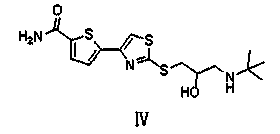
Preparation method of arotinolol hydrochloride
A technology of alololol hydrochloride and molar ratio, which is applied in the field of preparation of arololol hydrochloride, can solve the problems that the specific process of arololol hydrochloride is not announced, the product purity is not enough, and the process cycle is long, etc., to achieve easy post-processing Simple operation and post-processing, and the effect of improving product purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
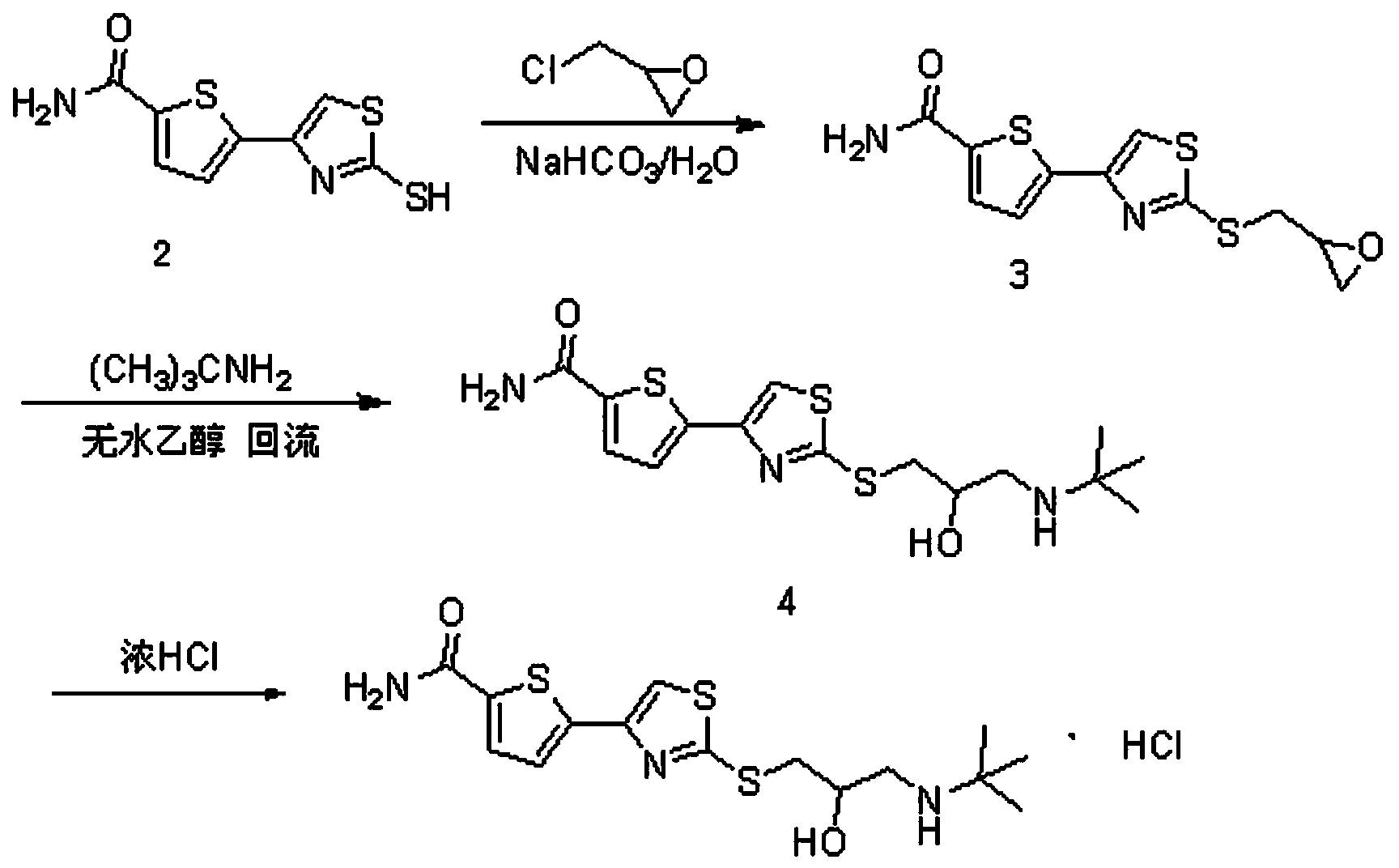
[0045] Prepare arolol hydrochloride as follows:
[0046] (1) Synthesis of 2-[2,3-epoxypropyl-4-(5-carbamoyl-2-thienyl)]thiazole
[0047]Dissolve sodium bicarbonate (3.36g, 0.04mol) into 290mL distilled water, add 5-(2-mercapto-4-thiazolyl)-2-thiophenecarboxamide (9.69g, 0.04mol) in batches, stir for 30 min, Add epichlorohydrin (3.70g, 0.04mol), react at 20°C for 4h, monitor the completion of the reaction with TCL, filter with suction, and filter the crude product with 71mL of methyl tert-butyl ether to obtain a pale yellow solid, which is dried to obtain 2-[2 , 3-epoxypropyl-4-(5-carbamoyl-2-thienyl)]thiazole (10.38 g, yield 87%).
