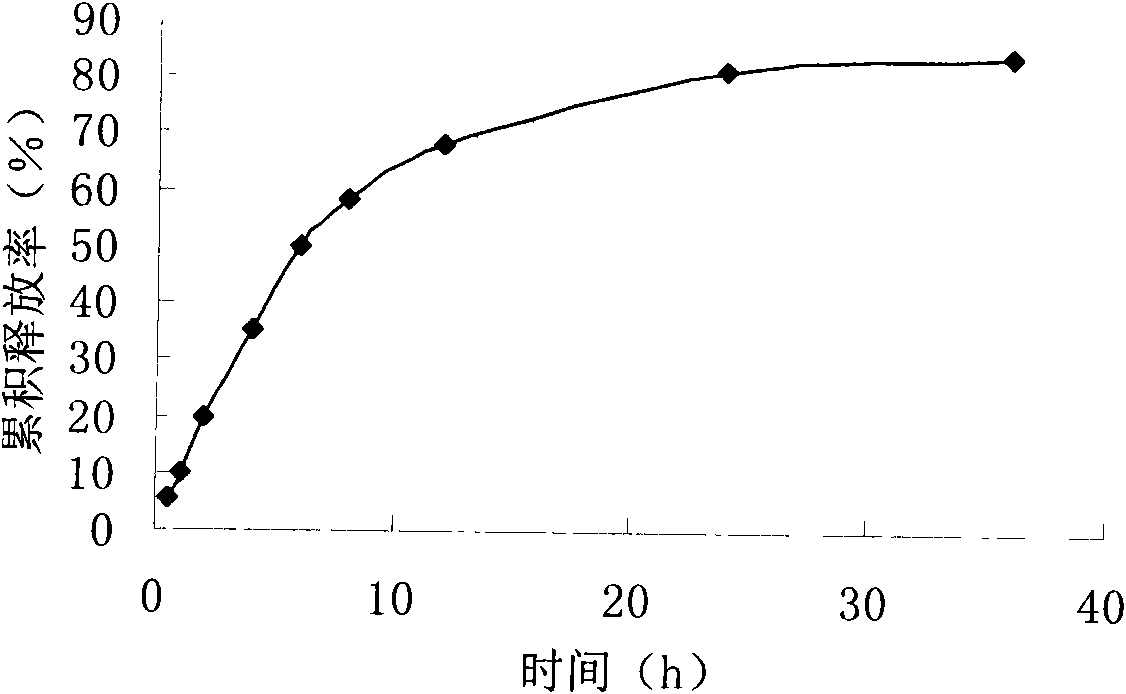
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
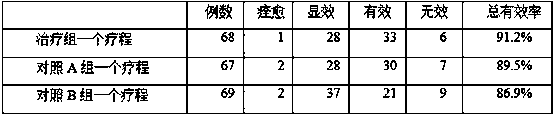
33results about How to "Eliminate allergic reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Long-circulating solid lipid docetaxel nanoparticles and preparation method thereof
InactiveCN101653414AGood water solubilityImprove stabilityOrganic active ingredientsAntineoplastic agentsLipid formationSolubility
The invention discloses long-circulating solid lipid docetaxel nanoparticles and a preparation method thereof. The long-circulating solid lipid docetaxel nanoparticles comprise the following materialsin therapeutic effective dose: docetaxel, lipid materials, long-circulating auxiliary materials and an emulsifier. The long-circulating solid lipid docetaxel nanoparticles have small particle size, high encapsulation rate and good stability, and not only improve the solubility and the stability of the docetaxel, reduce the toxicity of the docetaxel, but also prolong the circulating time of a medicament in blood, and improve the therapeutic index of the medicament, so that the preparation has the characteristics of low toxicity, low allergy, high efficiency and targeting in clinical application.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Amphiphilic tri-block copolymer taxol bonding medicament and synthesis method thereof
InactiveCN1961962ASolve the problem of insoluble in waterGood dispersionOrganic active ingredientsPharmaceutical non-active ingredientsPolyesterSynthesis methods
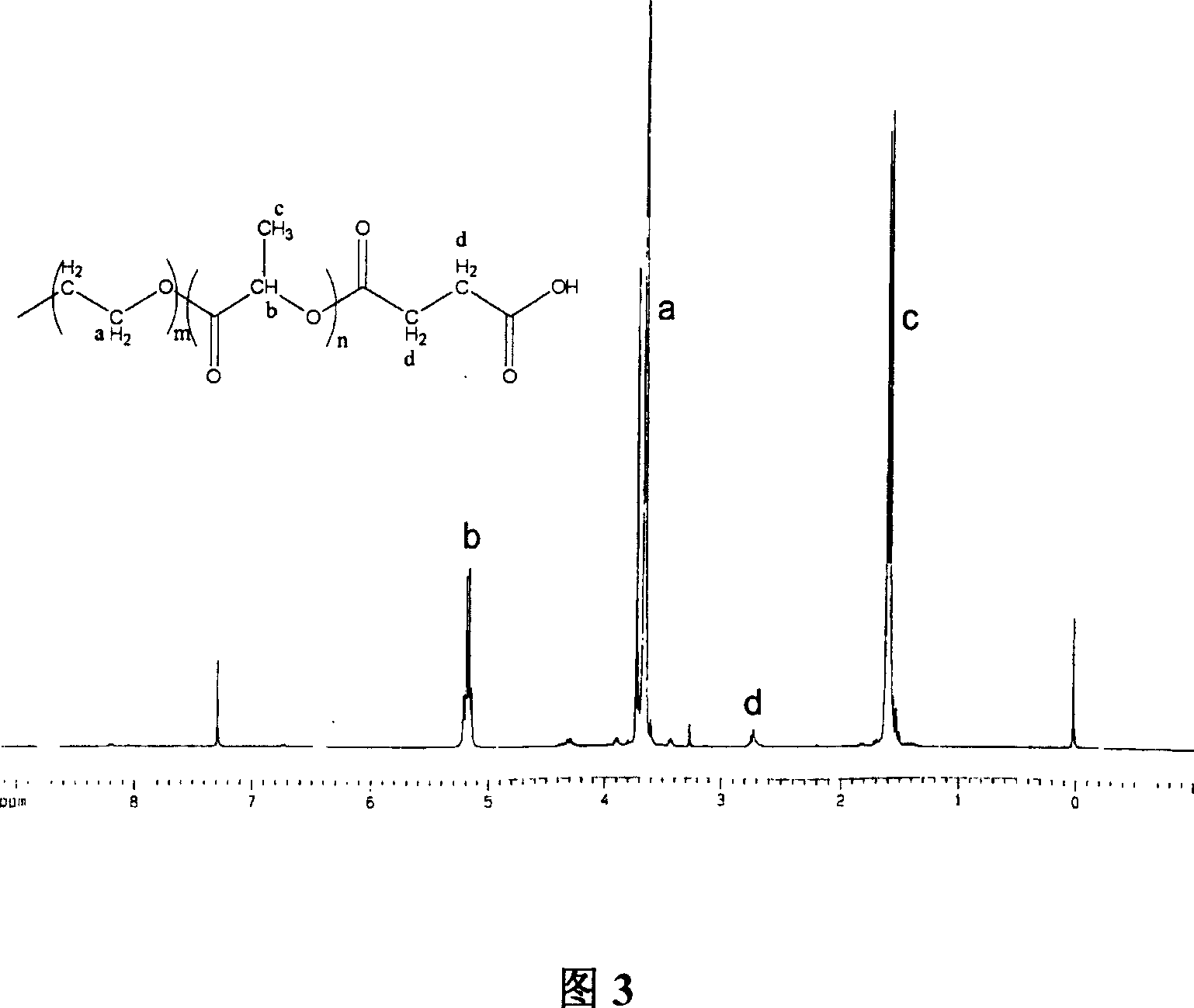
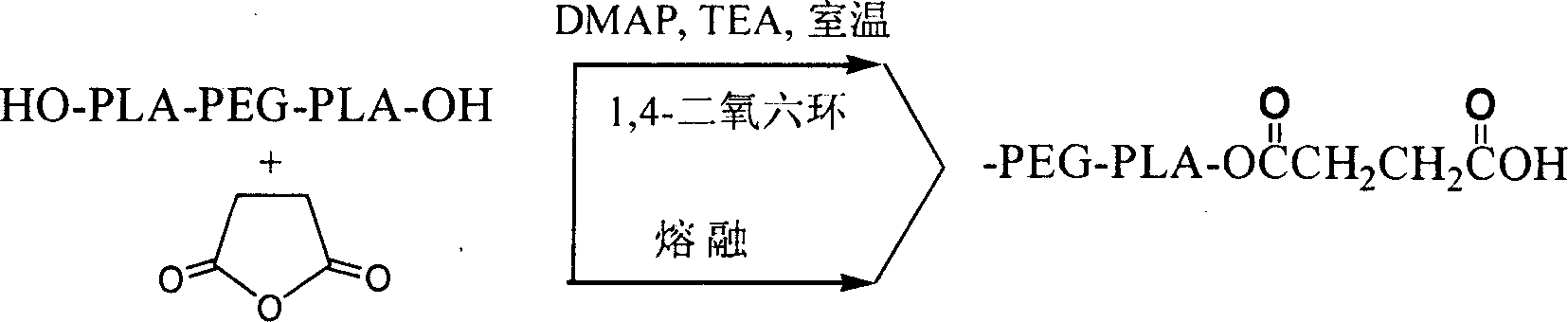
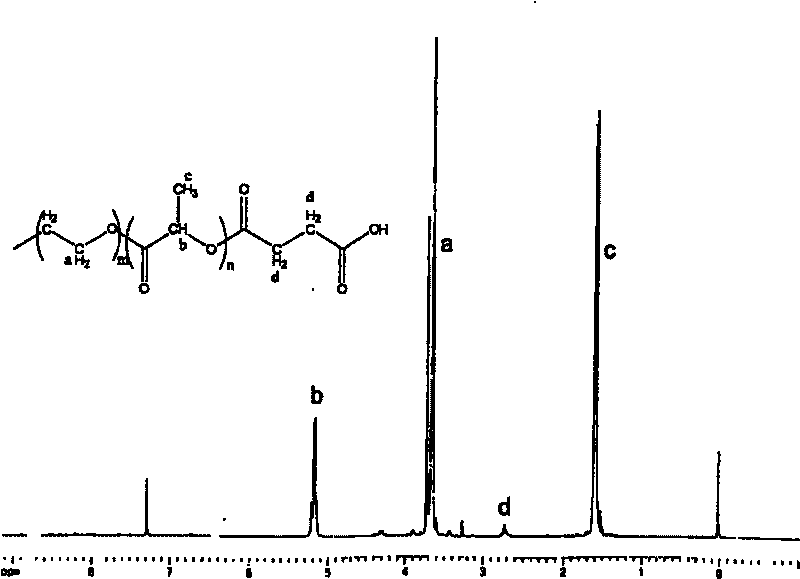
The invention relates to an amphipathy block copolymer-Paclitaxel compound and relative preparation, wherein said invention is formed by bonded aliphatic polyester-carbowax-aliphatic polyester block copolymer and Paclitaxel; with hydroxyl carbowax (PEG), solvent and catalyst, it processes the ring-opening polymerization of aliphatic ester to obtain the liphatic polyester-carbowax-aliphatic polyester block copolymer, then converting the hydroxyl grouyp into end carboxyl; with condensating agent, processing genate reaction with Paclitaxel, to obtain the inventive drug. The invention has amphipathy property, to be made into liquid agent or freeze dried. And its block structure can improve the Paclitaxel content, adjusted between 10-40%.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Cefquinome liposome
