A kind of preparation method of cetirizat
A technology of lixisenatide and hexadecane, which is applied to the preparation of cetilistat and the fields of drug preparation, can solve the problems of unfavorable industrial production, harsh reaction conditions, and high equipment requirements, and achieves easy industrial production, convenient preparation, and equipment requirements. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
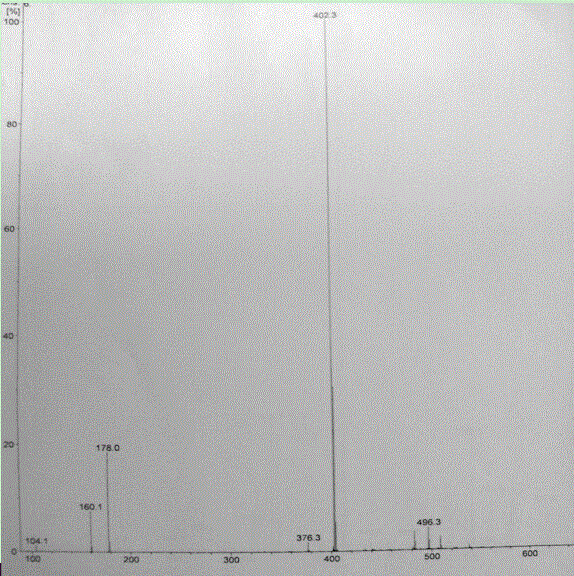
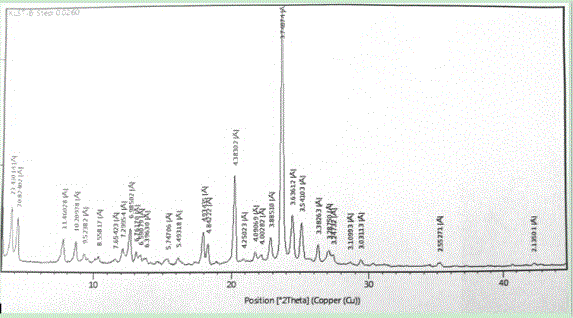
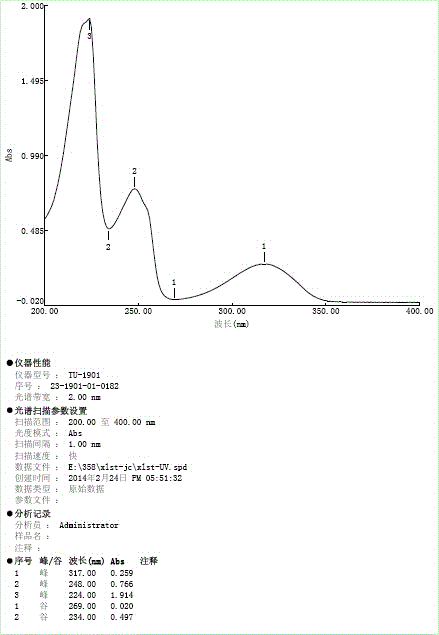
Image
Examples
Embodiment 1
[0053] Embodiment 1: Preparation of 2-(hexadecyloxycarbonylamino)-5-bromobenzoic acid methyl ester
[0054]
[0055] Add 4.9g of triphosgene to 50mL of dichloromethane, cool to 0°C, add dropwise a solution of 2-amino-5-bromobenzoic acid methyl ester (5g) and triethylamine (13.8mL) in dichloromethane (20mL) After the dropwise addition, keep at 0°C for 15 minutes, rise to room temperature and stir for 2 hours.
[0056] 5.26 g of cetyl alcohol was added to the above reaction liquid, and reacted at room temperature for 2 h. After the reaction is completed, filter, the filtrate is vacuum concentrated and spin-dried, the residue is beaten and washed with anhydrous methanol, filtered, and the filter cake is dried to constant weight. 9.1 g of white powder solid was obtained, which was methyl 2-(hexadecyloxycarbonylamino)-5-bromobenzoate; yield: 85%.
Embodiment 2
[0057] Embodiment 2: Preparation of 2-(hexadecyloxycarbonylamino)-5-methylbenzoate
[0058]
[0059] Under nitrogen protection, 10g of methyl 2-(hexadecyloxycarbonylamino)-5-bromobenzoate was dissolved in 1,4-dioxane (50mL) and water (5mL), and 11g of anhydrous potassium carbonate was added , 1.44g methylboronic acid, 0.731g Pd(dppf) 2 Cl 2 , and the mixture was reacted at 105°C for 3 hours. After the reaction is complete, cool down, filter, spin the filtrate to dry, wash the residue with anhydrous methanol, filter, and dry the filter cake to obtain 6.5 g of gray solid, which is 2-(hexadecyloxycarbonylamino)-5-methylbenzoic acid Methyl ester, yield 75%.
Embodiment 3
[0060] Embodiment 3: the preparation of 2-(hexadecyloxycarbonylamino)-5-methylbenzoic acid
[0061]
[0062] Add 7g of methyl 2-(hexadecyloxycarbonylamino)-5-methylbenzoate to a mixture of 35mL of tetrahydrofuran and 7mL of water, add 20.1g of lithium hydroxide, and react at 60°C for 3h. After the reaction was completed, the reaction solution was concentrated, the residue was added to 70 mL of ice water, the pH was adjusted to 7 with 6M hydrochloric acid, filtered, and the filter cake was dried to constant weight to obtain 6.2 g of a gray solid, namely 2-(hexadecyloxycarbonylamino)-5 -Methylbenzoic acid, yield 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com