Cetilistat crystal
A new Lilistat and crystal technology, applied in the field of crystals for obesity drugs, can solve problems such as affecting drug bioavailability and absorption, clinical efficacy differences, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Take 1ml of triethylamine and 19ml of dichloromethane and mix well, add 300mg of neolipristal, stir to dissolve at 50℃, filter to remove impurities while hot, and obtain a bright yellow clear solution, then add 20ml of methanol to mix well, and seal the resulting solution It was placed at 25°C for crystallization, and the solid was filtered out and dried to obtain 286 mg of neolipristal crystal I with a yield of 95.3% and a purity of 99.9%.
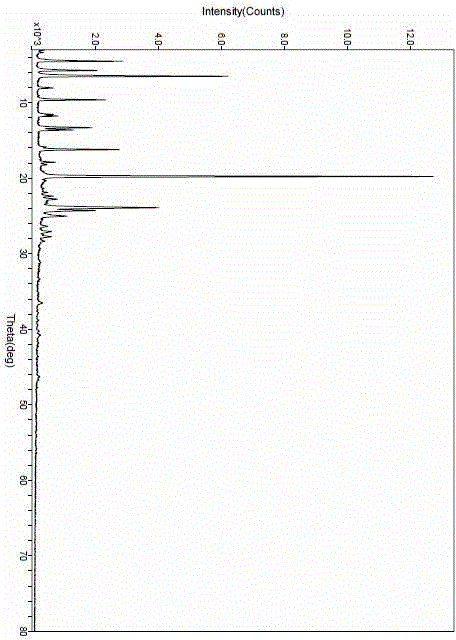
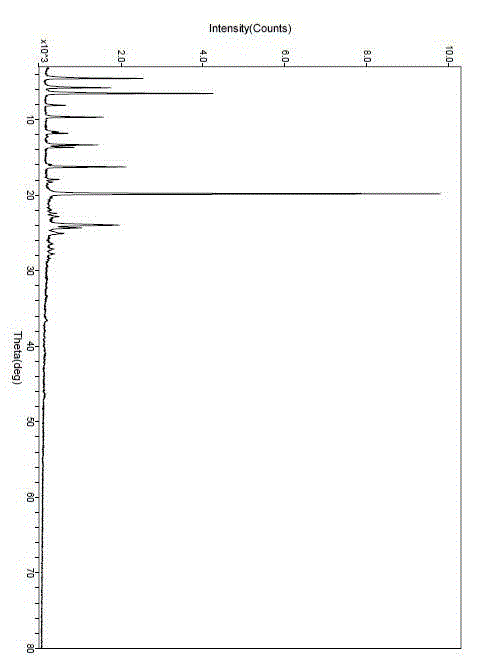
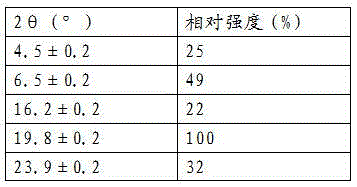
[0040] After determination, the X-ray powder diffraction pattern of the new lipristal crystal I obtained is as follows figure 1 As shown, it has the following X-ray powder diffraction data:
[0041]
Embodiment 2
[0043] Take 2ml of triethylamine and 18ml of acetone and mix well, add 300mg of neolipristal, stir to dissolve at 40℃, filter while hot to remove impurities, and obtain a bright yellow clear solution, then add 30ml of acetonitrile to mix well, and place the resulting solution sealed in It was allowed to stand for crystallization at 40°C, and the solid was filtered and dried to obtain 262 mg of neolipristal crystal I, with a yield of 87.3% and a purity of 99.7%. The X-ray powder diffraction pattern data is the same as that of Example 1.
Embodiment 3
[0045] Take 3ml of triethylamine and 17ml of dioxane and mix well, add 300mg of neolipristal, stir to dissolve at 50°C, filter while hot to remove impurities to obtain a bright yellow clear solution, then add 40ml of water to mix well, and the resulting solution It was sealed and placed at 20°C for crystallization, and the solid was filtered out and dried to obtain 269 mg of neolipristal crystal I, with a yield of 89.7% and a purity of 99.6%. The X-ray powder diffraction pattern data is the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com