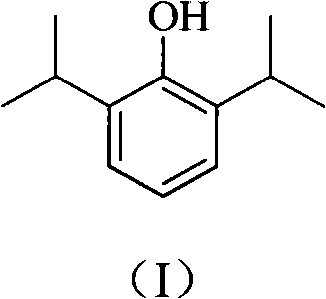
Novel method for preparing high-purity propofol
A propofol, high-purity technology, applied in the field of medicine, can solve the problems of high reaction cost, high equipment requirements, difficult removal, etc., and achieves the effects of simple post-processing, low equipment cost, and low environmental protection cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 1000 g (30%, 3.0 mol) of sulfuric acid into a 3 L flask, add 177 g (1.0 mol) of 2,6-diisopropylaniline under stirring, and stir to dissolve until clear. Cool to -5°C, slowly add dropwise a solution of 69g (1.0mol) sodium nitrite and 210ml of water, stir for about 20min after the addition, and store at -5°C for later use. Add 800 g of 25% sulfuric acid into the 3L flask, heat to 127°C, drop the diazonium salt solution in batches, and continue the reaction for about 2 hours. After the reaction solution was cooled, it was extracted three times with 80ml toluene, and the organic phases were combined and separated by distillation, and the 87-89°C / 2mmHg fraction was collected with a purity of >99% (GLC).
Embodiment 2
[0028] Add 910 g (10%, 2.5 mol) of hydrochloric acid into a 3 L flask, add 177 g (1.0 mol) of 2,6-diisopropylaniline under stirring, and stir to dissolve until clear. Cool to 15°C, slowly add dropwise 104g (1.5mol) of sodium nitrite and 155ml of water to obtain a solution, stir for about 20 minutes after the addition, and store at -5°C for later use. Add 500 g of 30% hydrochloric acid into a 3 L flask, heat to 105° C., drop the diazonium salt solution in batches, and continue the reaction for about 2 h. After the reaction solution was cooled, it was extracted three times with 80ml toluene, and the organic phases were combined and separated by distillation, and the 87-89°C / 2mmHg fraction was collected with a purity of >99% (GLC).
Embodiment 3
[0030] Add 245g (60%, 1.5mol) of phosphoric acid into a 3L flask, add 177g (1.0mol) of 2,6-diisopropylaniline under stirring, stir and dissolve until clear. Cool to 5°C, slowly add dropwise 55g (0.8mol) of sodium nitrite and 495ml of water to obtain a solution, stir for about 20 minutes after the addition, and store at -5°C for later use. Add 300 g of 70% phosphoric acid into a 3 L flask, heat to 120° C., drop the diazonium salt solution in batches, and continue the reaction for about 2 h. After the reaction solution was cooled, it was extracted three times with 80ml toluene, and the organic phases were combined and separated by distillation, and the 87-89°C / 2mmHg fraction was collected with a purity of >99% (GLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com