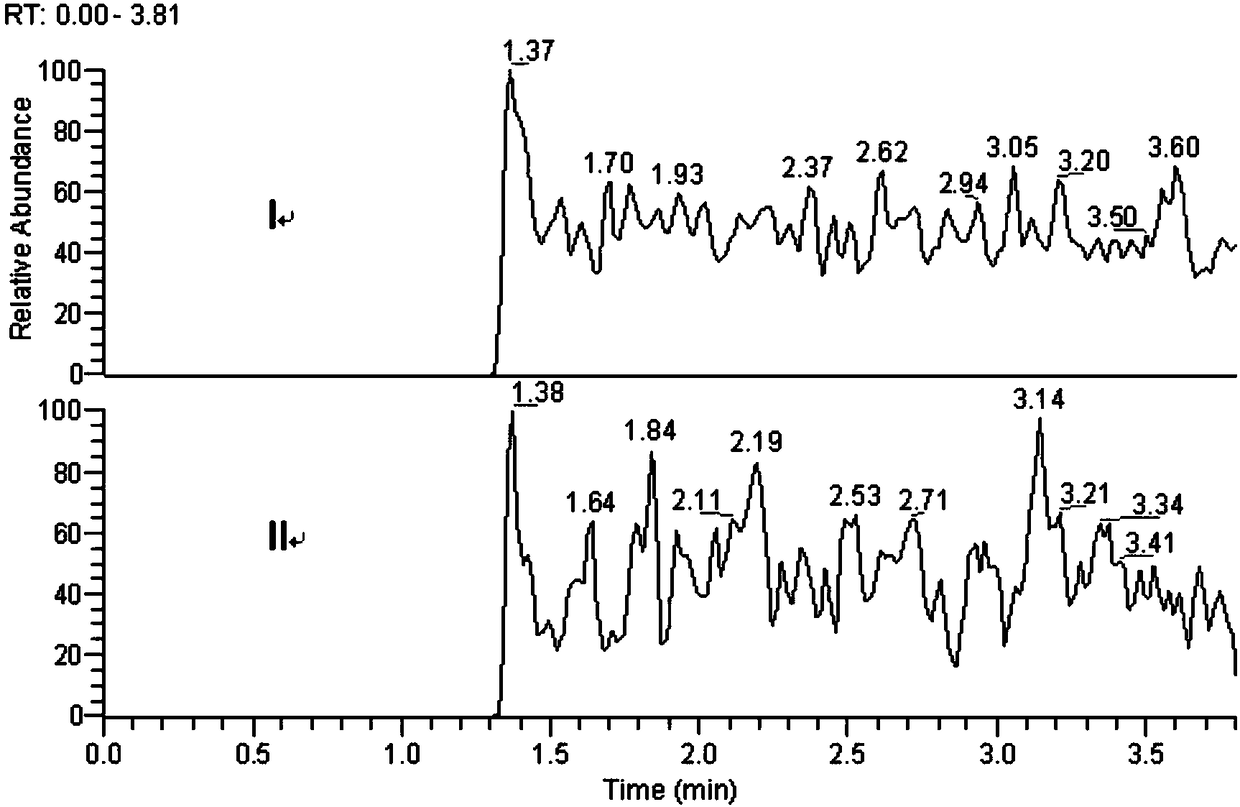
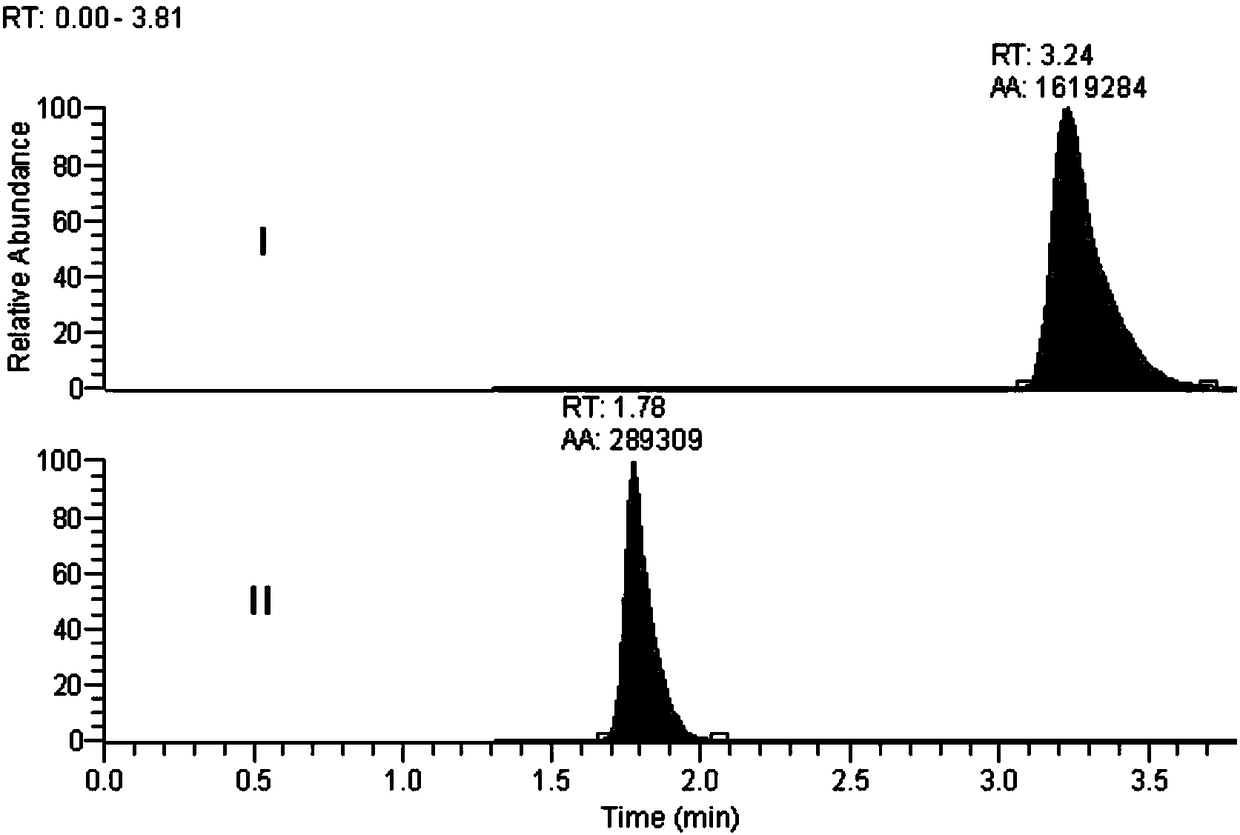
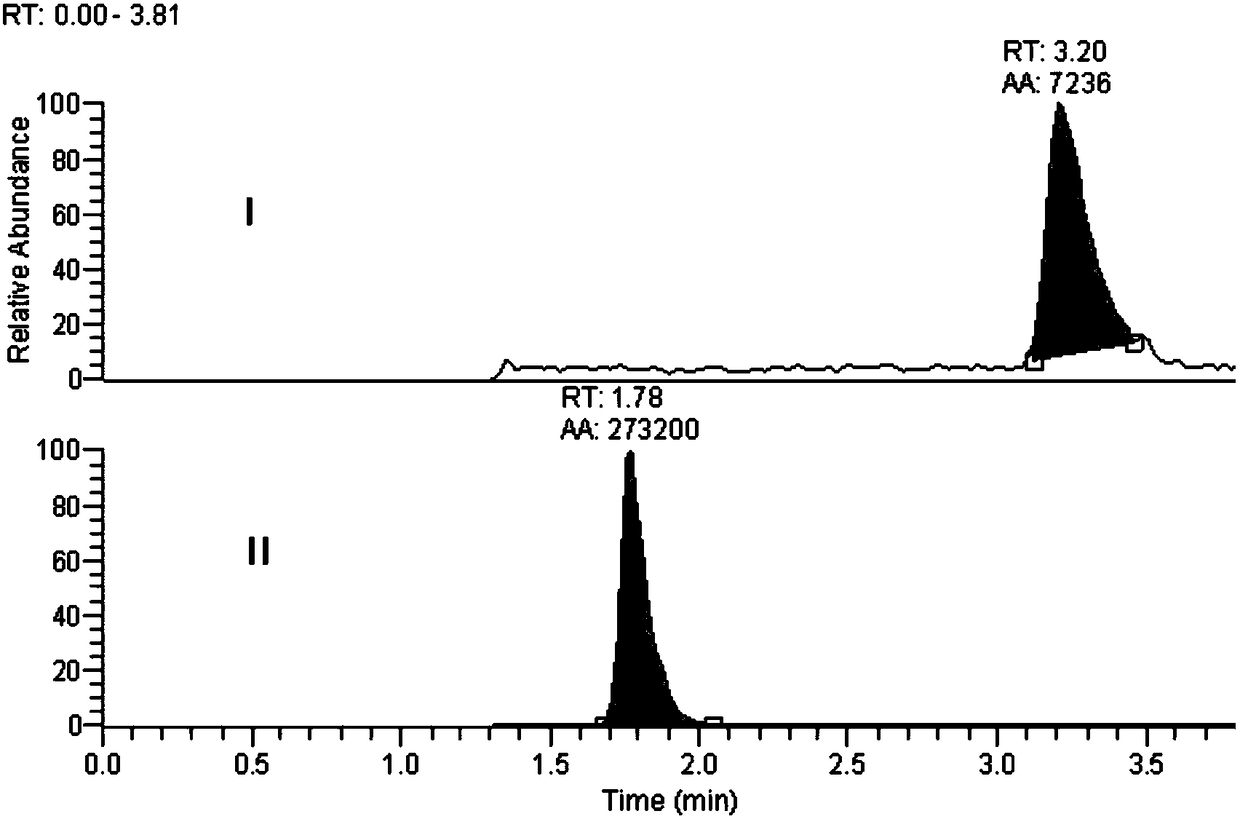
Method for determination of impurity A in Cetilistat in biological sample
A technology of biological samples and new listat, which is applied in the field of medicine to achieve the effect of improving analysis speed, ensuring accuracy and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The methodological investigation of embodiment 1 detection method of the present invention
[0024] 1. Preparation of standard solution
[0025] (1) Preparation of standard series solutions
[0026] The preparation method of the standard stock solution of the new lixistat impurity A: accurately weigh an appropriate amount of the new lixistat impurity A standard, put it in a 10mL volumetric flask, add