ActiveCN104000783AReduce toxicityTargetedAntibacterial agentsOrganic active ingredientsPhospholipinSterol
Cefquinome liposome, which belongs to the field of pharmaceutics, has a particle size of less than 1000 nm, and is mainly prepared from the following raw materials by weight: 1 part of cefquinome, 1-40 parts of phospholipid, 0-15 parts of sterol or soy isoflavone glucoside or soyasapogenol, and 0-15 parts of additives. The cefquinome liposome can be prepared into a liquid preparation, and can also be prepared into a solid preparation by adding a proper amount of a support agent. The cefquinome liposome or cefquinome long circulating liposome prepared in the invention contains no irritant substances, and can relieve anaphylactic reaction; the obtained preparation has good stability, has an average liposome particle size of less than 1000 nm, and has encapsulation efficiency of more than 80%. The preparation method is mature in process, simple, practical, and suitable for industrial production.
Owner:HENAN SOAR VETERINARY PHARMA
Human source anti- tetanus exotoxin antibody and preparation method and use thereof
InactiveCN1594361AAvoid allergic reactionsAddressing issues that predispose to allergic reactionsAntibacterial agentsImmunoglobulins against virusesEscherichia coliHypersensitive response
The invention discloses a human source anti-tetanus exotoxin antibody (HTAT-Fab) and preparation method, comprising: constructing human immunity phage antibody bank, screening phage positive clone, further getting HTAT-Fab gene with a specific neutralization activity and high affinity. The gene can be expressed in the procaryotic cell such as E.coli, eukaryotic cell such as microzyme, or mammalian cell such as CHO, purifying to get highly purified HTAT-Fab with a strong tissue penetrability, a high affinity. The HTAT-Fab product can not only eliminate the allergic reaction generated by horse serum anti-tatanus antitoxin (TAT) (foreign protein), but also avoid the blood source for producing human tetanus immunoglobulin (HTIG) and the latent virus pollution.
