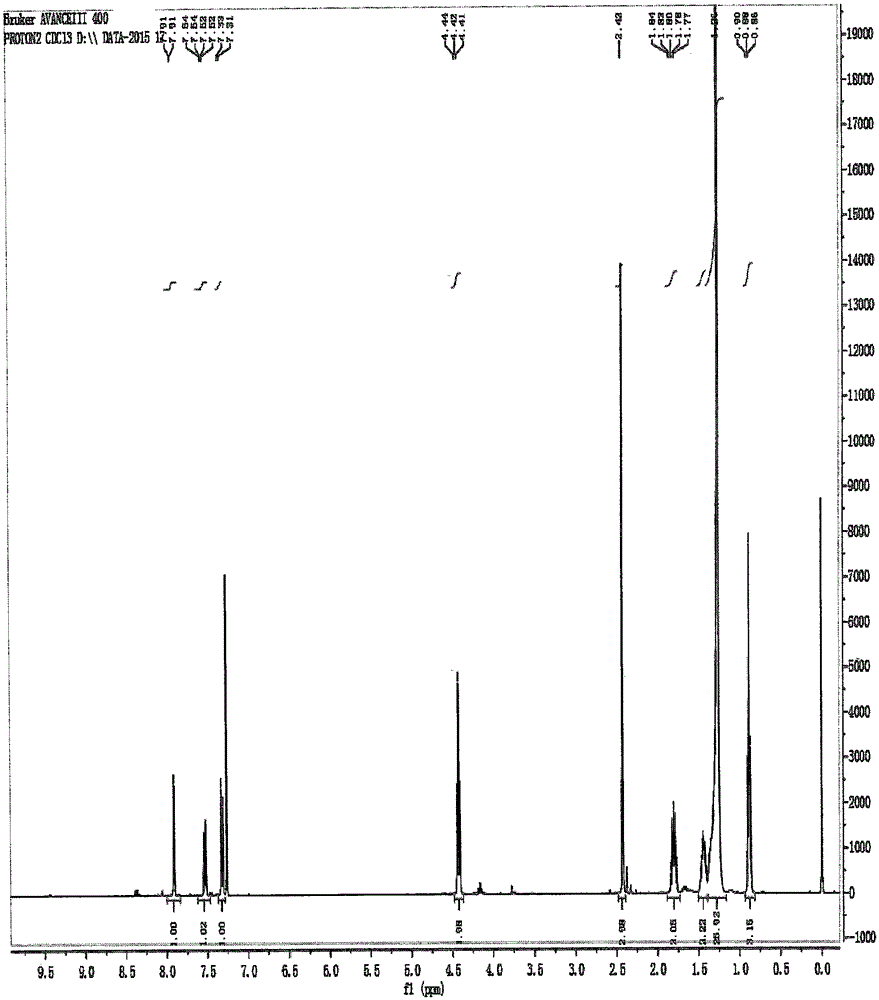
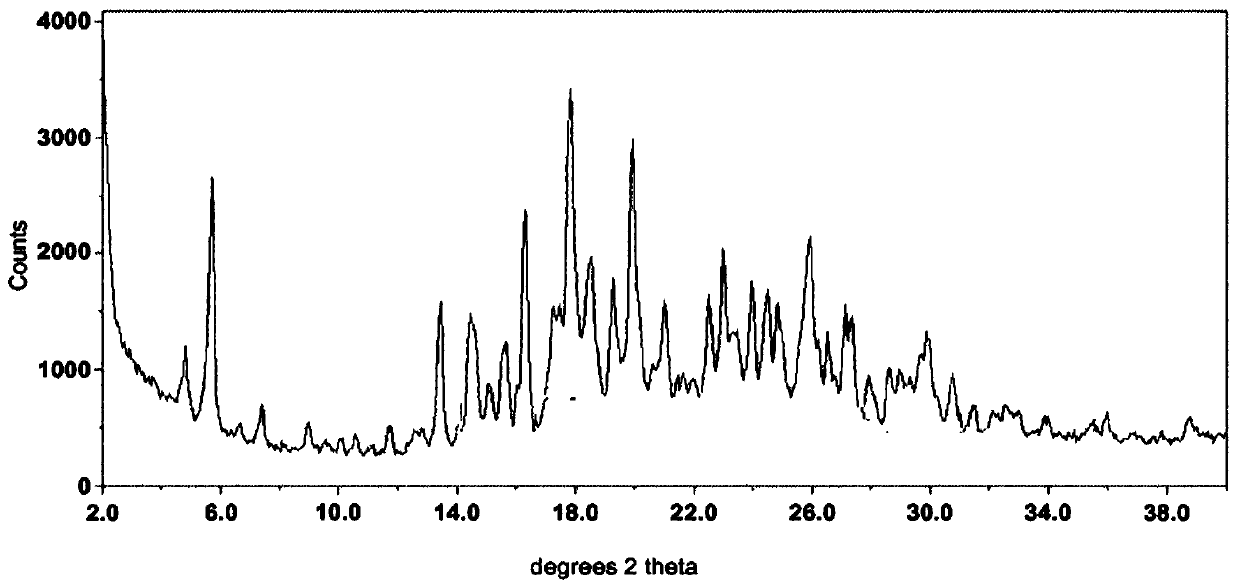
Novel crystal form cetilistat and preparation method thereof
InactiveCN105111162AImprove stabilityMetabolism disorderOrganic chemistry methodsX-rayCombinatorial chemistry
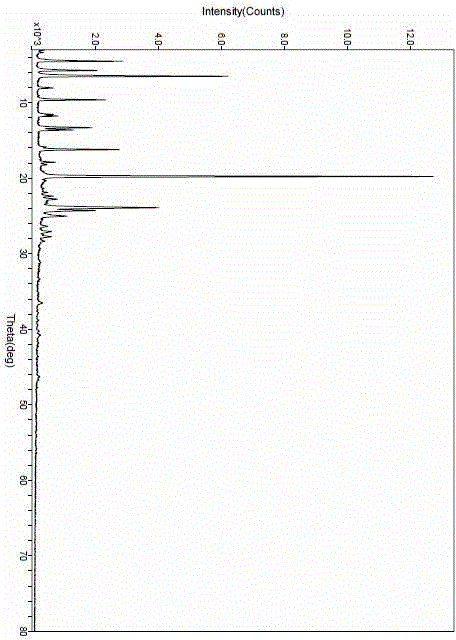
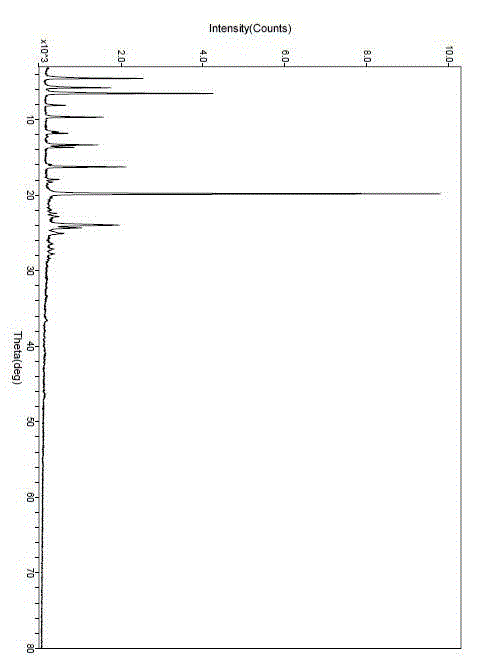
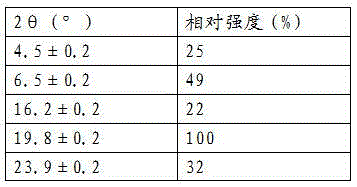
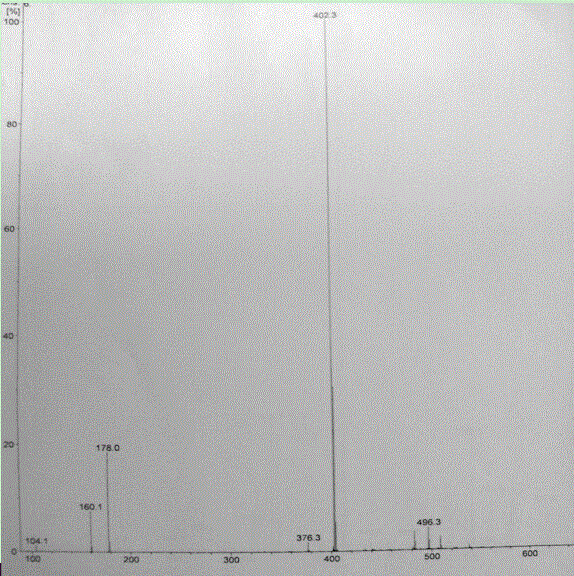
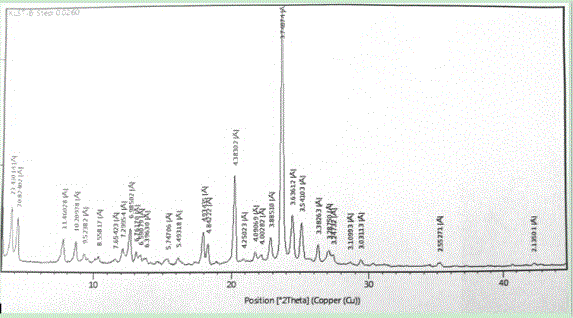
The invention relate to a crystal form of cetilistat, in particular to novel crystal form cetilistat and a preparation method thereof. The novel crystal form cetilistat is characterized in that X-ray powder diffraction expressed via Cu-Ka radiation at an angle 2Theta has characteristic peaks at 3.77+ / -0.2, 4.25+ / -0.2, 7.71+ / -0.2, 8.67+ / -0.2, 10.59+ / -0.2 and 23.7.71+ / -0.2. The invention further provides a preparation method of the novel crystal form cetilistat; the preparation method is simple, is easy to operate, good in reproducibility and high in purity, takes short time and consumes lower energy under dryness condition, and a solvent for use is low in toxicity. The novel crystal form cetilistat has better stability and is more suitable for uses in the predation of compositions used for treating obesity.
