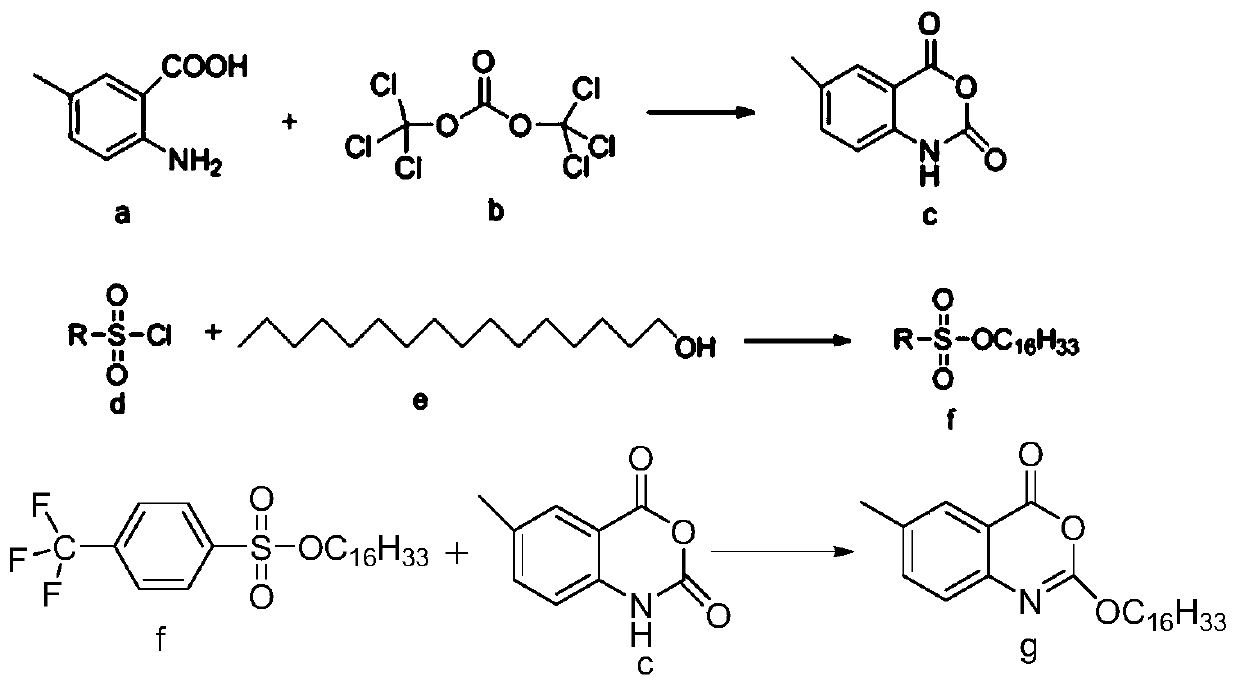
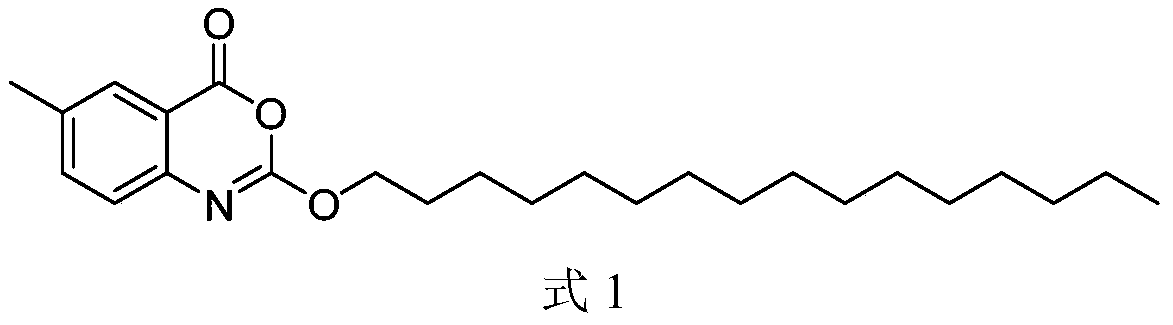
Process for efficiently synthesizing Cetilistat by taking 2-amino-5-methyl benzoic acid as raw material
A technology of methyl benzoic acid and raw materials, which is applied in the field of organic synthesis, can solve the problems of large-scale industrial production limitations, difficult separation and purification of intermediates, and difficult industrial production, etc., and achieve the effect of fewer reaction steps, short reaction routes, and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Synthesis of intermediate c (6-methyl-2,4-dihydro-1H--3,1-benzoxazine-2,4-dione)
[0055] Put 2-amino-5-methylbenzoic acid (4g, 0.026mol) into a 250ml reaction flask, add 150ml of acetonitrile, heat the oil bath to 52°C, and dissolve triphosgene (2.57g, 0.0087mol) with 40ml of acetonitrile , and slowly drop triphosgene and pyridine (4.1g, 0.052mol) into the reaction flask at the same time, stir at 52°C for 5h, remove the solvent under reduced pressure, add H 2 O (100ml), suction filtered, the filter cake was washed with water and glacial dichloromethane successively, and dried in vacuo to obtain 4.27g of the product with a yield of 93%. 1 H NMR (400MHz, d 6 -DMSO): δ11.63(s,1H),7.72(s,1H),7.58.7.55(dd,J 1 =4.0Hz,J 2 =8.0Hz, 1H), 7.06(d, J=8.0Hz, 1H), 2.33(s, 3H)
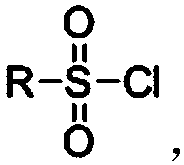
[0056] 2, the preparation of intermediate f (sulfonate)
[0057] Add cetyl alcohol (5g, 0.021mol) in a 250ml reaction flask, dissolve in 150ml of dichloromethane, then add p-trifluoromethylbenzenesulfo...
Embodiment 2
[0061] 1. Synthesis of intermediate c (6-methyl-2,4-dihydro-1H--3,1-benzoxazine-2,4-dione)
