Method for preparing cetilistat
A technology of Lise and compounds, which is applied in the field of drug preparation, can solve the problems of low yield and difficult separation and purification in the examples, and achieve the effects of excellent atom economy, high purity and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Synthesis 2- ((( Hexadecyloxy)carbonyl)amino)5-methylbenzoic acid
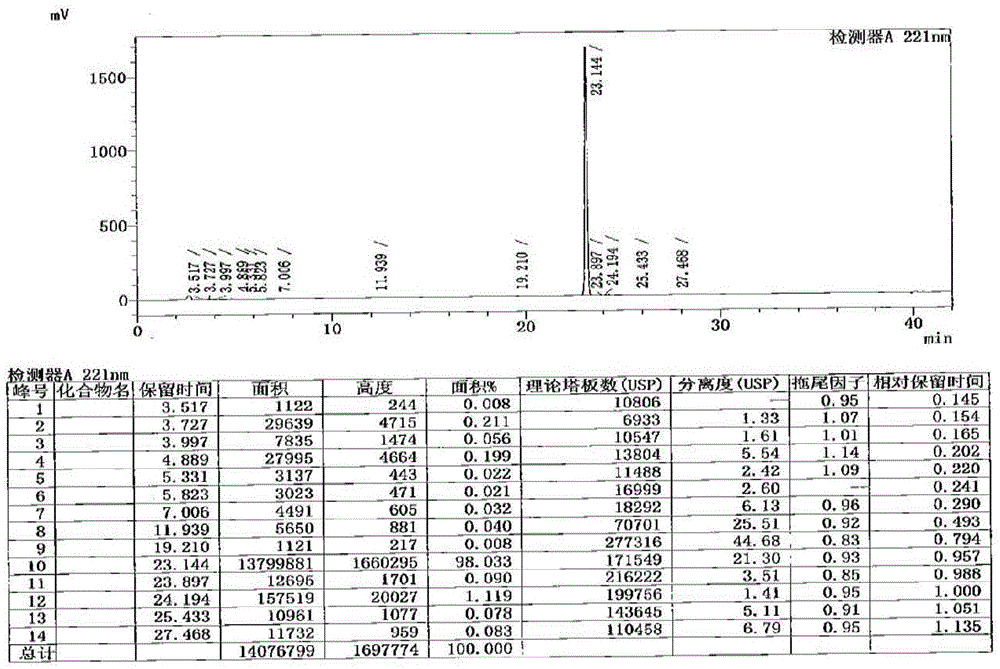
[0048] Put 2-amino-5-methylbenzoic acid (3.0 g, 19.8 mmol, 1.0 equivalent, hereinafter abbreviated as eq) into 24 mL of dichloromethane, suspend, and start stirring. Pyridine was added dropwise, followed by cetyl chloroformate slowly. The specific results are shown in Table 1. figure 1 It is the HPLC spectrogram of 4# reaction terminal point. It can be seen from the table below that when the feeding sequence is 2-amino-5-methylbenzoic acid, pyridine, and cetyl chloroformate, the product has high purity and no specific impurities are formed, and can be directly used for feeding in the next step.
[0049] Table 1 Cetyl Chloroformate and Pyridine Feeding Quantity Investigation Statistical Table
[0050]
[0051] *: Cetyl chloroformate dosage is the molar ratio of cetyl chloroformate to 2-amino-5-methylbenzoic acid;
[0052] **: Pyridine dosage is the molar ratio of pyridine to 2-am...
Embodiment 2
[0054] Embodiment 2: the preparation of west for Li Sita
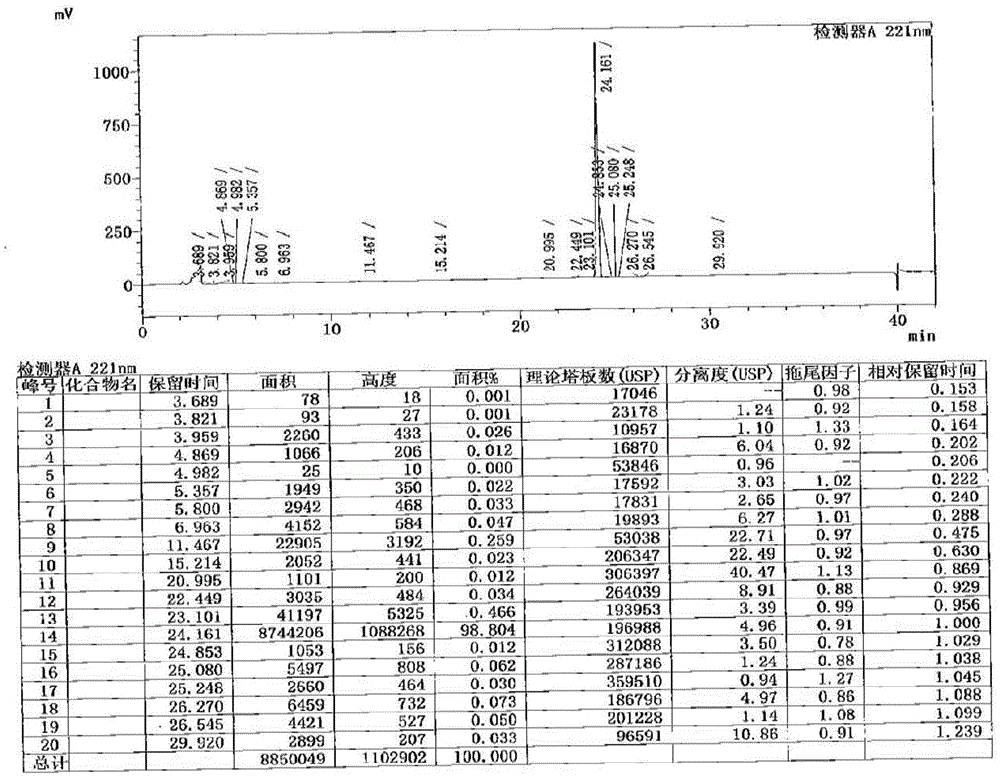
[0055] Add 36 mL of pyridine dropwise to the 6# reaction liquid in Example 1, and control the temperature at 0-5°C, then slowly add methyl chloroformate (7.5g, 79.2mmol, 4.0eq) dropwise, and control the internal temperature between 0-10°C, Insulated and stirred for 2.5 hours, at this time, the normalized content of cetiristat was 98.80%, such as figure 2 shown.