Owner:JINAN KANGHE MEDICAL TECH
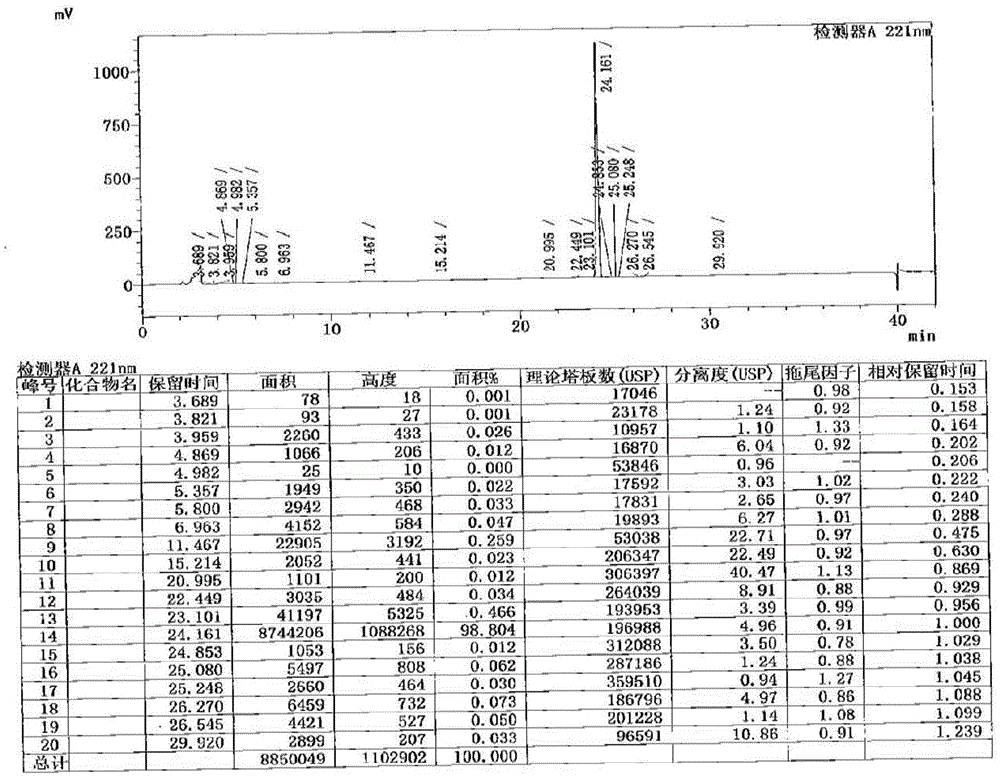
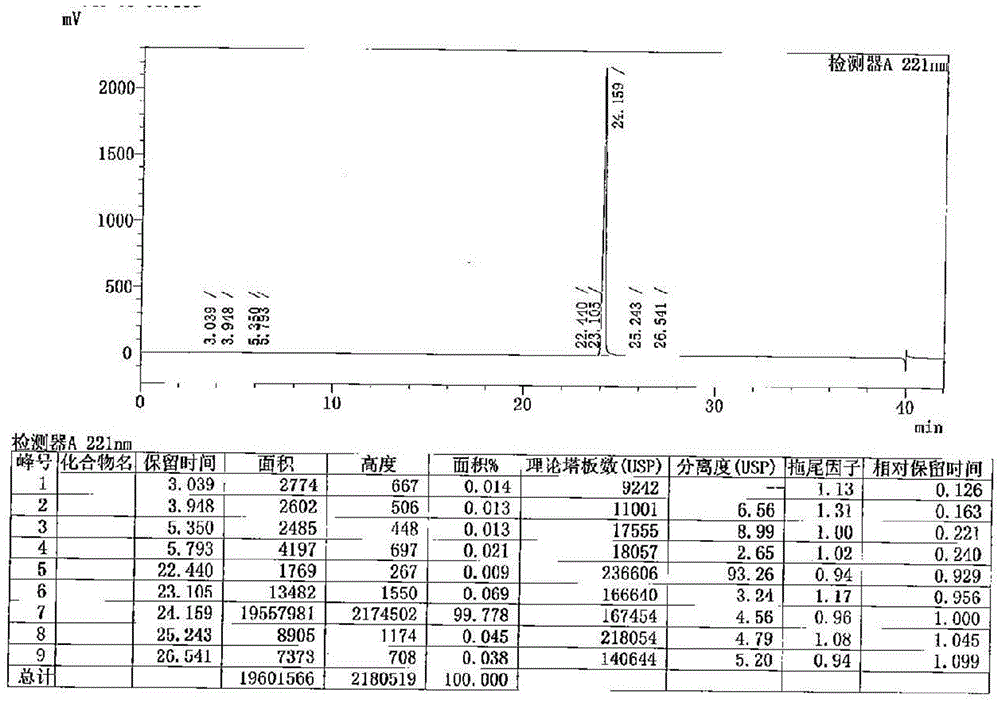
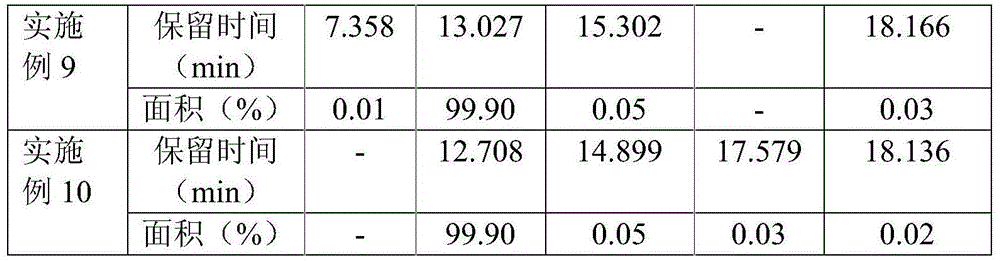
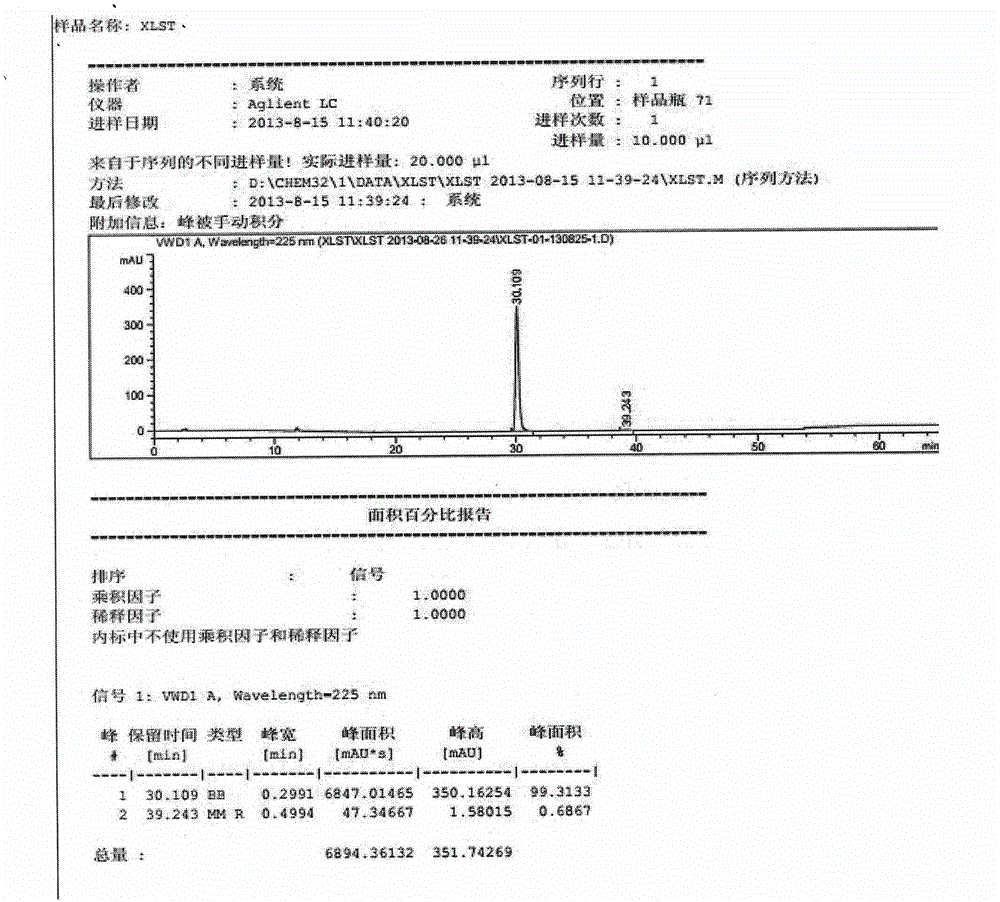
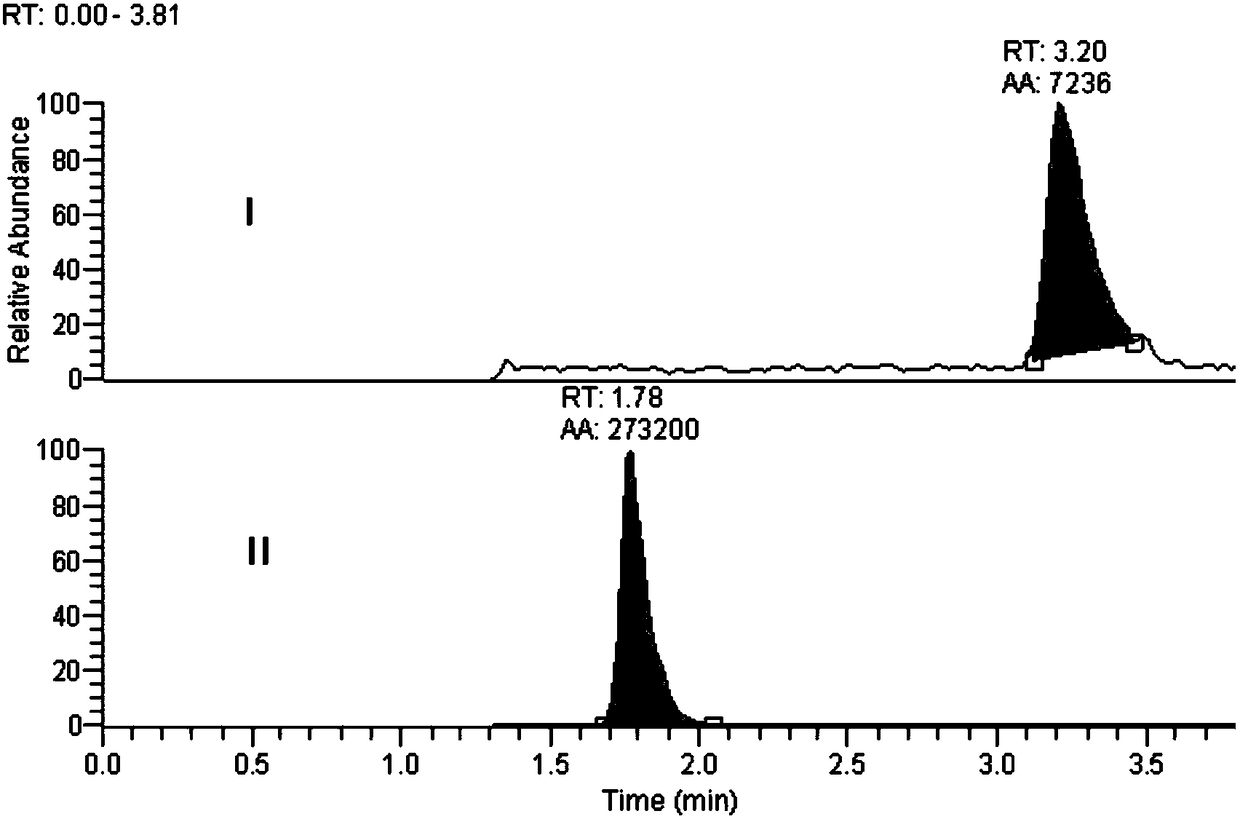
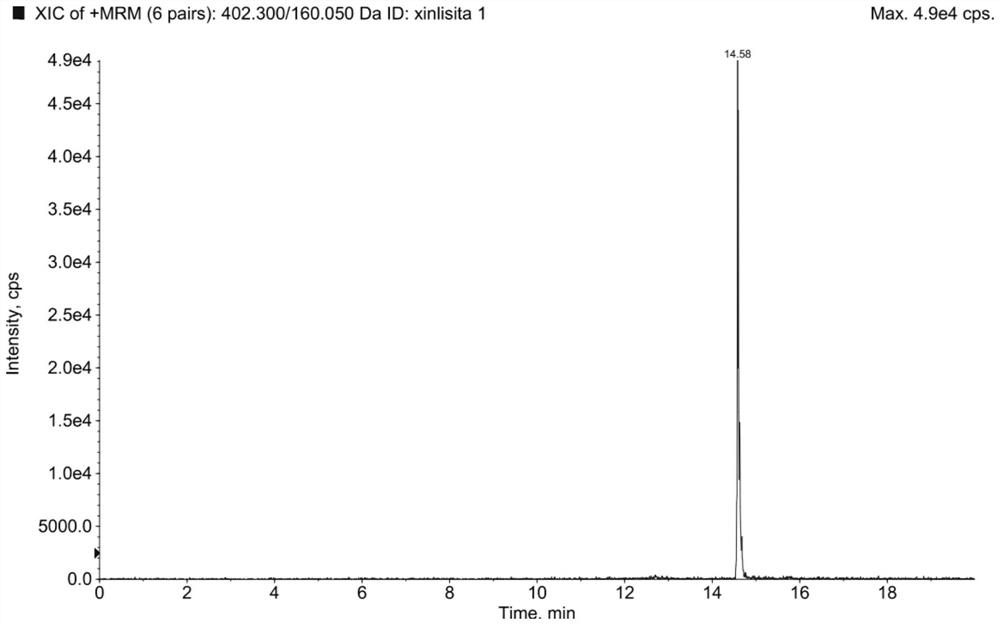
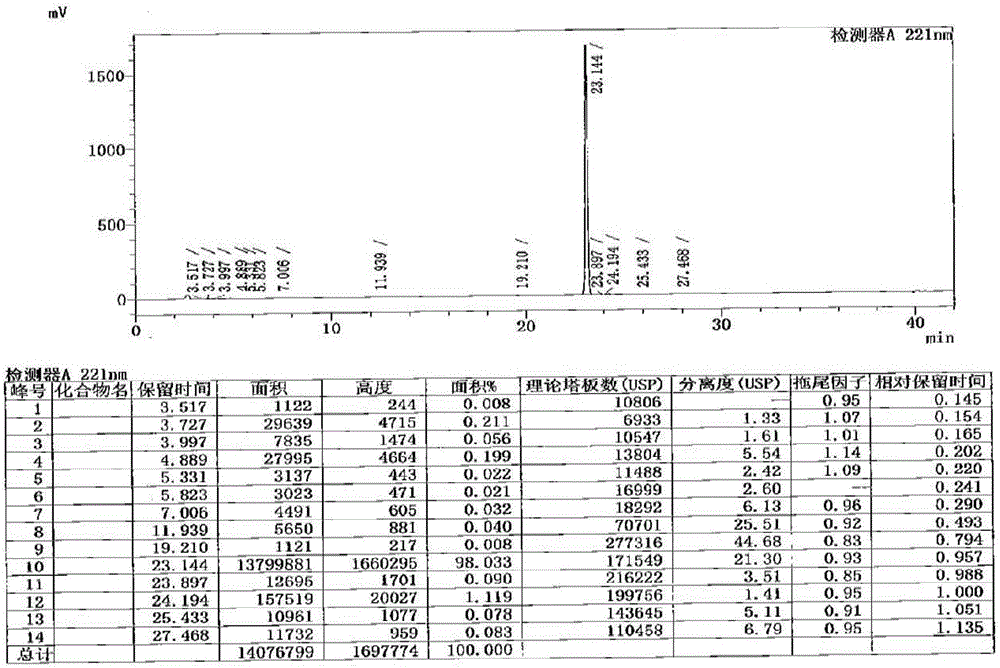
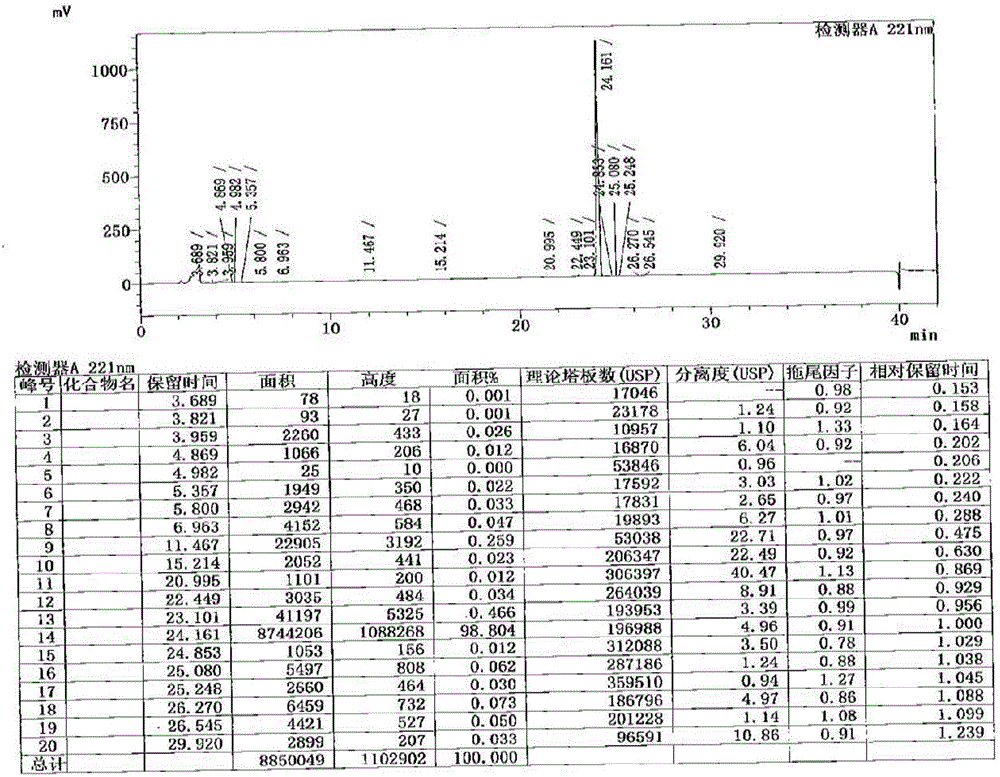
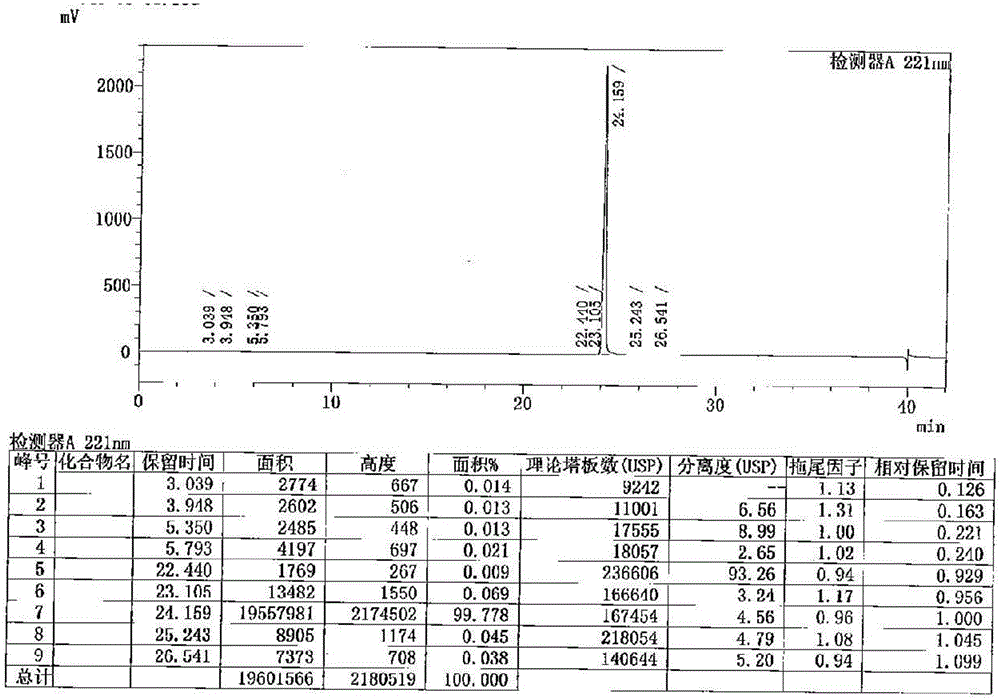
Method for determination of impurity A in Cetilistat in biological sample
ActiveCN109298081AAdvantages of assay methodImprove stabilityComponent separationBiological testingChemistryBiological organism
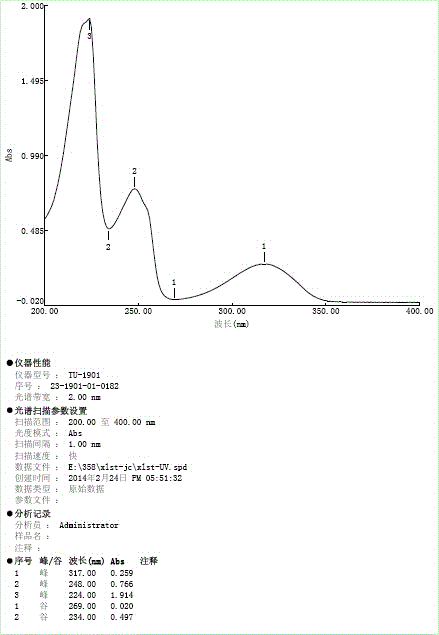
The invention relates to a method for determination of an impurity A in Cetilistat in a biological sample, and belongs to the technical field of