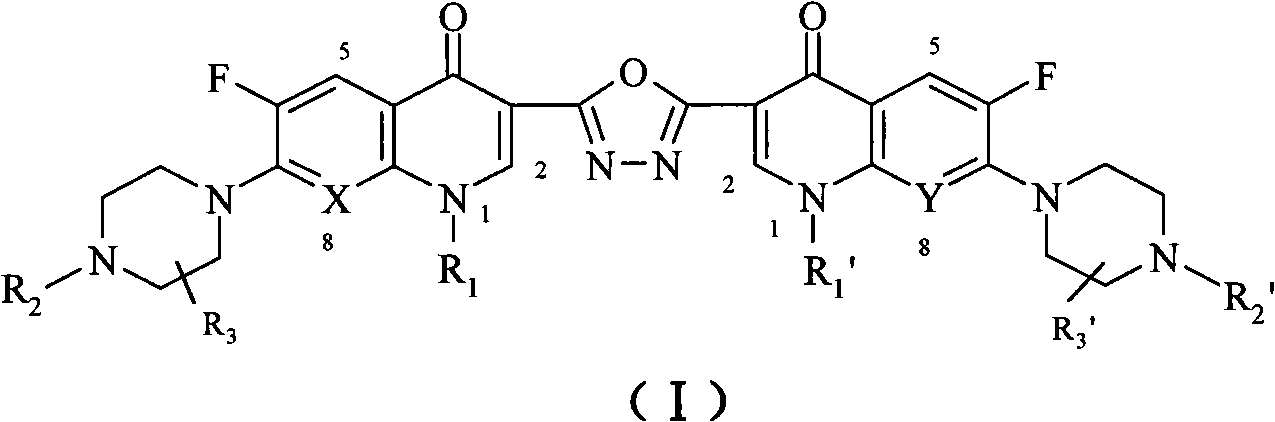
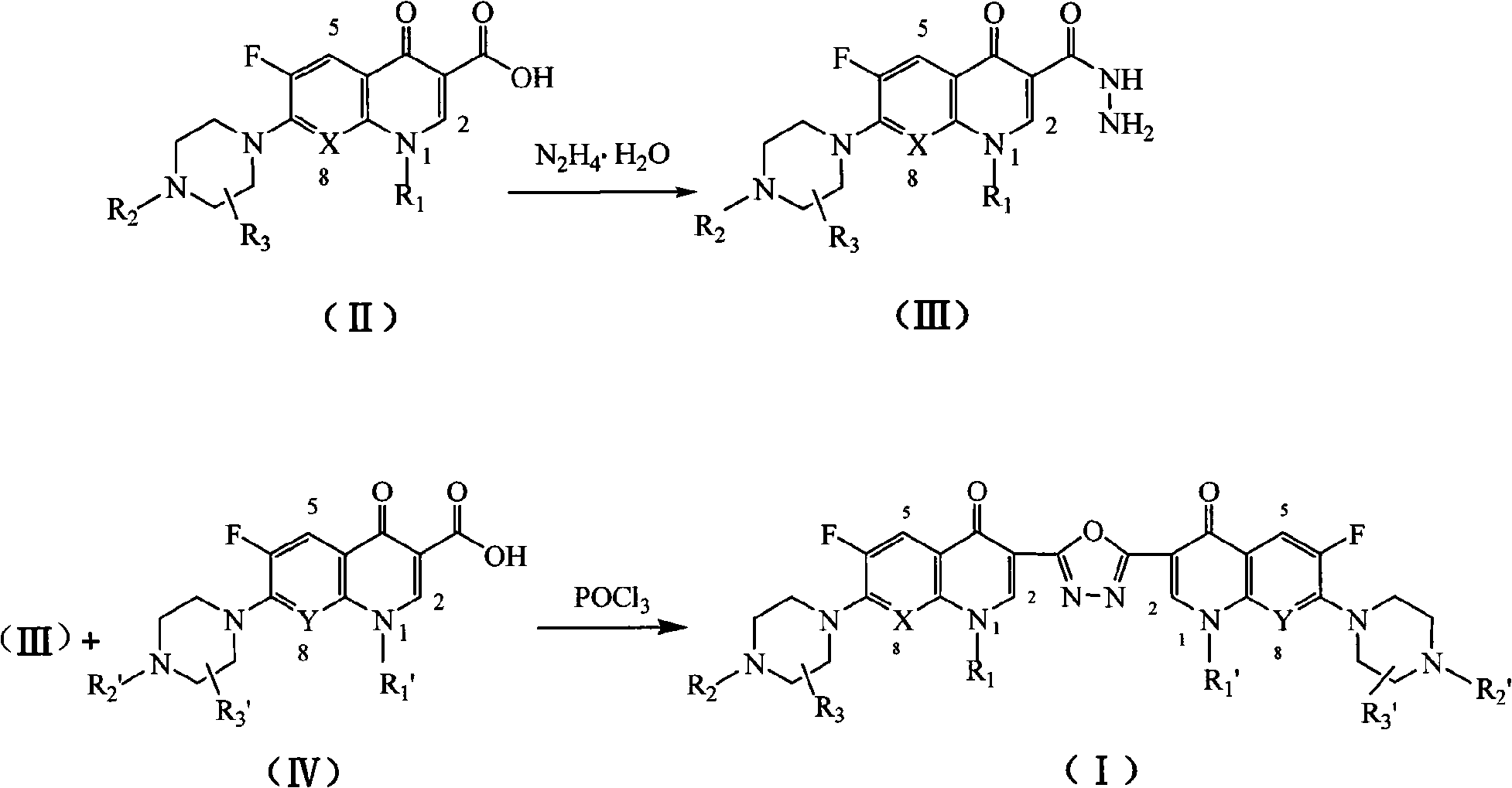
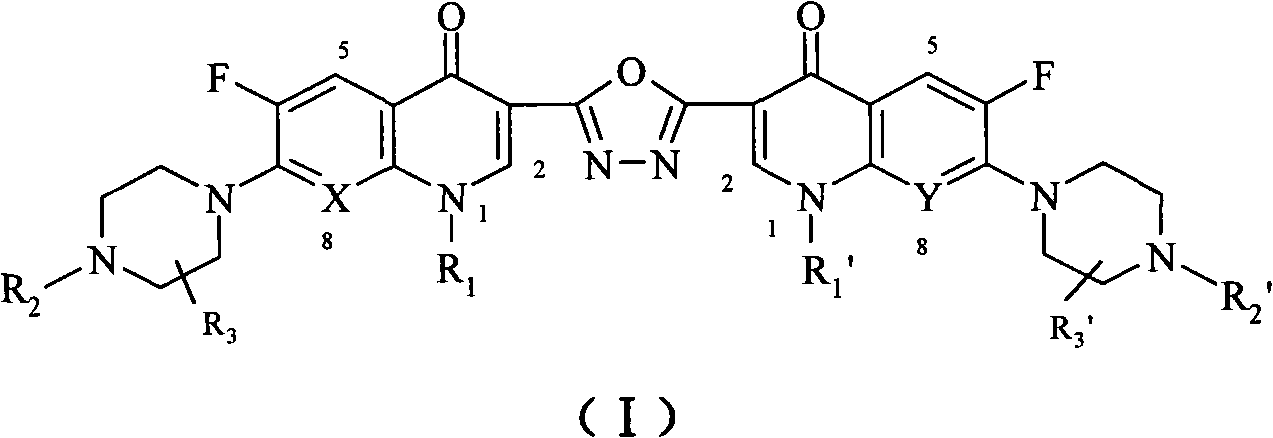
C3/C3 fluoroquinolone dimmer derivative using oxadiazole as connection chain as well as preparation method and application thereof
A technology of quinolone dimer and oxadiazole, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems that have not been reported, and achieve the effects of strong anti-tumor activity, strong growth inhibitory activity, and strong cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of 1-ethyl-6-fluoro-7-piperazin-1-yl-4(1H)-quinolinone-3-carboxhydrazide (norfloxacin hydrazide 1)
[0039] Norfloxacin (50 g, 157 mmol) was dissolved in 100 mL of 80% hydrazine hydrate, refluxed for 18 hours, cooled to room temperature, distilled under reduced pressure until evaporated to dryness, and the residue was recrystallized with 300 mL of absolute ethanol to obtain a yellow solid 1, Yield 74%. mp 186~187℃; IR(KBr)v: 3456, 2897, 1648, 1567, 1527, 1473, 1255, 869cm -1 ; 1 H NMR (DMSO-d 6 )δ: 10.56 (brs, 1H, CONH), 8.86 (s, 1H, 2-H), 7.86 (d, 1H, 5-H), 7.24 (d, 1H, 8-H), 4.53 (brs, 2H , NH 2 ), 4.62 (q, 2H, NCH 2 ), 3.32(t, 4H, piperazine-H), 2.67(t, 4H, piperazine-H), 1.36(t, 3H, CH 3 ); MS m / z: 356 (M+Na), 334 (M+H); Anal.calcd for C 16 h 20 FN5 o 2 : C 57.65, H 6.05, N 21.01; found C 57.84, H 6.15, N 21.24.
Embodiment 2
