Preparation method of etoposide, teniposide and analogs of etoposide and teniposide
A technology of teniposide and etoposide, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of difficult configuration control, high toxicity, low yield, etc., and achieves the scope of application Wide, easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
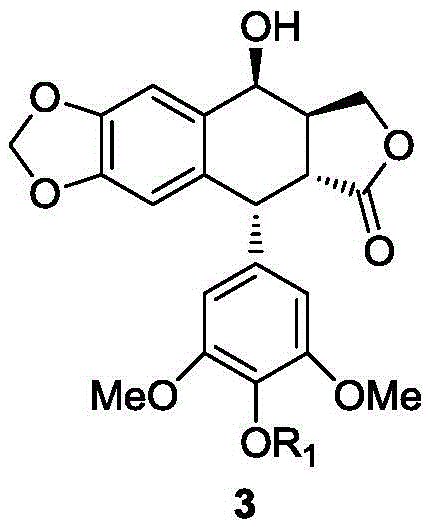
[0028] Preparation of podophyllotoxin 4-O-(2,3,4,6-tetra-O-benzoyl-β-D-glucose)podophyllotoxin glycoside
[0029]
[0030] Step 1: Synthesis of a perbenzoyl-protected glucose donor:
[0031]
[0032] Under the protection of nitrogen, 5g, 8.4mmol of fully Bz-protected glucose with exposed anomeric position and 1.87g, 10.1mmol of o-alkynylbenzoic acid were dissolved in dry 10mL DCM, and then 2g, 10.1mmol of EDCI, and 1g, 10mmol of DMAP were added to the system And DIPEA3ml, 16.7mmol, and stirred at room temperature for 3h, TLC tracking to the end of the reaction. The crude product was concentrated under reduced pressure in the reaction system, and then the glucosynyl ester donor (6.1 g, 95%) was obtained by column chromatography;
[0033] Step 2: Preparation of podophyllotoxin 4-O-(2,3,4,6-tetra-O-benzoyl-β-D-glucose)podophyllotoxin glycoside
[0034]
[0035] Under nitrogen protection, 115mg, 0.15mmol of whole benzoyl-protected glucose donor and 41mg, 0.1mmol of podo...
Embodiment 2
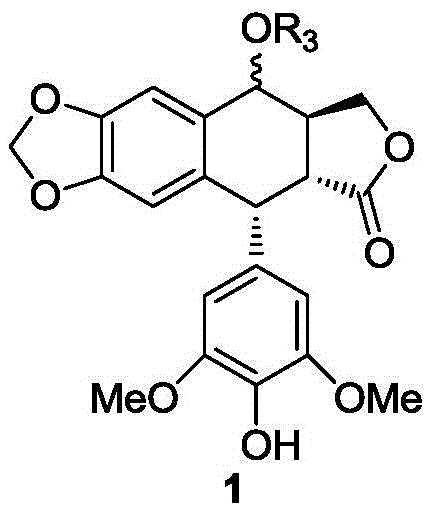
[0038] Preparation of epipodophyllotoxin 4-O-(2,3,4,6-tetra-O-benzoyl-β-D-glucose) epipodophyllotoxin glycoside
[0039]
[0040] Step 1: as shown in embodiment 1 step 1;
[0041] Step 2: Preparation of epipodophyllotoxin
[0042]
[0043] Under nitrogen protection, dissolve 1.0g, 2.41mmol of podophyllotoxin and 993.2mg, 9.65mmol of sodium bromide in 24mL of acetonitrile, then add 0.63mL of methanesulfonic acid, 9.65mmol of Water, then add barium carbonate 1.906g, 9.65mmol, stir at room temperature until the reaction is complete. The reaction system was extracted and dried, concentrated under reduced pressure to obtain a crude product, and purified by column chromatography to obtain 950 mg of the target product, 95%;
[0044] Step 3: Preparation of epipodophyllotoxin 4-O-(2,3,4,6-tetra-O-benzoyl-β-D-glucose) epipodophyllotoxin glycoside
[0045]
[0046] Under nitrogen protection, dissolve 230mg, 0.3mmol of whole benzoyl-protected glucose donor and 41mg, 0.1mmol of...
Embodiment 3
[0048] Preparation of podophyllotoxin 4-O-(2,3,6-tri-O-benzoyl-β-L-rhamnose)podophyllotoxin glycoside
[0049]
[0050] Step 1: Preparation of all-benzoyl-protected rhamnose donor;
[0051]
[0052] Under the protection of nitrogen, 5g, 10.5mmol of rhamnose and 2.3g, 12.6mmol of fully Bz-protected rhamnose exposed at the anomeric position and 2.3g, 12.6mmol of o-alkynylbenzoic acid were dissolved in dry 12mL DCM, and then 2.3g, 11.6mmol of EDCI was added to the system , DMAP1.2g, 11.6mmol and DIPEA3.5ml, 19.5mmol, and stirred at room temperature for 3h, followed by TLC until the end of the reaction. The reaction system was concentrated under reduced pressure to obtain the crude product, and then column chromatography yielded 6.1 g of the rhamnosynyl ester donor, 93%;
[0053] Step 2: Preparation of podophyllotoxin 4-O-(2,3,6-tri-O-benzoyl-β-L-rhamnose)podophyllotoxin glycoside
[0054]
[0055] Under the protection of nitrogen, dissolve 97mg, 0.15mmol of rhamnose do...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com