Owner:北京明新高科技发展有限公司 +2
Mumps vaccine freeze-drying protective agent without gelatin and human serum albumin components
InactiveCN104548110AImprove securityEliminate allergic reactionsViral antigen ingredientsAntiviralsUreaSucrose
The invention discloses a mumps vaccine freeze-drying protective agent without gelatin and human serum albumin components, and a formula and a preparation process of the mumps vaccine freeze-drying protective agent are provided. By adopting a technical scheme that the mumps vaccine freeze-drying protective agent is prepared from cane sugar, mycose, dextran, sodium glutamate, urea, arginine, a 199 culture medium, mannitol and sterile water for injection, a mumps live-attenuated vaccine freeze-drying preparation can avoid the defects of gelatin and human serum albumin, and the purposes of eliminating allergic reaction, removing hidden dangers of hepatitis infection and reducing cost can be achieved.
Owner:LIAONING EMMY BIOLOGICAL PHARMA
Humanized neutralizing antibody against rabies virus, method for preparing same and use
ActiveCN101337990AHigh activityHigh affinityImmunoglobulins against virusesAntiviralsEscherichia coliSide effect
The invention discloses a human anti-rabies virus neutralizing antibody as well as the preparation method and the application thereof. The heavy chain variable region and the light chain variable region of the antibody respectively have an amino acid sequence represented by sequence 2 and sequence 4 in a sequence list, and the heavy chain variable region and the light chain variable region of a modified body of the antibody irrespectively have an amino acid sequence represented by sequence 8 and sequence 10 in the sequence list. The antibody is prepared according to the following steps: firstly, preparing a CDR region, a variable region, IgG section or the whole genes of the antibody by molecular biology or other methods; expressing in prokaryotic cells such as Escherichia coli, eukaryotic cells such as yeast, insect cells, plant cells or mammal cells such as CHO; and purifying to obtain the antibody. The antibody has the advantages of high specific neutralization activity, high affinity and no toxic and side effects, suits prevention and emergency treatment of rabies as well as detection of rabies virus, and has excellent application prospect.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Taxotere nano preparation carried by albumin and phospholipid and method preparing same
InactiveCN102078301ASolve solubilityImprove compliancePowder deliveryOrganic active ingredientsSolubilityHemolysis
The invention provides a taxotere nano preparation with albumin and phospholipid acting as carriers and a preparation method thereof, which relate to an anticancer medicament preparation and preparation thereof and solve the problems that the taxotere medicament is poor in water solubility, has toxic and side effect, especially has high irritability and hemolysis, and can cause severe anaphylaxis symptoms when used in intravenous injection. The taxotere nano preparation comprises the following components in percentage by weight: docetaxel 1% to 15%, albumin of 10% to 43%, 10% to 43% of phospholipids, vitamin E about 1% to 10%, freeze-dried excipient about 1% to 20% and acid buffer salt about 1% to 15%, and is prepared by adding solvent in the compositions and using a high pressure homogenization method and a freeze-drying technology. In the taxotere nano preparation, auxiliaries and solvents which bring about an allergic reaction of a human body are not used, thereby eliminating the allergic reaction caused by solvents and Tween-80 accessories, greatly improving the safety of taxotere solid nano medicament preparation and patient medication compliance. By using the preparation method, the stability of pharmaceutical preparation is greatly improved, thereby being beneficial to storage and transportation of medicaments.
Owner:孙璐
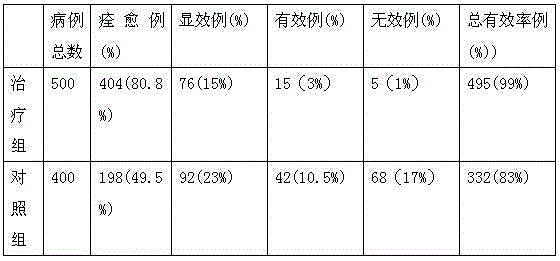
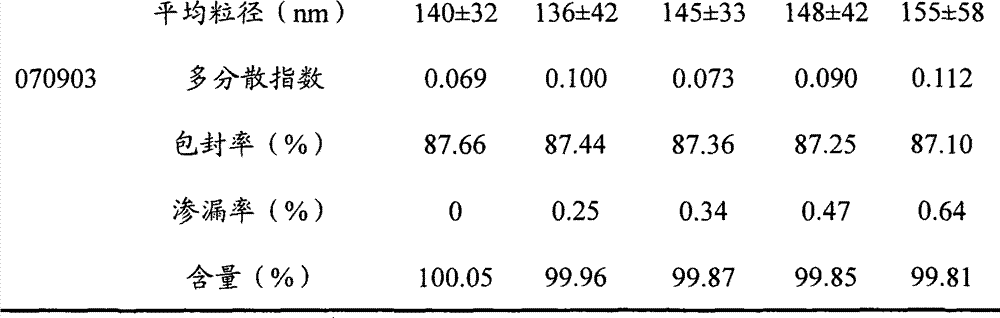
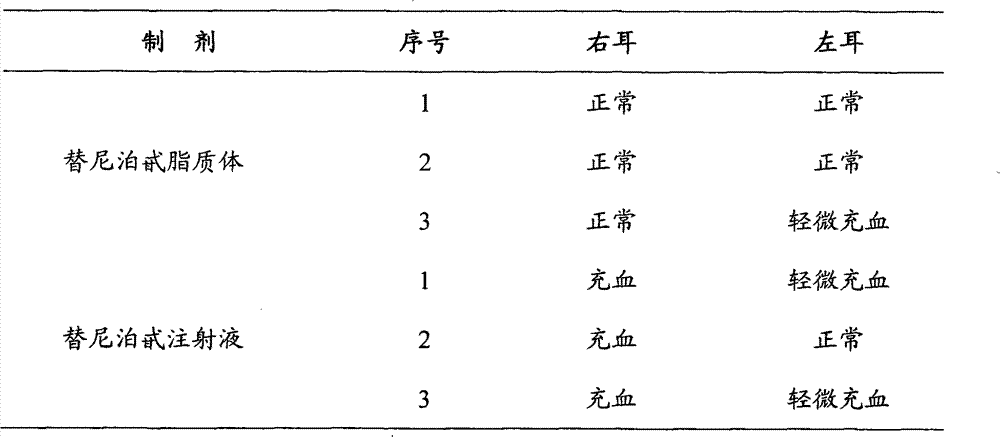
Teniposide solid lipid nanoparticle and preparation method thereof
InactiveCN101596155ALow toxicityEliminate allergic reactionsOrganic active ingredientsAntineoplastic agentsSolubilityMedicine
The invention discloses a teniposide solid lipid nanoparticle and a preparation method thereof in industrial application. The teniposide solid lipid nanoparticle comprises an effective treatment dose of teniposide, a lipid material and an emulsifier, has smaller grain diameter, high entrapment rate and good stability, improves the solubility and the stability of the teniposide, reduces the toxicity of the teniposide, prolongs the cycle time of medicaments in blood and improves the therapeutic index of the medicaments, thereby having the characteristics of low toxicity and irritability and high efficiency in clinical application.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
A kind of hepatocyte growth-stimulating factor, its preparation and application
ActiveCN102294014AAvoid the production of antihypertensive substancesEnergy savingPeptide/protein ingredientsDigestive systemUltrafiltrationHollow fibre
The invention relates to a hepatocyte growth-promoting factor. A preparation method for the hepatocyte growth-promoting factor comprises the following steps of: selecting materials; treating tissues; mixing the materials and injection water at room temperature, uniformly stirring, and freezing homogenate liquid to the temperature of between -20 and -35 DEG C; unfreezing the homogenate liquid which is subjected to freezing storage for at least seven days, then heating to the temperature of between 83 and 85 DEG C, immediately removing a heating source, filtering, and adjusting the pH value to be 6.5; filtering by using a hollow fiber column with the molecular weight cutoff of 20,000, then filtering by using an ultrafiltration membrane with the molecular weight cutoff of 10,000, freezing ultrafiltrate to the temperature of between -20 and -30 DEG C, and storing in a sealing way; and unfreezing the ultrafiltrate which is subjected to freezing storage for at least seven days, filtering, concentrating, filtering the concentrated solution by using a hollow fiber column with the molecular weight cutoff of 10,000, degerming by using a filtration membrane with the aperture of 0.22 micrometer, performing split charging, and storing at the temperature of below -18 DEG C. The invention also relates to a preparation and application of the hepatocyte growth-promoting factor. The hepatocyte growth-promoting factor has high activity and high yield and is safely used.
