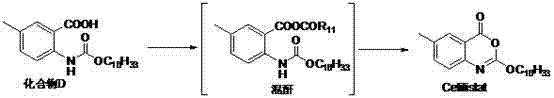
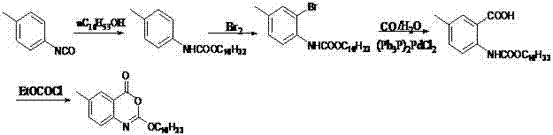
Method for preparing cetilistat
A technology of risstat and compound, which is applied in the field of synthesizing cetiristat, can solve the problems of high cost, difficult reaction conditions, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Put compound A (45.3g, 0.3mol) into a 1L reaction flask, add 500ml of methanol, add 30ml of concentrated sulfuric acid dropwise under stirring, stir overnight at room temperature, concentrate and recover most of the methanol, add 200ml of acetone, and stir in an ice bath for 2h. Suction filtration and drying gave 73.4 g of 2-amino-5-methylbenzoic acid methyl ester hydrogen sulfate, with a yield of 93%.
Embodiment 2
[0063] Put compound A hydrochloride (56.3g, 0.3mol) into a 1L reaction flask, add 600ml tetrahydrofuran and tert-butanol (26.7g, 0.36mol), add DCC (61.9g, 0.3mol) in batches under stirring, and Stir for 2h, then add DMPA (0.5g), stir overnight, concentrate and recover most of THF, add 600ml ethyl acetate and 30g sodium bicarbonate, stir for 1h, filter with suction, the filtrate is sequentially washed with 1% acetic acid solution, water and saturated saline Fully stirred, washed, separated, concentrated under reduced pressure to a slurry, added 500ml of petroleum ether, stirred for 2h in an ice bath, filtered with suction, and dried to obtain 55.9g of tert-butyl 2-amino-5-methylbenzoate. The yield was 90%.
Embodiment 3
[0065] Put compound A (45.3g, 0.3mol) into a 1L reaction flask, add 500ml of toluene and thionyl chloride (53.6g, 0.45mol), heat up to reflux, stir for 2h, cool down to room temperature, drop benzyl alcohol (43.2g , 0.4mol), stirred overnight at room temperature, concentrated most of the solvent under reduced pressure to form a slurry, stirred for 2h under ice bath, filtered with suction, and dried to obtain 79.2g of benzyl 2-amino-5-methylbenzoate hydrochloride , The yield is 95%.
[0066] Preparation of Compound C:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com