Cetilistat solid dispersion and medicinal preparation prepared from the solid dispersion
A solid dispersion, the technology of new Lilistat, applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of affecting drug efficacy, low bioavailability, Problems such as low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
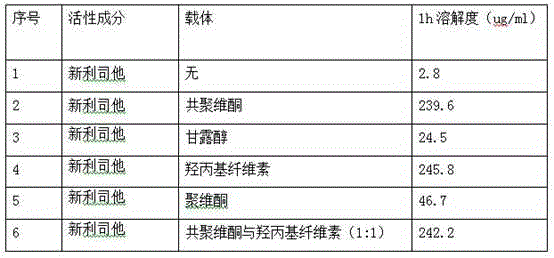
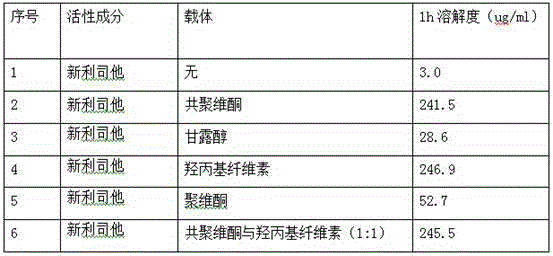
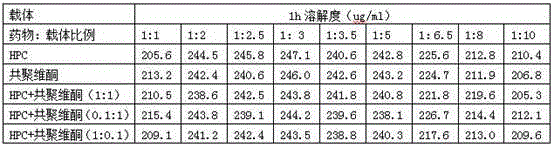
[0059] Mix 120mg of Neolistat and 240mg of hydroxypropyl cellulose evenly, heat in an oil bath, raise the temperature to 120°C, the mixture melts into a viscous shape, and cool rapidly; crush the cooled mixture and pass through a 60-mesh sieve to obtain Solid dispersion.
Embodiment 2
[0061] Dissolve 120 mg neolistat and 120 mg copovidone in 150 ml acetone, and dry in vacuum to obtain a solid dispersion.
Embodiment 3
[0063] Mix 120mg of Neolistat, 300mg of hydroxypropyl cellulose, and 300mg of copovidone evenly, heat in an oil bath, and raise the temperature to 110°C. 60 mesh sieve to obtain a solid dispersion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com