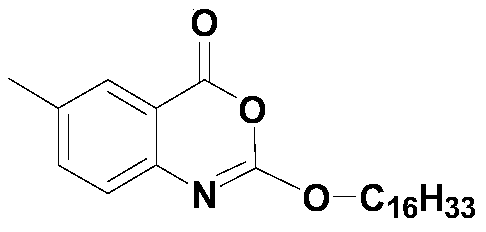
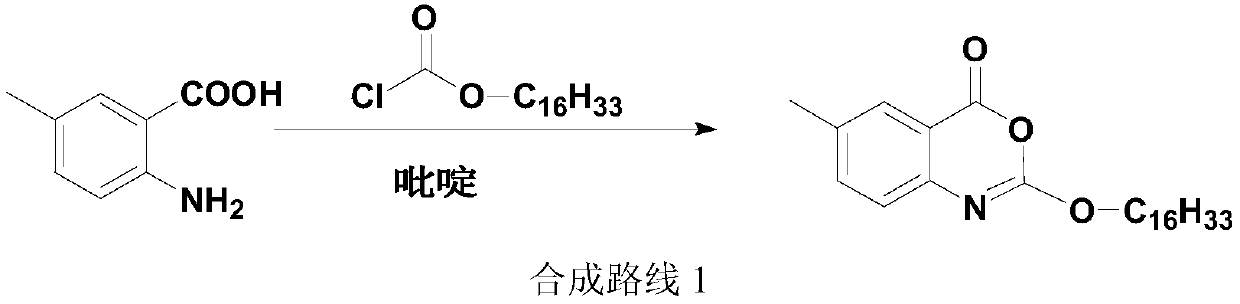
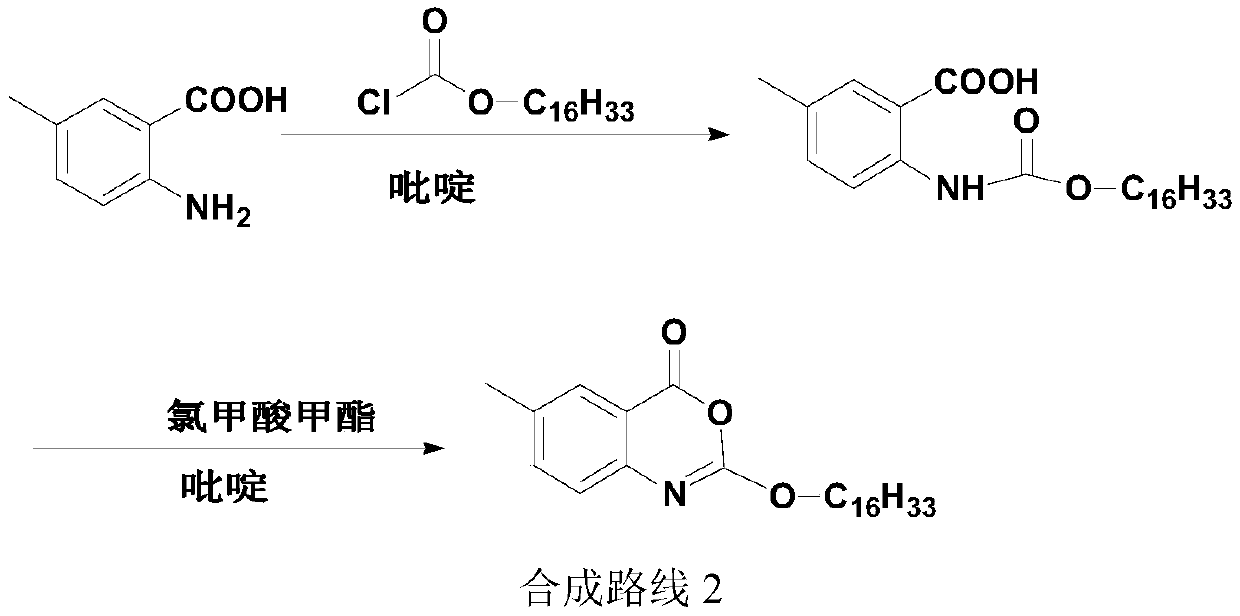
A kind of one-pot method prepares the method for new lixistat
A new Lilistat and synthetic method technology, applied in the field of simple and efficient preparation of new Lilistat, can solve the problems of high equipment requirements, complicated operations, cumbersome steps, etc., and achieve simple and not cumbersome processes, simple post-processing, and product purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 5g (33.08mmol, 1.0eq) of 2-amino-5-methylbenzoic acid, pyridine (80ml) and tetrahydrofuran (40ml) were added in a 500ml three-necked flask, stirred and dissolved, the dissolution was complete, and the temperature was lowered. When the temperature of the reaction system was At 5°C, start to slowly add 12.10g (39.70mmol, 1.2eq) of hexadecyl chloroformate dropwise, control the temperature not to exceed 10°C, and complete the dropwise addition in about 30 minutes. 30°C, heat preservation reaction for 30 minutes, after the heat preservation is over, lower the temperature of the system to 0-5°C, start to slowly add 15.1ml (186.55mmol, 2.5eq) of methanesulfonyl chloride dropwise, and the dropwise addition is completed in about 20 minutes. The temperature does not exceed 10°C. After dropping, continue to keep warm and stir for 30 minutes. When the keep warm is over, move the reaction system to a water bath, slowly raise the temperature to 25°, keep warm for 1 hour, and when the ...
Embodiment 2
[0031] 5g (33.08mmol, 1.0eq) of 2-amino-5-methylbenzoic acid, pyridine (60ml) and tetrahydrofuran (60ml) were added in a 500ml three-necked flask, stirred and dissolved, the dissolution was complete, and the temperature was lowered. When the temperature of the reaction system was At 5°C, start to slowly add 12.10g (39.70mmol, 1.2eq) of hexadecyl chloroformate dropwise, control the temperature not to exceed 10°C, and complete the dropwise addition in about 30 minutes. 30°C, heat preservation reaction for 30 minutes, after the heat preservation is over, lower the temperature of the system to 0-5°C, start to slowly add 15.1ml (186.55mmol, 2.5eq) of methanesulfonyl chloride dropwise, and the dropwise addition is completed in about 20 minutes. The temperature does not exceed 10°C. After dropping, continue to keep warm and stir for 30 minutes. When the keep warm is over, move the reaction system to a water bath, slowly raise the temperature to 25°, keep warm for 1 hour, and when the ...
Embodiment 3
[0033] 5g (33.08mmol, 1.0eq) of 2-amino-5-methylbenzoic acid, pyridine (40ml) and tetrahydrofuran (80ml) were added in a 500ml three-necked flask, stirred and dissolved, the dissolution was complete, and the temperature was lowered. When the temperature of the reaction system was At 5°C, start to slowly add 12.10g (39.70mmol, 1.2eq) of hexadecyl chloroformate dropwise, control the temperature not to exceed 10°C, and complete the dropwise addition in about 30 minutes. 30°C, heat preservation reaction for 30 minutes, after the heat preservation is over, lower the temperature of the system to 0-5°C, start to slowly add 15.1ml (186.55mmol, 2.5eq) of methanesulfonyl chloride dropwise, and the dropwise addition is completed in about 20 minutes. The temperature does not exceed 10°C. After dropping, continue to keep warm and stir for 30 minutes. When the keep warm is over, move the reaction system to a water bath, slowly raise the temperature to 25°, keep warm for 1 hour, and when the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com