medicines. The method adopts ultra-high performance liquid chromatography-electrospray ionization tandem mass spectrometry (UPLC-MS / MS) to detect the concentration of the Cetilistat impurity A in the biological sample. The established LC-MS / MS determination method for a Cetilistat impurity A plasma sample meets the analyzing requirement of 2015 edition of Chinese pharmacopoeia and (chemical drug non-clinical pharmacokinetics research technique governing principle) issued by SFDA in 2014 for the biological sample in the aspects of accuracy, precision, specialization, stability, the extraction recovery rate, the matrix effect and the like; and a powerful guarantee is provided for development of new drugs of Cetilistat and guidance of clinical rational drug use.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of method for preparing cetirizat
Owner:CHONGQING TOPTECH PHARMA TECH
Medical composition for losing weight or treating hyperlipidemia
ActiveCN101756990BReduce adverse reactionsReduce drug riskOrganic active ingredientsMetabolism disorderSustained Release TabletChemical composition
The invention discloses a medical composition for losing weight or treating hyperlipidemia, belonging to the field of medicines, and in particular relates to a medical composition containing orlistat or cetilistat and rosuvastatin calcium, wherein the composition is solid preparations such as compressed tablets, dispersible tablets, sustained release tablets, capsules, granules, and the like. In experiments, the medical composition containing the orlistat or the cetilistat and the rosuvastatin calcium is accidentally found to have obvious synergistic effect on the aspects of reducing the serum total cholesterol, the serum triglyceride and the low density lipoprotein cholesterin.
Owner:LUNAN PHARMA GROUP CORPORATION
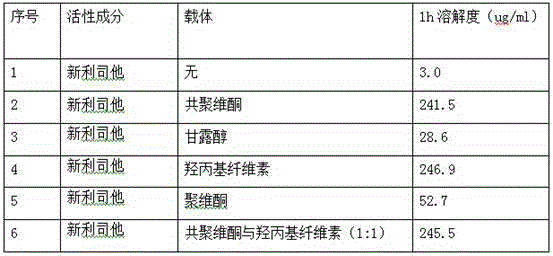
Neolistat solid dispersion and pharmaceutical preparation thereof
The invention relates to cetilistat solid dispersion, and a medicinal preparation prepared from the solid dispersion. The cetilistat and one or both of the carriers hydroxypropyl cellulose and copovidone are prepared into a solid dispersion to significantly improve the solubility of the cetilistat, and overcome low solubility of cetilistat, so that the prepared medicinal preparation achieves good dissolution, and has increased bioavailability.