[0041] Preparation of 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4(1H)-quinolinone-3-carboxhydrazide (pefloxacin hydrazide 2)
[0042] Using pefloxacin as raw material, compound 2 was obtained by the same preparation method as in Example 1, with a yield of 68%. mp192~194℃; IR(KBr)v: 3387, 3025, 1638, 1557, 1525, 1456, 1255, 886cm -1 ; 1 H NMR (DMSO-d 6 )δ: 10.46 (brs, 1H, CONH), 8.87 (s, 1H, 2-H), 7.82 (d, 1H, 5-H), 7.32 (d, 1H, 8-H), 4.56 (brs, 2H , NH 2 ), 4.42 (q, 2H, NCH 2 ), 3.35(t, 4H, piperazine-H), 2.66(t, 4H, piperazine-H), 2.37(s, 3H, N-CH 3 ), 1.34(t, 3H, CH 3 ); MS m / z: 348 (M+H); Anal.calcd for C 17 h 22 FN 5 o 2 : C58.78, H 6.38, N 20.16; found C 58.94, H 6.20, N 20.40.
Embodiment 3
[0044] Preparation of 1-cyclopropyl-6-fluoro-7-piperazin-1-yl-4-(1H)-quinolinone-3-carboxhydrazide (ciprofloxacin hydrazide 3)
[0045] Using ciprofloxacin as raw material, compound 3 can be obtained according to the same preparation method as in Example 1, with a yield of 70%. mp226~227℃; IR(KBr)v: 3428, 3309, 2940, 2834, 1661, 1626, 1471cm -1 ; 1 H NMR (DMSO-d 6 )δ: 1.20~1.35(m, 4H, cyclopropane-H), 3.09~3.12(m, 4H, piperazine-H), 3.25~3.27(m, 4H, piperazine-H), 3.45~3.51(m, 1H , cyclopropane-H), 4.45 (brs, 2H, NH 2 ), 7.32(d, 1H, 8-H), 8.01(d, 1H, 5-H), 8.77(s, 1H, 2-H), 10.83(s, 1H, CONH); MS m / z: 346 (M+H); Anal.calcd for C 17 h 20 FN 5 o 2 : C 59.12, H 5.84, N 20.28; found C 59.36, H 5.67, N 20.46.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com