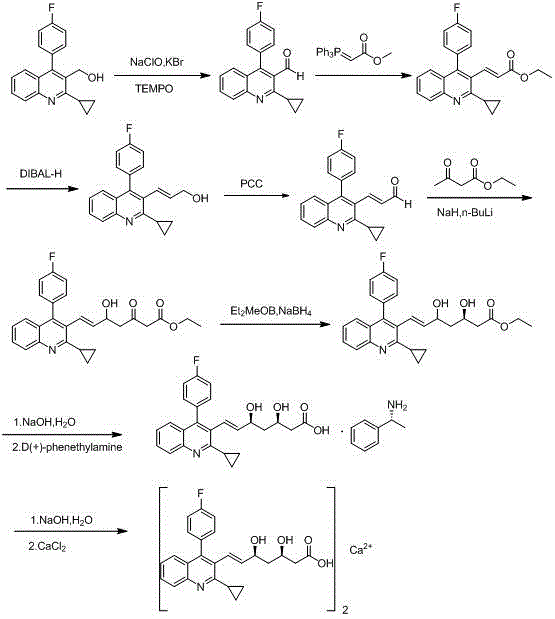
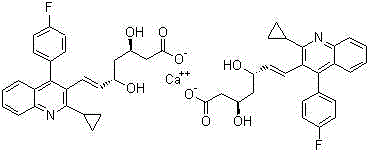
Method for preparing intermediate of pitavastatin calcium
A technology for pitavastatin calcium and intermediates, applied in the field of blood lipid-lowering drugs, can solve the problems of many reaction steps, unsuitable for industrial production, difficult purification of intermediates, etc., and achieve the effect of reducing purification steps and simplifying reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of preparation method of pitavastatin calcium intermediate of the present invention, comprises the steps:
[0033] Preparation of Intermediate 1:
[0034] Add 26g 2-cyclopropyl-4-(4-fluorophenyl) quinoline-3-carbaldehyde, 37.3g (triphenyl-orthophosphoryl) methyl acetate and 300mL DMF respectively into a 500mL reaction flask, stir and heat up React at 80°C, monitor the reaction by TLC; after the reaction is complete, cool to room temperature, add 800mL of water, stir and separate the layers, extract the aqueous layer with ethyl acetate (300mL×3), combine the organic phases, and wash with 500mL of water and 500mL of saturated sodium chloride The organic phase was washed with the solution, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 63.2g of a brownish-yellow solid residue. Add 300mL of methanol, heat to dissolve, cool down and stir to crystallize, filter with suction, rinse, and dry, etc. 26.9g Pale yellow s...
Embodiment 2
[0046]A kind of preparation method of pitavastatin calcium intermediate of the present invention, comprises the steps:
[0047] Preparation of Intermediate 1:
[0048] Add 39g 2-cyclopropyl-4-(4-fluorophenyl) quinoline-3-carbaldehyde, 78.3g (triphenyl-orthophosphoryl) methyl acetate and 600mL toluene respectively in a 1000mL reaction flask, stir and heat up React at 90°C, monitor the reaction by TLC; after the reaction is completed, cool to room temperature, wash the organic phase with 300mL water and 300mL saturated sodium chloride solution, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and obtain 94.8g of brown solid Add 450mL of methanol to the residue, heat to dissolve, cool down and stir to crystallize, filter with suction, rinse, and dry to obtain 42.3g of a light yellow solid product. Yield: 87%.
[0049] Preparation of intermediate 2:
[0050] At room temperature, put 35g of intermediate 1 in 300mL of acetonitrile, stir for 20 minu...
Embodiment 3
[0060] A kind of preparation method of pitavastatin calcium intermediate of the present invention, comprises the steps:
[0061] Preparation of Intermediate 1:
[0062] Add 20g 2-cyclopropyl-4-(4-fluorophenyl)quinoline-3-carbaldehyde, 50.3g (triphenyl-orthophosphoryl) methyl acetate and 400mL DMSO to a 1000mL reaction flask respectively, stir and heat up React at 90°C, monitor the reaction by TLC; after the reaction is complete, cool to room temperature, add 800mL of water, stir and separate the liquids, extract the aqueous layer with ethyl acetate (200mL×3), combine the organic phases, and use 200mL of water and 200mL of saturated chlorine for the reaction solution The organic phase was washed with sodium chloride solution, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 47.5 g of a brownish-yellow solid residue. Add 250 mL of methanol, heat to dissolve, cool down and stir to crystallize, filter with suction, rinse, dry, et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com