Owner:BEIJING WINSUNNY PHARMA CO LTD
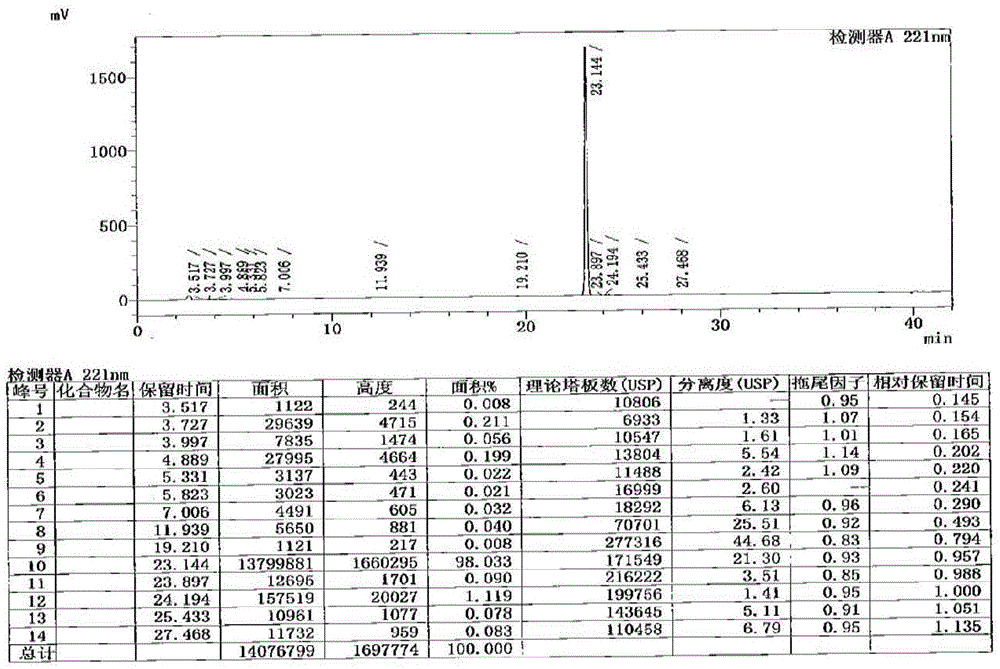
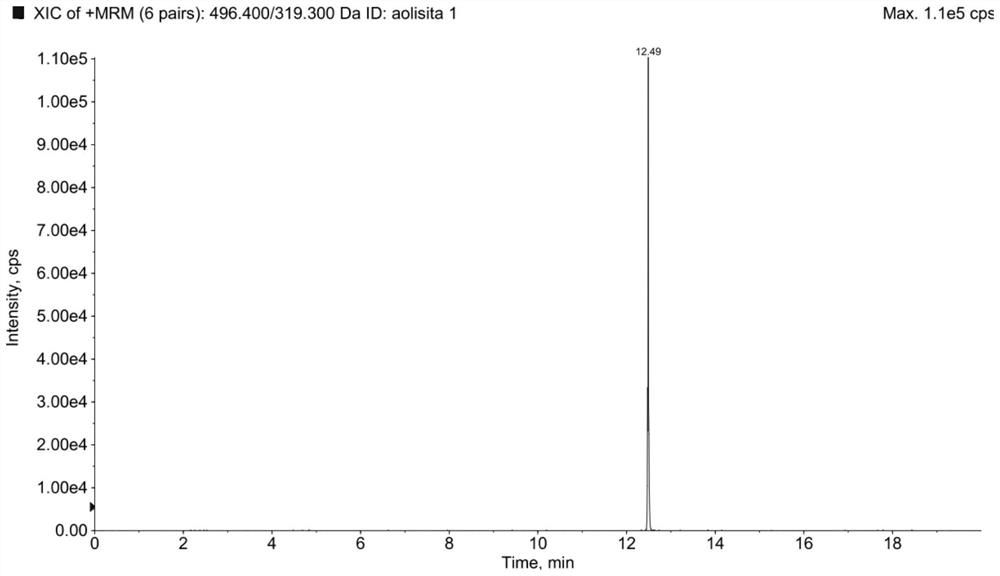
Method for detecting cetilistat in weight-losing health-care product
PendingCN114720597ARealize separation detectionMeet the requirements of the methodologyComponent separationFluid phaseMass Spectrometry-Mass Spectrometry
The invention relates to a method for detecting cetilistat in a weight-losing health-care product. The method comprises the following steps: providing a reference substance solution, wherein a reference substance in the reference substance solution contains cetilistat; taking a weight-losing health-care product sample, extracting with methanol, collecting an extracting solution, and preparing a test solution; and detecting the reference solution and the test solution by adopting a high performance liquid chromatography-tandem mass spectrometry method, and determining whether the weight-losing health-care product sample contains the cetilistat or / and the content of the cetilistat according to a detection result. According to the method disclosed by the invention, the separation and detection of the cetilistat in the weight-losing health-care product are realized by adopting a proper extraction solvent and matching with proper detection conditions, the technical blank of the detection of the cetilistat in the weight-losing health-care product is filled, and a theoretical basis is laid for applying a high performance liquid chromatography-tandem mass spectrometry technology to the detection of the cetilistat in the weight-losing health-care product.
Owner:GUANGZHOU INSPECTION TESTING & CERTIFICATION GRP CO LTD
A kind of new lixistat pellet and preparation method thereof
ActiveCN103393608BWarrantyRapid dissolutionOrganic active ingredientsMetabolism disorderCetilistatDissolution
The invention discloses a cetilistat pellet and a preparation method thereof. The pellet comprises a pill core, then the pill core is loaded with drugs to prepare a cetilistat drug-loaded pill, and finally the pill is coated with a gastric-soluble coating layer. The cetilistat pellet prepared by the preparation method not only effectively maintains the original properties of the citilistat, but also guarantees the quick and even dissolution of drugs, and the pellet is capable of being prepared into various common dosage forms in pharmacy.