[0048] (2) Synthesis of Alololol
[0049] Dissolve 2-[2,3-epoxypropyl-4-(5-carbamoyl-2-thienyl)]thiazole (11.94 g, 0.04 mol) and tert-butylamine (8.78 g, 0.12 mol) in absolute ethanol After reflux at 70~75°C for 14 hours, the reaction liquid was concentrated to remove absolute ethanol, the residue was added to 89 mL of toluene and slurried for 2...
Embodiment 2
[0053] Prepare arolol hydrochloride as follows:
[0054] (1) Synthesis of 2-[2,3-epoxypropyl-4-(5-carbamoyl-2-thienyl)]thiazole
[0055] Dissolve sodium bicarbonate (6.72g, 0.08mol) into 290mL distilled water, add 5-(2-mercapto-4-thiazolyl)-2-thiophenecarboxamide (9.69g, 0.04mol) in batches, stir for 30 min, Add epichlorohydrin (3.70g, 0.04mol), react at 20°C for 4h, monitor the completion of the reaction with TCL, filter with suction, and filter the crude product with 71mL of methyl tert-butyl ether to obtain a pale yellow solid, which is dried to obtain 2-[2 , 3-epoxypropyl-4-(5-carbamoyl-2-thienyl)]thiazole (10.74 g, yield 90%).
[0056] (2) Synthesis of Alololol
[0057] Dissolve 2-[2,3-epoxypropyl-4-(5-carbamoyl-2-thienyl)]thiazole (11.94 g, 0.04 mol) and tert-butylamine (14.63 g, 0.20 mol) in absolute ethanol After reflux at 70-75°C for 14 hours, the reaction solution was concentrated to remove absolute ethanol, the residue was added to 89 mL of toluene to make a slur...
Embodiment 3
[0061] Prepare arolol hydrochloride as follows:
[0062] (1) Synthesis of 2-[2,3-epoxypropyl-4-(5-carbamoyl-2-thienyl)]thiazole
[0063] Dissolve sodium bicarbonate (6.72g, 0.08mol) into 290mL distilled water, add 5-(2-mercapto-4-thiazolyl)-2-thiophenecarboxamide (9.69g, 0.04mol) in batches, stir for 30 min, Add epichlorohydrin (3.70g, 0.04mol), react at 40°C for 4h, monitor the completion of the reaction with TCL, filter with suction, and filter the crude product with 71mL of methyl tert-butyl ether to obtain a light yellow solid, which is dried to obtain 2-[2 , 3-epoxypropyl-4-(5-carbamoyl-2-thienyl)]thiazole (11.22 g, yield 94%).
[0064] (2) Synthesis of Alololol
[0065] Dissolve 2-[2,3-epoxypropyl-4-(5-carbamoyl-2-thienyl)]thiazole (11.94 g, 0.04 mol) and tert-butylamine (14.63 g, 0.20 mol) in absolute ethanol After reflux at 75-80°C for 20 h, the reaction solution was concentrated to remove absolute ethanol, the residue was added to 89 mL of toluene and slurried for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com