Medical composition for losing weight or treating metabolic syndromes
A technology for metabolic syndrome and composition, applied in the directions of drug combination, metabolic disease, pharmaceutical formulation, etc., can solve the problems of enhancing insulin resistance, no report of orlistat, no report of the synergistic effect of the two drugs, etc., to achieve enhanced diabetes. The effect of good blood pressure, systolic blood pressure and pulse pressure difference, and systolic blood pressure and pulse pressure difference reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
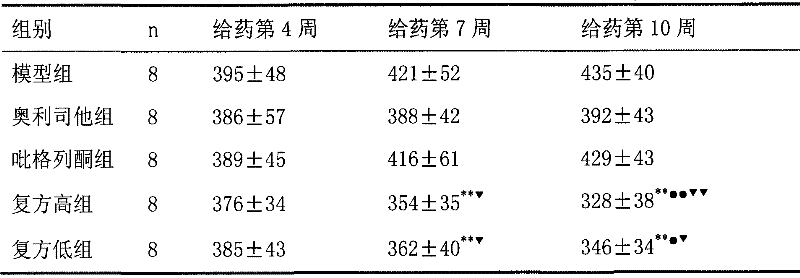
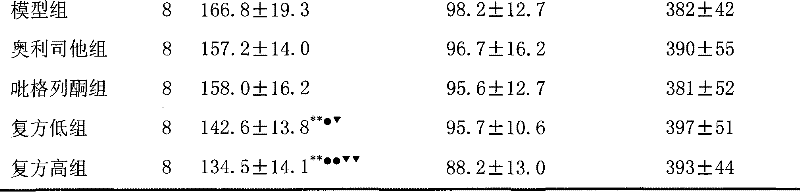
Image
Examples
Embodiment 1
[0011] prescription
[0012] Orlistat 125g
[0013] Pioglitazone Hydrochloride 5g
[0014] Microcrystalline Cellulose 230g
[0015] 8% hydroxypropyl methylcellulose solution appropriate amount
[0016] Low-substituted hydroxypropyl cellulose 150g
[0017] Magnesium Stearate 4.5g
[0018] Preparation process: Orlistat and pioglitazone hydrochloride pass through a 100-mesh sieve, microcrystalline cellulose and low-substituted hydroxypropyl cellulose pass through a 80-mesh sieve, and weigh the prescribed amount of pioglitazone hydrochloride, orlistat and low-substituted hydroxypropyl cellulose Mix the cellulose and microcrystalline cellulose evenly, add an appropriate amount of 8% hydroxypropyl methylcellulose solution to granulate, dry at 60°C, sieve the granules with 16 meshes, add the prescribed amount of magnesium stearate to the dry granules, mix evenly, press Slice and serve.
Embodiment 2
[0020] Orlistat 60g
[0021] Pioglitazone Hydrochloride 15g
[0022] 90g pregelatinized starch
[0023] Mannitol 70g
[0024] Lactose 40g
[0025] 6% PVP in 95% ethanol solution
[0026] Micronized silica gel 5g
[0027] Preparation process: Pass orlistat, pioglitazone hydrochloride, mannitol, lactose and micronized silica gel in the prescription through a 100-mesh sieve, mix well, add 6% PVP in 95% ethanol solution to granulate in an appropriate amount, dry at 60°C, and dry for 18 Mesh sieve for granulation, then fill the capsules.
Embodiment 3
[0029] Orlistat 60g
[0030] Pioglitazone Hydrochloride 10g
[0031] Croscarmell Starch Sodium 140g
[0032] Microcrystalline Cellulose 85g
[0033] Ethylcellulose 3g
[0034] Vitamin E 7g
[0036] Preparation process: The sustained-release tablet can be prepared according to the preparation process of the sustained-release tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com