Owner:GUANGZHOU YIPINHONG PHARMA +4
Method for preparing sponge products from tannic acid modified natural latex
ActiveCN105295127AEliminate allergic reactionsFacilitate dissociationPillowsStuffed mattressesAntibacterial propertyRaw material
The invention discloses a method for preparing sponge products from tannic acid modified natural latex. The method comprises the following steps: the first step: treating tannic acid by using soft water, strong ammonia water and a nonionic surfactant or sodium dodecyl sulfate so as to obtain a tannic acid solution, wherein the tannic acid solution is prepared from the following ingredients in mass ratio: 20 parts of tannic acid, 3-8 parts of strong ammonia water, 3-5 parts of the nonionic surfactant or sodium dodecyl sulfate and 70-75 parts of soft water; the second step: with natural latex as a raw material, uniformly adding and dispersing the tannic acid solution into natural latex according to the mass ratio that 0.5-3.0 parts of the tannic acid solution is added into each 100 parts of dry natural latex in a raw material preparation process; the third step: bubbling, carrying out injection molding, carrying out gelatinization, vulcanizing, demoulding, washing with water and drying so as to obtain the sponge products. According to the invention, since tannic acid is added into the natural latex, anaphylaxis caused by water soluble protein as well as influences of variable-valence metal ions on ageing performance of the products are eliminated, and ageing resistance and antibacterial properties of the natural latex sponge products are improved.
Owner:温州市鑫时利实业有限公司
Emulsifiable paste for treating urticaria papulosa and preparation method thereof
InactiveCN104095869AInhibit migrationInhibition formationAntibacterial agentsOrganic active ingredientsTherapeutic effectTime effect
The invention discloses an emulsifiable paste for treating urticaria papulosa. The emulsifiable paste is prepared from the following raw materials in parts by weight: 1 to 2 parts of butyric acid hydrocortisone, 13 to 17 parts of erythromycin lactobionate, 7 to 9 parts of chlorpheniramine maleate and 1000.5 to 1071.5 parts of auxiliary materials. The preparation method comprises the following steps: A) substrate preparation: taking the auxiliary materials of 63 parts of glycerinum, 150 parts of vaseline, 100 parts of cetanol, 100 parts of liquid paraffin, 1 part of hydroxy benzene acetic acid and 600 parts of purified water, placing into a vessel, heating at the temperature of 85 to 90 DEG C, then, adding 25 parts of leveling agent O, stopping heating, and stirring uniformly until generation of condensation so as to acquire O / W type emulsifiable paste substrate; B) emulsifiable paste preparation: taking powder raw materials of 1.5 parts of the butyric acid hydrocortisone, 15 parts of the erythromycin lactobionate and 8 parts of the chlorpheniramine maleate, adding into the O / W type emulsifiable paste substrate in step A, and stirring uniformly; C) packaging: respectively taking 15 parts of the emulsifiable paste, and sub-packaging with plastic cases. According to the emulsifiable paste, stimulation reaction and anaphylactic reaction of skin can be avoided; besides, the emulsifiable paste is short in therapy time-effect, high in cure rate, simple in preparation process, convenient to produce and process and definite in therapy effect.