Owner:杭州高成生物营养技术有限公司
A kind of preparation method of cetirizat
The invention discloses a preparation method of cetiristat, which has the advantages of high yield, mild reaction conditions, and easy industrial production. The steps are as follows: (1) methyl 2-amino-5-halobenzoate and Sanko gas reaction to obtain 2-methoxycarbonyl-4-halogenated phenyl isocyanate; then add cetyl alcohol to generate 2-(hexadecyloxycarbonylamino)-5-halogenated methyl benzoate; (2) 2-( Hexadecyloxycarbonylamino)-5-halobenzoic acid methyl ester, and methyl boronic acid, alkali join in water and organic solvent, add palladium catalyst, reaction obtains 2-(hexadecyloxycarbonylamino)-5-methyl (3) Methyl 2-(hexadecyloxycarbonylamino)-5-methylbenzoate undergoes ester hydrolysis reaction to obtain 2-(hexadecyloxycarbonyl)-5-methylbenzoic acid ; (4) Suspend 2-(hexadecyloxycarbonylamino)-5-methylbenzoic acid in pyridine, and close the ring under the action of a dehydrating agent; purify and crystallize to obtain cetilistat.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
Method for preparing important intermediate 2-(hexadecyloxy carbonyl)-amino-5-methylbenzoic acid of Cetilistat
InactiveCN105646287AAddress the impact of low conversion ratesImprove conversion rateCarbamic acid derivatives preparationOrganic compound preparationCetilistatChloroformate
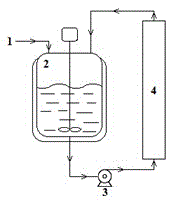
The invention relates to a method for preparing an important intermediate 2-(hexadecyloxy carbonyl)-amino-5-methylbenzoic acid (I) of Cetilistat and mainly aims to eliminate the influence of low conversion rate of a reactant hexadecyl chloroformate (II) by water produced in a compound I production process in the prior art. According to the method, an external-circulating water removing device is adopted to remove water produced in a nucleophilic substitution reaction of a compound II and 2-amino-5-methylbenzoic acid (III) in time, so that the conversion rate of the reaction material compounds II and III is greatly increased, simple and fast continuous production with low energy consumption is realized, the yield of the reaction product I is higher than 90%, and the chemical purity is higher than 99%.
Owner:NANJING UNIV OF TECH
A kind of preparation method of cetirizat
The invention provides a method for preparing cetilistat. According to the method, 2-amidogen-5-methyl benzoic acid is adopted as an initial raw material, under existence of pyridine, the 2-amidogen-5-methyl benzoic acid reacts with chlorine acid cetyl alcohol ester in the first place, a midbody 2-(((hexadecane oxygroup) carbonyl) amidogen)-5-methyl benzoic acid is obtained, then dehydrogenation cyclization reagent is utilized to obtain the cetilistat, and the feeding sequence in step 1 is the 2-amidogen-5-methyl benzoic acid, alkali and the chlorine acid cetyl alcohol ester in sequence. The method has the remarkable advantages that the route is simple, operation is less, atom economy is better than that of other routes, and the cetilistat is suitable for large-scale production; the purity of the cetilistat at the reaction endpoint is larger than 98%, and through simple postprocessing, a final product which meets medical standards can be obtained, wherein the purity is larger than 99.5%, and the single impurity is smaller than 0.1%; the utilized solvent including dichloromethane and pyridine can be recycled for mechanical application, and the method is environmentally friendly.
Owner:ZHONGSHAN WANHAN PHARM CO LTD +1
A kind of one-pot method prepares the method for new lixistat
The invention discloses a method of preparing Cetilistat through a one-pot method. The method comprises the following steps that 2-amino-5-toluic acid is sequentially reacted with cetyl chloroformate and methane sulfonyl chloride in a mixed solution, and the Cetilistat is obtained through the one-pot method. According to the method, with selection of a mixed solution system as a reaction medium and the methane sulfonyl chloride as a lactonization reagent and adoption of the one-pot method to prepare the Cetilistat, an obtained product is high in yield and purity; the process is simple and not tedious, separation and purification of an intermediate are not needed, postprocessing is liable to operate, requirement on equipment is not high, the industrial production is facilitated.
Owner:LUNAN PHARMA GROUP CORPORATION
Medical composition for losing weight or treating metabolic syndromes
The invention discloses a medical composition for treating metabolic syndromes, belonging to the field of medicines, and in particular relates to a medical composition containing orlistat or cetilistat and heparin, wherein the composition is solid preparations such as compressed tablets, dispersible tablets, sustained release tablets, capsules, granules, and the like. In experiments, the medical composition containing the orlistat or the cetilistat and the heparin is accidentally found to have obvious synergistic effect on the aspects of reducing the blood pressure, the serum total cholesterol, the serum triglyceride and the low density lipoprotein cholesterin and enhancing the carbohydrate tolerance.
Owner:LUNAN PHARMA GROUP CORPORATION
Medical composition for losing weight or treating metabolic syndromes
ActiveCN101756993BReduce adverse reactionsReduce drug riskOrganic active ingredientsMetabolism disorderTG - TriglycerideLost Weight
The invention discloses a medical composition for treating metabolic syndromes, belonging to the field of medicines, and in particular relates to a medical composition containing orlistat or cetilistat and actos, wherein the composition is solid preparations such as compressed tablets, dispersible tablets, sustained release tablets, capsules, granules, and the like. In experiments, the medical composition containing the orlistat or the cetilistat and the actos is accidentally found to have obvious synergistic effect on the aspects of reducing the blood pressure, the serum total cholesterol, the serum triglyceride and the low density lipoprotein cholesterin and enhancing the carbohydrate tolerance.
Owner:LUNAN PHARMA GROUP CORPORATION
One-pot method for high-yield preparation of new lisstat
The invention relates to a new process for one-pot preparation of cetilistat; the one-pot reaction is adopted, separation steps are sample, the product yield is high, the product quality is good, and the new process is especially suitable for industrialized production.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com