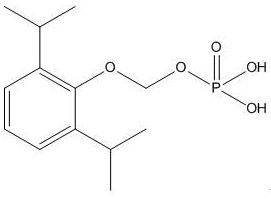
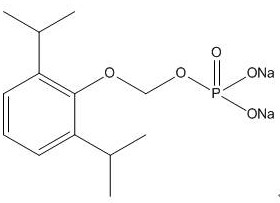
Synthesis method and application of fospropofol sodium and intermediates thereof
A technology of fospropofol sodium and its synthesis method, which is applied in the field of drug synthesis and application, can solve the problems of large side effects and low biological safety, and achieve the effects of simple operation, high yield and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
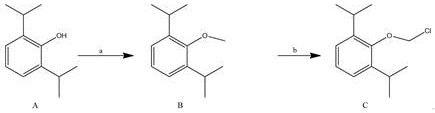
[0044] A kind of preparation method of water-soluble propofol prodrug fospropofol sodium, its preparation method comprises the following steps:
Embodiment 1-a
[0046] Dissolve compound A (propofol) in DMF (5V: the volume mass ratio of DMF to compound A is 5 mL / g), add potassium carbonate (2.0eq: the molar ratio of potassium carbonate to compound A is 2.0), Cool the reaction solution to 0-5°C, add CH dropwise under stirring 3 I(2.0eq:CH 3 The molar ratio of I to Compound A is 2.0) in DMF (1V: DMF vs. CH 3 The volume to mass ratio of I is 1mL / g) solution. After the dropwise addition, it was raised to room temperature for 2-3h reaction, monitored by TLC plate layer. After the reaction was completed, the reaction solution was diluted into ice water, and a large amount of solid was precipitated, filtered and dried to obtain the compound The crude product of B, with a mass yield of 106.8%, was directly used in the next reaction without further purification.
Embodiment 1-b
[0048] Dissolve compound B in DMF (5V: the volume mass ratio of DMF to compound B is 5 mL / g), add phosphorus pentachloride (1.0eq: the molar ratio of phosphorus pentachloride to compound B is 1.0), and heat React at 70°C for 3-4 hours, monitor with TLC plate, filter after the reaction is completed, concentrate the filtrate, add water and ethyl acetate to the remaining liquid, separate the liquid, and use 1M HCl solution and saturated NaHCO for the organic phase respectively 3 The solution was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain a crude compound C with a mass yield of 115.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com