Owner:王卫武
Teniposide liposome and preparation method thereof
InactiveCN101579312BTargetedGood curative effectOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityTherapeutic effect
The invention provides teniposide liposome capable of being used for injection or oral administration, which is characterized in that teniposide is encapsulated by phospholipids, and the teniposide liposome with small grain diameter, high encapsulation rate, good stability and low toxic and side effect is prepared. The teniposide liposome prepared by the method improves the solubility and the stability of the teniposide, lowers the toxicity and prolongs the circulation time of drug in blood, thereby improving the treatment effect of the drug, and leading the preparation prepared by the liposome to have the characteristics of low toxicity, low hypersensitivity and high efficiency. The invention also relates to a preparation method of the teniposide liposome, which has simple process and lowcost and is suitable to industrial production.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Human source anti-A botulinum neurotoxin genetic engineering antibody and preparation method and use thereof
ActiveCN101045751AStrong characteristicHigh activityImmunoglobulins against animals/humansAntinoxious agentsEscherichia coliSide effect
This invention relates to human source, a type kreotoxin resistant neurotoxin monoclonal antibody. This antibody possesses amino acid sequence as 35 in sequence table. This invention also discloses isomeride of above antibody, its amino acid sequence of heavy chain as 36 in sequence table, amino acid sequence of light chain as 37 in sequence table. Above antibody through following method preparing: first by molecular biology or other method prepare HuAb - BNa gene that possess idiosyncratic neutralize activity and high affinity, express in prokaryotic cell such as colibacillus, eukaryon as microzyme, mammal cell as CHO and so on, after purification to obatin homogeneous HuAb - BNa protein. This HuAb - BNa possess trait of strong idiosyncratic neutralize activity, high affinity, and no side effect, lend itself to first-aid treatment of A type kreotoxism neurotoxin, at the same time lend itself to detect of A type kreotoxism neurotoxin, possess favorable application prospects.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Analgesic and detumescence plaster, and preparation method and application thereof
InactiveCN110742993AIncrease contentImprove efficacyAnthropod material medical ingredientsAntipyreticMyrrhAngelica Sinensis Root
The invention relates to the field of external application of traditional Chinese medicine preparations, in particular to an analgesic detumescence plaster, a preparation method and application thereof. The analgesic detumescence plaster is prepared by mixing a medicinal extract A and powder B, the medicinal extract A is prepared from the following bulk pharmaceutical chemicals in parts by weight:20-90 parts of stephania terandra, 20-90 parts of cassia twig, 20-90 parts of radix angelicae pubescentis, 20-90 parts of herba asari, 20-90 parts of radix aconiti, 20-90 parts of radix aconiti agrestis, 20-90 parts of pericarpium zanthoxyli, 20-90 parts of radix aconiti lateralis preparata, 20-90 parts of rhizoma arisaematis, 20-90 parts of rhizoma curcumae longae, 20-90 parts of peach kernels,20-90 parts of angelica sinensis, 20-90 parts of olibanum and 20-90 parts of myrrh; and the powder B is prepared from the following bulk pharmaceutical chemicals in parts by weight: 6-20 parts of semen brassicae, 6-20 parts of ground beeltle, 6-20 parts of pseudo-ginseng, 6-20 parts of the olibanum, 6-20 parts of the myrrh and 6-20 parts of the pericarpium zanthoxyli. The analgesic detumescence plaster is used for treating rheumatoid arthritis, osteoproliferation, synovitis, lumbar intervertebral disc prolapse and various joint pain and swelling, the analgesic and detumescence effects are good, operation is easy, carrying is convenient, toxic side effects are avoided, the pesticide effect of a composition traditional Chinese medicine material is proportionately developed, the medicine utilization is high, and the treatment effect is good.
Owner:FIRST AFFILIATED HOSPITAL OF LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Immunoglobulin for resisting O and Asia I type foot and mouth disease used for cow and pig
