Fat emulsion pre-emulsifying concentrated solution for teniposide intravenous injection and preparation method thereof
A teniposide and pre-emulsification technology, applied in the directions of emulsion delivery, oil/fat/wax inactive ingredients, and inactive medical formulations, etc., can solve problems such as strong side effects, extend the validity period, and increase storage. Effects of stability, ease of transport and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
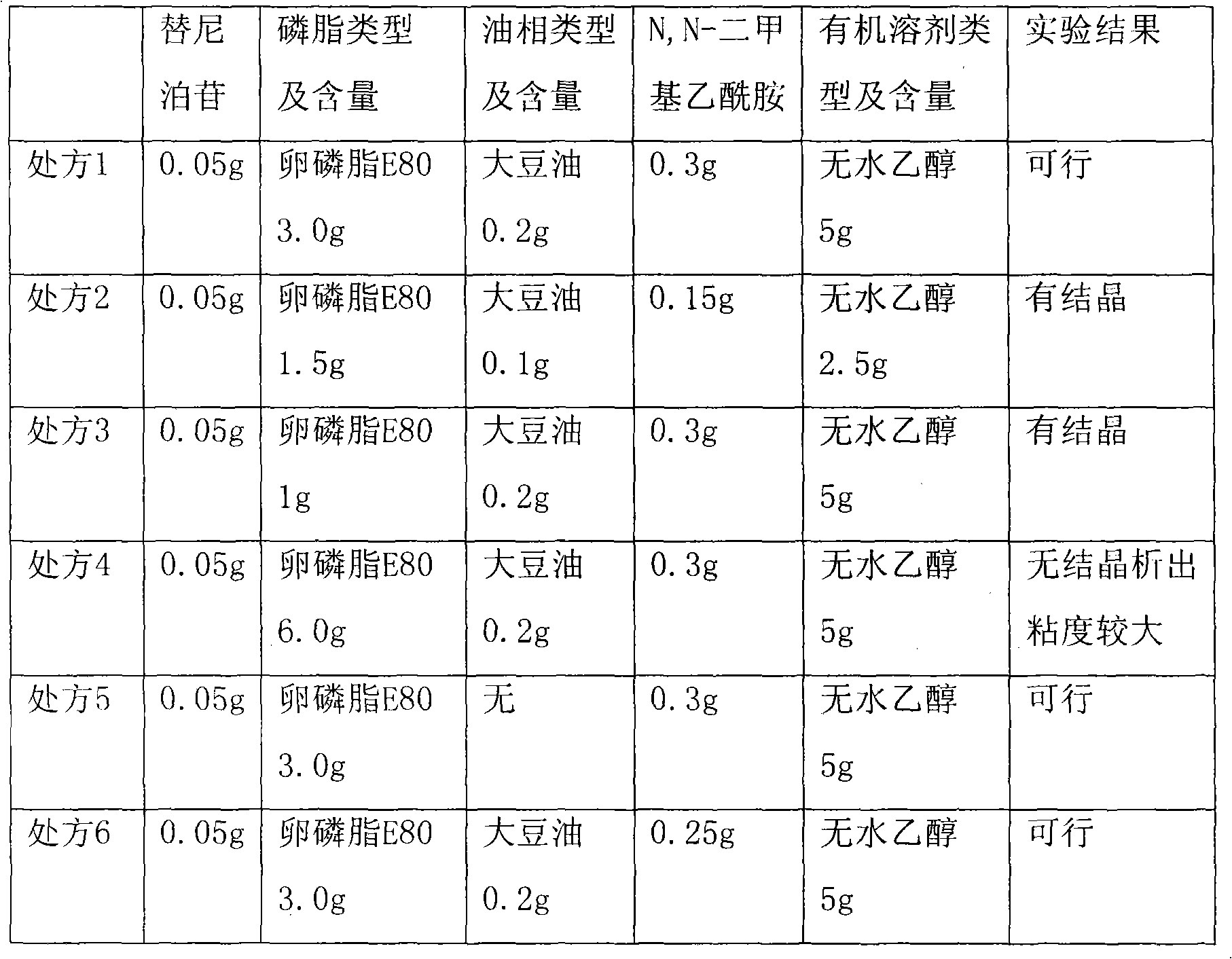
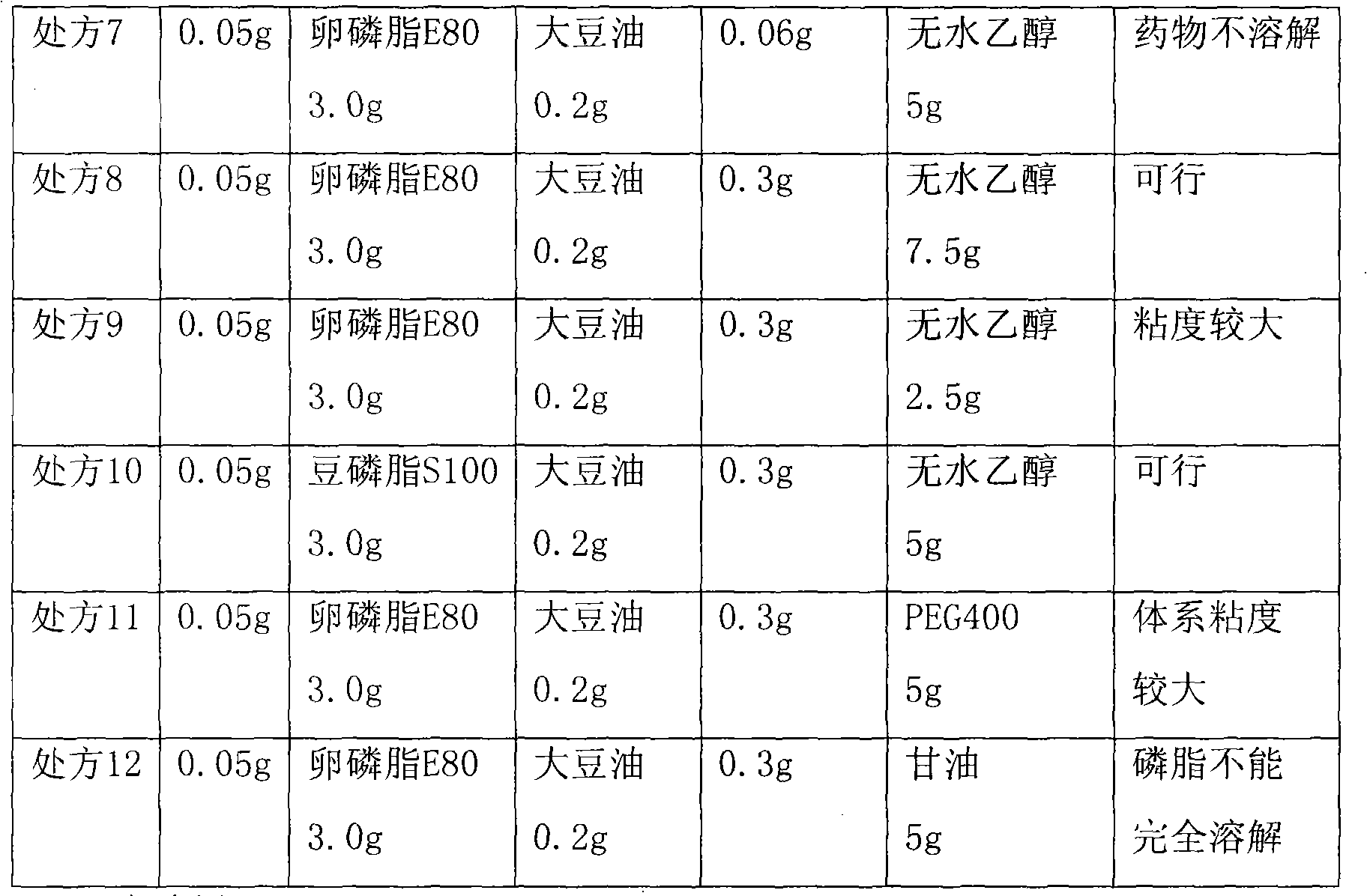
[0046] Embodiment 1, prescription screening of teniposide injection
[0047] Prepare the solution as above, and observe the result after 8 hours. The prescription composition and screening results of teniposide are shown in Table 1.
[0048] Table 1. Prescription composition and screening results of teniposide
[0049]
[0050]
Embodiment 2
[0052] prescription:
[0053] Teniposide 0.05g
[0054] Lecithin 3.0g
[0055] soybean oil 0.2g
[0056] N,N-Dimethylacetamide 0.3g
[0057] Absolute ethanol 5g
[0058] Preparation:
[0059] Add 0.05g of teniposide into 0.3g of N,N-dimethylacetamide, after it is completely dissolved, add 3g of lecithin, 0.2g of soybean oil and 5g of absolute ethanol, stir to make them evenly mixed Forms a clear, clear pre-emulsion concentrate.
Embodiment 3
[0061] prescription:
[0062] Teniposide 0.05g
[0063] Lecithin 3.0g
[0064] soybean oil 0.2g
[0065] N,N-Dimethylacetamide 0.3g
[0066] Absolute ethanol 5g
[0067] Preparation:
[0068] Teniposide 0.05g is added to the mixed solution of 0.3g of N,N-dimethylacetamide and 0.2g of soybean oil, after completely dissolving, add lecithin 3g and dehydrated alcohol 5g, stir to make They are mixed well to form a clear and clear pre-emulsion concentrate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com