[0062] Put 2-amino-5-methylbenzoic acid (4g, 0.026mol) into a 250ml reaction flask, add 150ml of acetonitrile, and dissolve triphosgene (2.57g, 0.0087mol) with 40ml of acetonitrile, and slowly drop it into the reaction flask Add triphosgene (2.57g, 0.0087mol) and pyridine (4.1g, 0.052mol), at room temperature for 5h, after removing the solvent under reduced pressure, add H 2 O (100ml), suction filtered, the filter cake was washed with water and ice dichloromethane successively, and dried in vacuo to obtain 3.0g of the product with a yield of 65%. 1 H NMR (400MHz, d 6 -DMSO): δ11.63(s,1H),7.72(s,1H),7.58.7.55(dd,J 1 =4.0Hz,J 2 =8.0Hz,1H),7.06(d,J=8.0Hz,1H),2.33(s,3H),1.69-1.64(m,3H),1.33-1.15(m,25H),0.84(t,J= 7.0Hz, 3H)
[0063] The synthesis of intermediate f and final product g is the same as in Example 1.
Embodiment 3
[0065] 1. The synthesis of intermediate c is the same as in Example 1;
[0066] 2, the preparation of intermediate f (sulfonate)
[0067] Add p-hexadecanol (5g, 0.021mol) into a 250ml reaction flask, add 150ml of dichloromethane to dissolve, then add p-toluenesulfonyl chloride (5.5g, 0.028mol), pyridine (4.8g, 0.0616mol) in sequence , DMAP (0.18g, 1.5mmol), after dropwise addition, heat up and reflux for 12h, after the reaction is completed, use successively the hydrochloric acid of 1mol / L of 50ml, the 1% NaOH of 50ml, the water washing reaction solution of 50ml to neutrality, The liquid was separated, and the aqueous layer was sequentially extracted three times with 40 ml of dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate, filtered with suction, and the solvent was removed in vacuo to obtain 5.95 g of off-white solid with a yield of 72%. 1 H NMR (400MHz, CDCl 3 ):d 7.78(d, J=8.4Hz, 2H), 7.34(d, J=8.0Hz, 2H), 4.02(t, J=6.4Hz, 2H), 2.44(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com