InactiveCN1966079APrevent invasionThe protective effect is indeedAntiviralsAntibody ingredientsType antigenGlycoside formation
The invention relates to an immunity globulin used to prevent O-type and I-type foot-and-mouth diseases, of cow and pig, wherein it mixes foot-and-mouth disease O-type and I-type antigens in 1-2:1-2 volume ratio; mixes Freund's adjuvant with immunogen at 1:1 volume ratio; uses glycosides injection as immunity strengthener. The inventive production meets the standard of national animal biological product standard (2000 version). And it can prevent O-type and I-type foot-and-mouth diseases.
Owner:张中洋
Air purifier
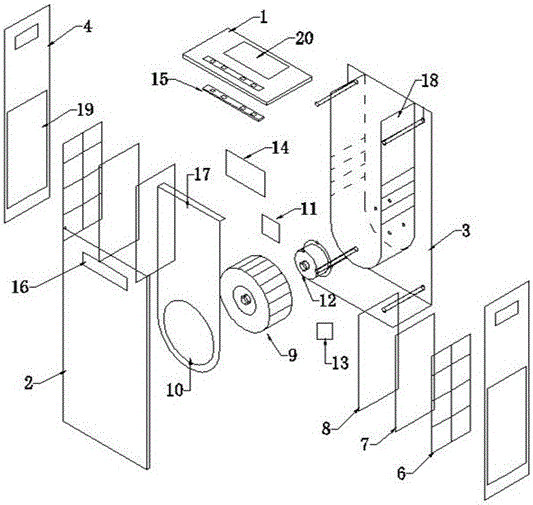
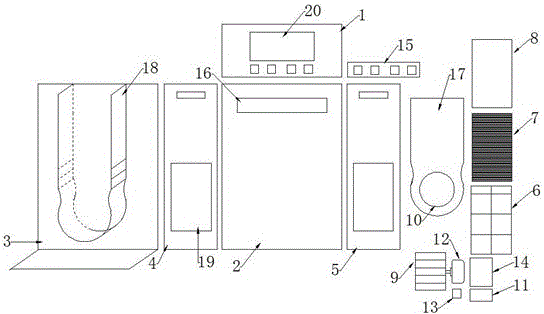
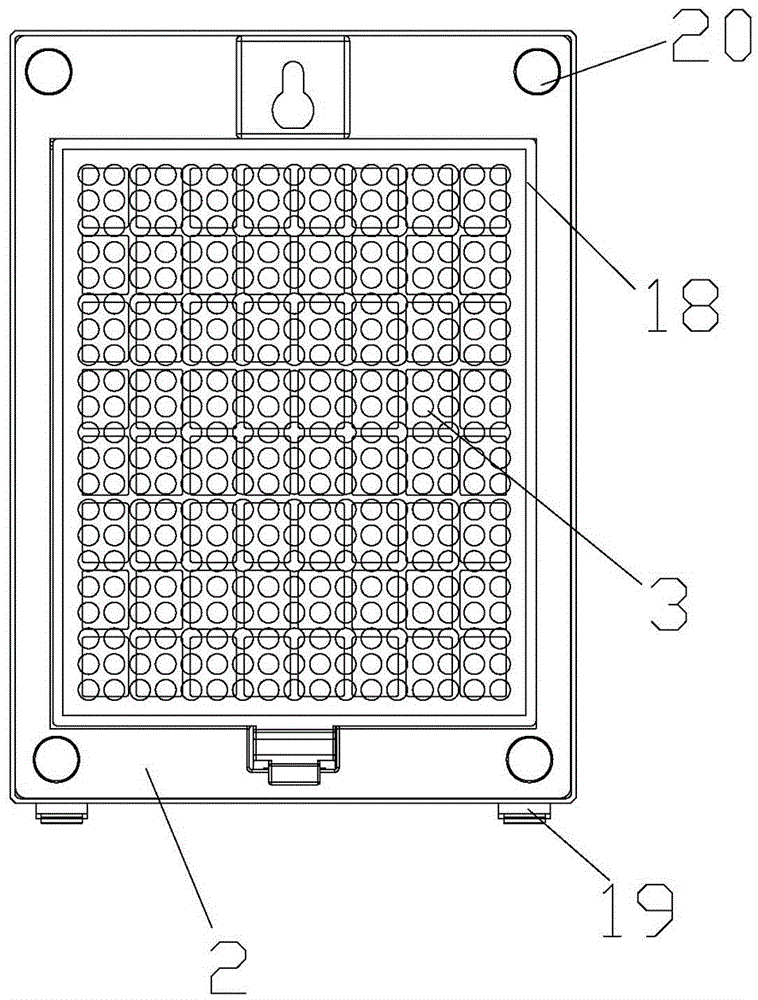
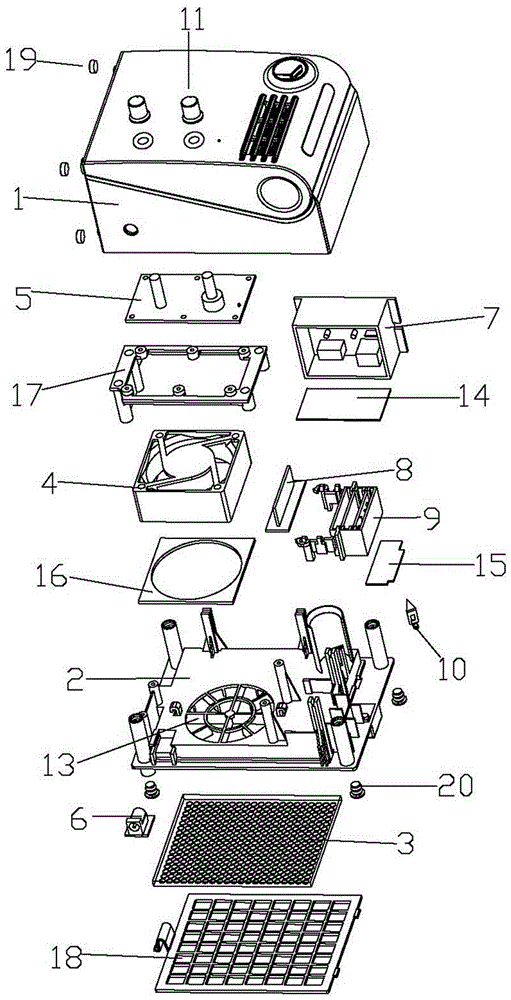
ActiveCN105042708AEasy to carryEasy to operateMechanical apparatusSpace heating and ventilation safety systemsAir cycleAir purifiers
The invention discloses an air purifier. The air purifier comprises a case, a filter system and a power system. The case comprises a front shell, a rear shell, a left cover plate, a right cover plate and an upper cover plate. The power system comprises an air wheel, a motor and a transformer. A U-shaped air blocking groove is formed in the rear shell, a U-shaped air blocking plate matched with the U-shaped air blocking groove is arranged on the U-shaped air blocking groove, the air wheel and the motor are fixedly installed at the bottom of the air blocking groove, a round air guiding opening is formed in the bottom of the air blocking plate, an air outlet is formed in the upper cover plate and connected with the top of the air blocking groove, air inlets are formed in the lower portion of the left cover plate and the lower portion of the right cover plate respectively, and the filter system is arranged between the air inlet and the air blocking groove. In operation of the air purifier, air in a room or a vehicle enters from the air inlets in the two sides of the air purifier and is exhausted from the air outlet in the top of the case after a series of treatment. The air purifier is simple in structure and can efficiently remove various pollutants in the room, the circulation speed of the air in the room is high, and filter efficiency is high.
Owner:JIANGSU ZHONGKE RUISAI POLLUTION CONTROL ENG
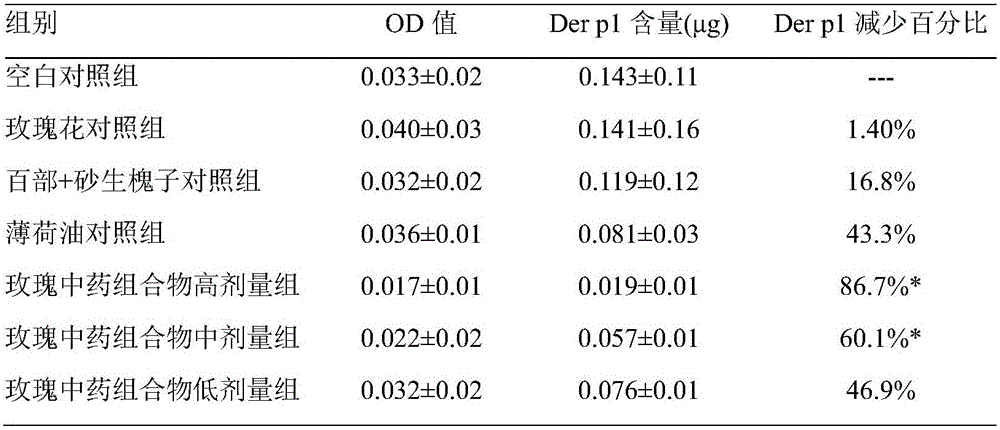
Rose traditional Chinese medicine composition for eliminating dust mites and preparation method and application thereof
ActiveCN105794883AReasonable compositionEliminate allergic reactionsBiocideDead animal preservationDrugDust mites
The invention belongs to the field of biological medicine, and particularly relates to a rose traditional Chinese medicine composition for eliminating dust mites and a preparation method and application thereof. The rose traditional Chinese medicine composition is prepared from rose flowers, jasmine flowers, mint oil, radix stemonae and seeds of sophora moorcroftiana. The rose traditional Chinese medicine composition has the effect of eliminating dust mites, and can be applied in preparation of medicine for killing mites.
Owner:GANSU DONGFANG TIANRUN ROSE TECH DEV CO LTD
Human source anti- tetanus exotoxin antibody and preparation method and use thereof
InactiveCN100391975CAvoid allergic reactionsAddressing issues that predispose to allergic reactionsAntibacterial agentsImmunoglobulins against virusesEscherichia coliHypersensitive response
The invention discloses a human source anti-tetanus exotoxin antibody (HTAT-Fab) and preparation method, comprising: constructing human immunity phage antibody bank, screening phage positive clone, further getting HTAT-Fab gene with a specific neutralization activity and high affinity. The gene can be expressed in the procaryotic cell such as E.coli, eukaryotic cell such as microzyme, or mammalian cell such as CHO, purifying to get highly purified HTAT-Fab with a strong tissue penetrability, a high affinity. The HTAT-Fab product can not only eliminate the allergic reaction generated by horse serum anti-tatanus antitoxin (TAT) (foreign protein), but also avoid the blood source for producing human tetanus immunoglobulin (HTIG) and the latent virus pollution.