[0056] After the reaction was completed, 18 mL of water and 18 mL of dichloromethane were added, vigorously stirred for 10 minutes, then allowed to stand and separated. The organic phase was washed twice with 30 mL of 1 mol / L dilute hydrochloric acid, and then washed with purified water until pH = 6-7. The organic phase was concentrated under reduced pressure to a small volume, and then put into n-heptane to completely replace the dichloromethane. Then concentrated under reduced pressure to about 15-20mL, a large amount of solids precipitated, filtered, ...
Embodiment 3
[0058] Embodiment 3: the preparation of west for Li Sita
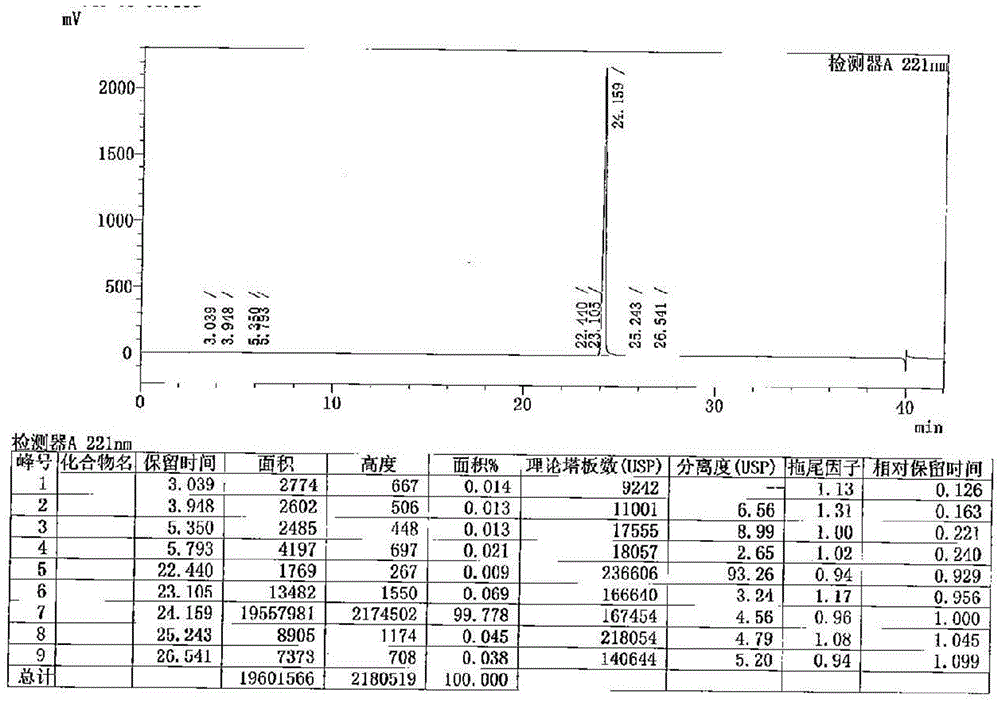
[0059] To the 4# reaction solution in Example 1, 33 mL of recovered pyridine was added dropwise, and the temperature was controlled at 0-5°C, and then ethyl chloroformate (10.7g, 99mmol, 5.0eq) was slowly added dropwise, and the internal temperature was controlled at 0-10°C. Insulated and stirred for 1 hour, at this time the normalized content of cetiristat was 98.86%.
[0060] After the reaction was completed, 18 mL of water and 18 mL of methylene chloride were recovered, vigorously stirred for 10 minutes, and then left to stand and separated. The organic phase was washed twice with 30 mL of 1 mol / L dilute hydrochloric acid, and then washed with purified water until pH = 6-7. The organic phase was concentrated under reduced pressure to a small volume, and then put into n-heptane to replace the dichloromethane. Then concentrated under reduced pressure to about 15-20mL, a large amount of solids precipitated, filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com