tetrahydrofuran to dissolve it to a constant volume, and make a new lixistat with a concentration of 1000mg / L Impurity A standard stock solution.
[0027] Dilute the standard stock solution of the new lixistat impurity A with 20% tetrahydrofuran acetonitrile according to a certain ratio to prepare standard working solutions with concentration gradients of 100, 50.0, 20.0, 4.00, 1.00, 0.400 and 0.200 mg / L.
[0028] (2) Preparation of internal standard solution
[0029] Preparation method of internal standard orlistat standard stock solution: Accurately weigh an appropriat...
Embodiment 2
[0059] Embodiment 2 new lixistat impurity A suspension rat pharmacokinetic test
[0060] Take 12 SD rats with a body weight of about 300g and divide them into 3 groups, half male and half male; respectively intragastric administration of the new lisstat impurity A suspension, low, medium and high dose groups were 20mg / kg, 80mg / kg, respectively. kg, 320mg / kg. On the day of administration, the body weight was weighed, and the dosage and corresponding volume were accurately calculated according to the body weight. Rats in each group took blank blood from the subclavian vein before administration, and 0.2 mL of blood at 0.5h, 0.25, 0.5, 1, 3, 6, 9, 12 and 24h after administration, centrifuged the blood at 4000rpm for 10min, and took the upper layer Plasma was clarified and immediately stored in a -20°C refrigerator for determination.
[0061] A high-throughput method of ultra-high performance liquid chromatography-tandem mass spectrometry (LC-MS / MS) was used to determine the new...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com