Owner:北京明新高科技发展有限公司 +2
Preparation method of hepatocyte growth-promoting factors employing ultrasonic treatment
InactiveCN104388509AHigh activityEliminate allergic reactionsPeptide preparation methodsFermentationWater bathsFiber
The invention discloses a preparation method of hepatocyte growth-promoting factors employing ultrasonic treatment. The method comprises the following steps: 1) pretreatment of porcine hepatocytes; 2) homogenizing; 3) ultrasonic treatment, namely processing porcine hepatocytes homogenate in an ultrasonic treatment machine in an ice bath by virtue of an ultrasonic wave to obtain ultrasonic homogenate; 4) enzymolysis, putting the ultrasonic homogenate into a reaction container, dropwise adding a hydrochloric acid until the pH value is 2.5-3.5, adding pepsase and stirring, and then putting the reaction container and contents of the reaction container in a water bath kettle to hydrolyze to obtain an enzymolysis material; 5) inactivation; 6) centrifuging; 7) coarse filtration, namely filtering a neutral centrifugal liquid by virtue of a hollow fiber ultrafiltration device of which the molecular weight cut off is 100KD and a hollow fiber ultrafiltration device of which the molecular weight cut off is 10KD to obtain coarse filtrate; and 8) ultrafiltration, namely carrying out ultrafiltration on the coarse filtrate by virtue of an ultrafiltration membrane of which the molecular weight cut off is 6,000D, so as to obtain a hepatocyte growth-promoting factor liquid. The method disclosed by the invention is simple and mild in process, and high in extraction rate; and the final product is relatively high in activity and high in safety.
Owner:HEFEI PINGGUANG PHARMA
High-extraction rate preparation method of high-activity hepatocyte growth-promoting factor
InactiveCN104388510APromote fragmentationPromote dissolutionPeptide preparation methodsFermentationPig liverFiber
The invention discloses a high-extraction rate preparation method of a high-activity hepatocyte growth-promoting factor. The preparation method comprises the following steps: 1) performing pretreatment on a suckling pig liver; 2) homogenizing; 3) preparing pepsin; 4) performing enzymolysis: putting a homogenate of the suckling pig liver into a reaction container, dropping a hydrochloric acid solution till the pH value is 2.5-3.5, further adding the pepsin, stirring and then putting the reaction container and contents thereof into a water bath pot for hydrolysis to obtain an enzymolyzed material; 5) inactivating; 6) centrifuging; 7) performing coarse filtration: sequentially filtrating neutral centrifugal liquid with a hollow fiber ultrafiltration device with molecular weight cutoff of 100KD and a hollow fiber ultrafiltration device with the molecular weight cutoff of 10KD to obtain coarse filtrate; and 8) performing ultrafiltration: performing ultrafiltration on the coarse filtrate with an ultrafiltration membrane with the molecular weight cutoff of 6000D to obtain a hepatocyte growth-promoting factor solution. The preparation method disclosed by the invention has the advantages of simple and mild process, energy conservation, environment friendliness and high extraction rate; and the prepared hepatocyte growth-promoting factor is relatively high in activity and high in safety.
Owner:HEFEI PINGGUANG PHARMA
Amphiphilic tri-block copolymer taxol bonding medicament and synthesis method thereof
InactiveCN1961962BSolve the problem of insoluble in waterEliminate allergic reactionsOrganic active ingredientsPharmaceutical non-active ingredientsPolyesterSynthesis methods
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Cefquinome liposome
ActiveCN104000783BReduce toxicityTargetedAntibacterial agentsOrganic active ingredientsSterolSOY ISOFLAVONES
Cefquinome liposome, which belongs to the field of pharmaceutics, has a particle size of less than 1000 nm, and is mainly prepared from the following raw materials by weight: 1 part of cefquinome, 1-40 parts of phospholipid, 0-15 parts of sterol or soy isoflavone glucoside or soyasapogenol, and 0-15 parts of additives. The cefquinome liposome can be prepared into a liquid preparation, and can also be prepared into a solid preparation by adding a proper amount of a support agent. The cefquinome liposome or cefquinome long circulating liposome prepared in the invention contains no irritant substances, and can relieve anaphylactic reaction; the obtained preparation has good stability, has an average liposome particle size of less than 1000 nm, and has encapsulation efficiency of more than 80%. The preparation method is mature in process, simple, practical, and suitable for industrial production.
Owner:HENAN SOAR VETERINARY PHARMA
Long-circulating solid lipid docetaxel nanoparticles and preparation method thereof
InactiveCN101653414BGood water solubilityImprove stabilityOrganic active ingredientsAntineoplastic agentsSolubilityLipid formation
The invention discloses long-circulating solid lipid docetaxel nanoparticles and a preparation method thereof. The long-circulating solid lipid docetaxel nanoparticles comprise the following materials in therapeutic effective dose: docetaxel, lipid materials, long-circulating auxiliary materials and an emulsifier. The long-circulating solid lipid docetaxel nanoparticles have small particle size, high encapsulation rate and good stability, and not only improve the solubility and the stability of the docetaxel, reduce the toxicity of the docetaxel, but also prolong the circulating time of a medicament in blood, and improve the therapeutic index of the medicament, so that the preparation has the characteristics of low toxicity, low allergy, high efficiency and targeting in clinical application.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Human source anti-A botulinum neurotoxin genetic engineering antibody and preparation method and use thereof
ActiveCN100575363CStrong characteristicHigh activityImmunoglobulins against animals/humansAntinoxious agentsEscherichia coliSide effect
This invention relates to human source, a type kreotoxin resistant neurotoxin monoclonal antibody. This antibody possesses amino acid sequence as 35 in sequence table. This invention also discloses isomeride of above antibody, its amino acid sequence of heavy chain as 36 in sequence table, amino acid sequence of light chain as 37 in sequence table. Above antibody through following method preparing: first by molecular biology or other method prepare HuAb - BNa gene that possess idiosyncratic neutralize activity and high affinity, express in prokaryotic cell such as colibacillus, eukaryon as microzyme, mammal cell as CHO and so on, after purification to obatin homogeneous HuAb - BNa protein. This HuAb - BNa possess trait of strong idiosyncratic neutralize activity, high affinity, and no side effect, lend itself to first-aid treatment of A type kreotoxism neurotoxin, at the same time lend itself to detect of A type kreotoxism neurotoxin, possess favorable application prospects.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Hepatocyte growth-promoting factor and preparation and application thereof
ActiveCN102294014BIncrease content concentrationIncreased biological activityPeptide/protein ingredientsDigestive systemHollow fibreFiber
The invention relates to a hepatocyte growth-promoting factor. A preparation method for the hepatocyte growth-promoting factor comprises the following steps of: selecting materials; treating tissues; mixing the materials and injection water at room temperature, uniformly stirring, and freezing homogenate liquid to the temperature of between -20 and -35 DEG C; unfreezing the homogenate liquid which is subjected to freezing storage for at least seven days, then heating to the temperature of between 83 and 85 DEG C, immediately removing a heating source, filtering, and adjusting the pH value to be 6.5; filtering by using a hollow fiber column with the molecular weight cutoff of 20,000, then filtering by using an ultrafiltration membrane with the molecular weight cutoff of 10,000, freezing ultrafiltrate to the temperature of between -20 and -30 DEG C, and storing in a sealing way; and unfreezing the ultrafiltrate which is subjected to freezing storage for at least seven days, filtering, concentrating, filtering the concentrated solution by using a hollow fiber column with the molecular weight cutoff of 10,000, degerming by using a filtration membrane with the aperture of 0.22 micrometer, performing split charging, and storing at the temperature of below -18 DEG C. The invention also relates to a preparation and application of the hepatocyte growth-promoting factor. The hepatocyte growth-promoting factor has high activity and high yield and is safely used.
