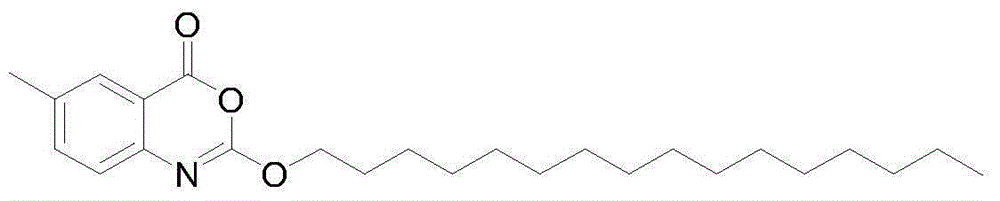
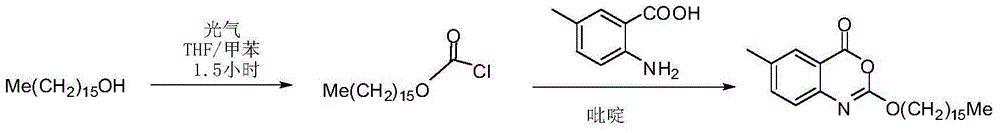
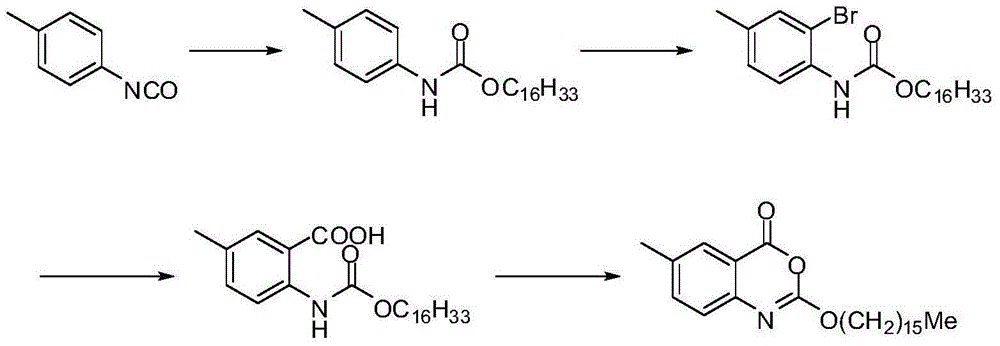
Novel crystal form cetilistat and preparation method thereof
A technology of celistat and its crystal form, which is applied in the field of crystallization of pharmaceutical compounds, can solve the problems of unsuitability for large-scale industrial production, cumbersome operation steps, and long preparation time, and achieve excellent stability, simple process, and good reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of new crystal form of Selista
[0038] The crude product of Selistat was prepared according to the route described in the patent US2003027821A.
[0039] 1) The preparation method of the new crystal form of Selista is as follows:
[0040] (1) Add 10 g of the crude selestat obtained by the above method into absolute ethanol, wherein the mass ratio of the crude selestat to absolute ethanol is 1:6, heat up to 45° C. and stir to dissolve;
[0041] (2) After dissolving, cool down to 10°C for crystallization at a cooling rate of 10°C / h, and stir at this temperature for 0.5 hours to obtain crystallization;
[0042] (3) Filtration, washing with absolute ethanol, and air-drying at 30° C. for 4 hours to obtain 8.8 g of the new crystal form of selista, with a yield of 88.0%.
[0043] After testing, the melting point of the new crystal form of Selista is 73.5-74.8°C.
[0044] 2) Detection conditions and results
[0045] (1) The X-ray powder diffraction te...
Embodiment 2
[0060] Embodiment 2: Preparation of new crystal form of Selista
[0061] The crude product of Selistat was prepared according to the route described in the patent US2003027821A.
[0062] 1) The preparation method of the new crystal form of Selista is as follows:
[0063] (1) Add 10 g of the crude selestat obtained by the above method into methanol, wherein the mass ratio of the crude selestat to methanol is 1:5, heat up to 50° C. and stir to dissolve;
[0064] (2) After dissolving, cool down to 15°C at a cooling rate of 10°C / h to crystallize, and stir at this temperature for 0.5 hours to obtain crystallization;
[0065] (3) Filtration, washing with methanol, and air-drying at 30° C. for 4 hours to obtain 8.5 g of the new crystal form of selistastat with a yield of 85.0%.
[0066] After testing, the melting point of the new crystal form of Selista is 73.6-74.7°C.
[0067] 2) Detection conditions and results
[0068] The instrument used in this embodiment is the same as embo...
Embodiment 3
[0074] Example 3: Preparation of new crystal form of Selista
[0075] The crude product of Selistat was prepared according to the route described in the patent US2003027821A.
[0076] The preparation method of the new crystal form of Selista is as follows:
[0077] (1) Add 10 g of the crude selestat obtained by the above method into ethyl acetate, wherein the mass ratio of the crude selestat to ethyl acetate is 1:3, heat up to 55° C. and stir to dissolve;
[0078] (2) After dissolving, cool down to 12°C to crystallize at a cooling rate of 20°C / h, and stir at this temperature for 0.5 hours to obtain crystallization;
[0079] (3) Filtration, washing with ethyl acetate, and air-drying at 30°C for 4 hours to obtain 8.6 g of the new crystalline form of selestat, with a yield of 86.0%.
[0080] After testing, the melting point of the new crystal form of Selista is 73.5-74.9°C.
[0081] The instrument used in this embodiment is the same as that in Example 1.
[0082] As detected ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com