Owner:GUANGZHOU YIPINHONG PHARMA +4
A kind of preparation method of fermented coconut milk
ActiveCN107095099BIncrease application pathPromote leachingFood ingredient functionsBiotechnologyNutrition
The invention mainly relates to the technical field of food processing, and discloses a preparation method of fermented coconut milk, comprising: raw material crushing, raw material enzymatic hydrolysis, raw material ripening, primary fermentation, secondary fermentation, nutrition strengthening, and packaging; simple preparation, rich raw materials, Balanced nutrition, rich flavor, sweet and sour taste, appropriate concentration, fat content reduced to 4.6%, selenium content reached 17.32μg / 100g; Flammulina velutipes Poria can increase fragrance and nutrition, strengthen the body, and reduce the allergic reaction of the body; combine all After adding water to the solid raw material, carry out two enzymatic hydrolysis to decompose the macromolecular structure of the raw material, decompose the protein into amino acids, reduce allergic reactions, increase the juice yield, and accelerate the leaching of active ingredients; Fusion makes the fragrance rich and harmonious, protects gastrointestinal function, and is easy to digest and absorb.
Owner:阜南椰枫食品有限公司
A kind of method of tannic acid modified natural latex sponge product
ActiveCN105295127BEliminate allergic reactionsFacilitate dissociationPillowsStuffed mattressesPolymer scienceAdhesive
A method for modifying natural rubber latex sponge products with tannic acid, the first step: utilize soft water, concentrated ammonia water, nonionic surfactant or sodium lauryl sulfate to treat tannic acid to obtain a tannic acid solution, the tannic acid The nic acid solution is prepared according to the following mass proportions: tannic acid: 20 parts, concentrated ammonia water: 3-8 parts, nonionic surfactant or sodium lauryl sulfate: 3-5 parts, soft water: 70-75 parts. Step 2: using natural rubber latex as raw material, in the raw material preparation process, the tannic acid solution obtained above is evenly added in a mass ratio of 0.5 to 3.0 dry parts per 100 dry rubber, and dispersed in the natural rubber latex. The third step: Foaming, injection molding, gelling, vulcanization, demoulding, washing and drying to prepare sponge products. Adding tannic acid to natural rubber latex can eliminate the allergic reaction caused by water-soluble protein and the influence of variable price metal ions on the aging performance of products, and improve the aging resistance of natural latex sponge products and the antibacterial performance of natural latex sponge products.
Owner:温州市鑫时利实业有限公司
Gelatin-free bivalent renal syndrome vaccine freeze-drying protective agent
InactiveCN105435235AHarm reductionImprove securitySsRNA viruses negative-sensePowder deliveryProtective antigenArginine
The invention discloses a gelatin-free bivalent renal syndrome vaccine freeze-drying protective agent, and provides a formula and a preparation technology of the freeze-drying protective agent. According to the bivalent renal syndrome vaccine freeze-drying protective agent prepared from saccharose, glycine, human serum albumin, arginine, mannitol and a phosphate buffer solution, the problems and defects that in the prior art, an anaphylactic reaction and a fever reaction exist are avoided, so that for the freeze-drying protective agent of a bivalent inactivated vaccine for a hemorrhagic fever with renal syndromes, the defect of gelatin is overcome, and the purposes of eliminating the anaphylactic reaction and the fever reaction are achieved.
Owner:ZHEJIANG VACIN BIO PHARMA LTD
Air purifier
ActiveCN103100106AEliminate allergic reactionsKill mitesDispersed particle separationGaseous substancesAllergic reactionPrinted circuit board
The invention belongs to the technical field of a household electrical appliance product and particularly relates to an air purifier for purifying air. The air purifier comprises a front cover and a rear cover, wherein a containing space is formed by the front cover and the rear cover; the containing space is internally provided with a direct-current fan, an electronic control board, a power supply, a main board, a PCB (Printed Circuit Board) panel, a voltage multiplier and an insertion piece, wherein the voltage multiplier, the direct-current fan, the power supply, the insertion piece and the main board are arranged on the inner surface of the rear cover; the electronic control board is arranged in front of the direct-current fan; a filter is arranged on the outer surface of the rear cover; the front cover is provided with a knob switch and an air outlet; and an air inlet is formed in the position of the rear cover and relative to the position of the direct-current fan. Compared with the prior art, the air purifier disclosed by the invention not only can kill harmful bacteria, moulds and funguses in indoor air, but also can effectively eliminate awful smell dispersed by tobaccos, lampblack and garbage, and dispersed in the cooking process; and the air purifier eliminates odors dispersed by indoor ammonia, carbon monoxide, marsh gas and dispersed from a sewer exhaust pipe, can eliminate an allergic reaction of people to pollen and can also effectively kill acarids.
Owner:东莞冠唯电子科技有限公司
an air purifier
ActiveCN103100106BElimination is effectiveReduce the smellDispersed particle separationGaseous substancesEngineeringAllergic reaction
The invention belongs to the technical field of a household electrical appliance product and particularly relates to an air purifier for purifying air. The air purifier comprises a front cover and a rear cover, wherein a containing space is formed by the front cover and the rear cover; the containing space is internally provided with a direct-current fan, an electronic control board, a power supply, a main board, a PCB (Printed Circuit Board) panel, a voltage multiplier and an insertion piece, wherein the voltage multiplier, the direct-current fan, the power supply, the insertion piece and the main board are arranged on the inner surface of the rear cover; the electronic control board is arranged in front of the direct-current fan; a filter is arranged on the outer surface of the rear cover; the front cover is provided with a knob switch and an air outlet; and an air inlet is formed in the position of the rear cover and relative to the position of the direct-current fan. Compared with the prior art, the air purifier disclosed by the invention not only can kill harmful bacteria, moulds and funguses in indoor air, but also can effectively eliminate awful smell dispersed by tobaccos, lampblack and garbage, and dispersed in the cooking process; and the air purifier eliminates odors dispersed by indoor ammonia, carbon monoxide, marsh gas and dispersed from a sewer exhaust pipe, can eliminate an allergic reaction of people to pollen and can also effectively kill acarids.
Owner:东莞冠唯电子科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com