Mecano growth factor peptides and their use
A growth factor, mechanical technology, applied in the direction of insulin-like growth factor, hormone peptide, specific peptide, etc., can solve the problem of expression decline and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0152] Preparation of Polypeptides or Extended Polypeptides of the Invention
[0153] Polypeptides or extended polypeptides of the invention can be prepared using standard techniques. In general, they can be obtained by standard peptide synthesis techniques and, if desired, suitable chemical modifications (eg pegylation) of the resulting amino acid sequences can be carried out. In the absence of D-amino acids, polypeptides and extended polypeptides can also be obtained by recombinant expression in host cells from appropriate coding DNA by standard techniques.
[0154] It can also be isolated and purified to any desired degree using standard techniques. Polypeptides or extended polypeptides of the invention will usually be completely or partially isolated or purified. The preparation of the isolated polypeptide or the extended polypeptide may be any preparation as long as the preparation contains the polypeptide or the extended polypeptide at a higher concentration than the p...
Embodiment
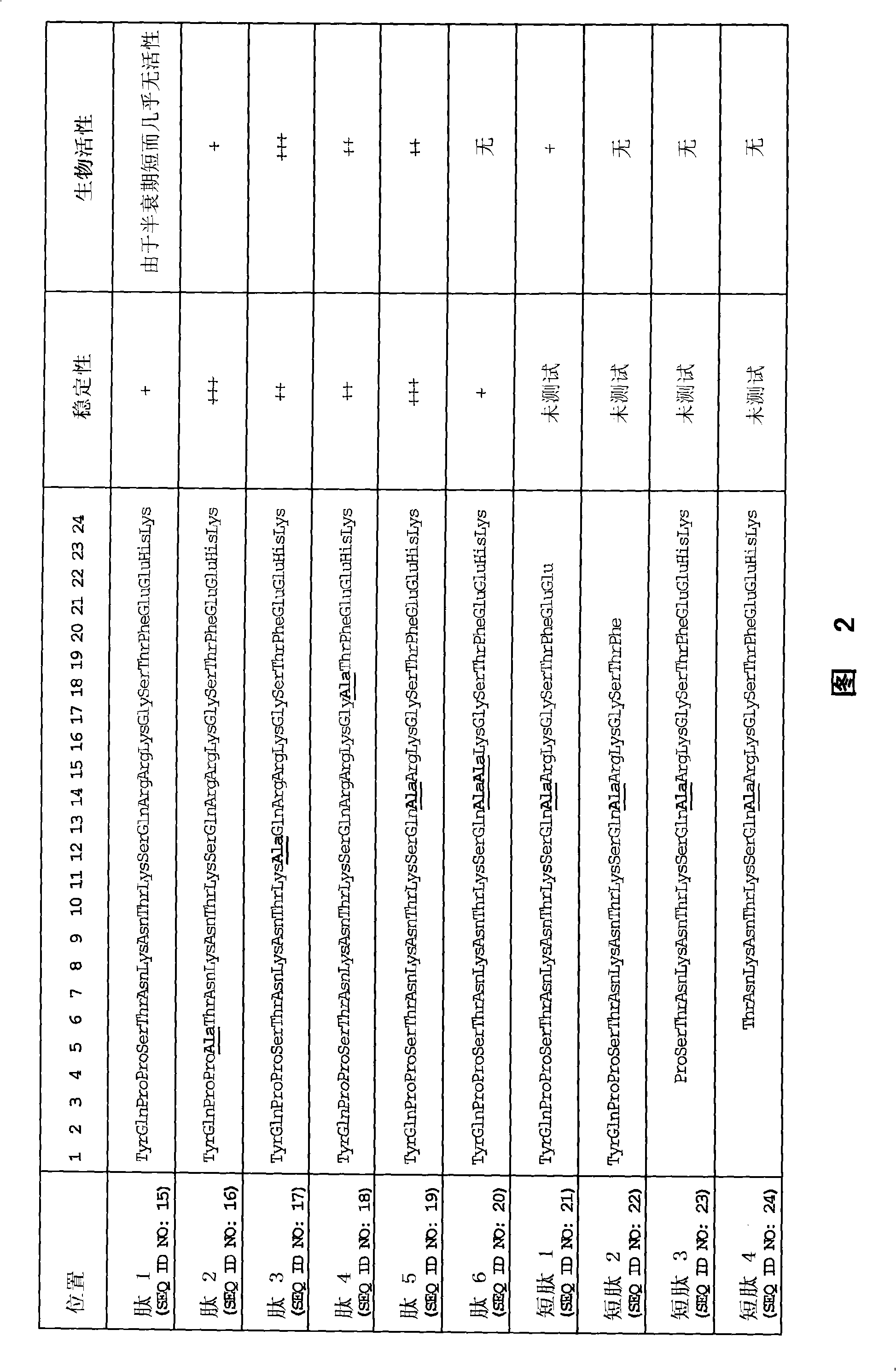
[0170] 1. Peptides
[0171] 1.1 Peptides of Examples 2, 3, 4 and 6
[0172] The peptide used in Examples 2, 3, 4 and 6 has the sequence of SEQ ID NO: 15, wherein the penultimate arginine in the native sequence (see SEQ ID NO: 1, 2 and 27) is replaced by histidine and by replacing the naturally occurring L-arginine with D-arginine at positions 14 and 15, the N-terminus is covalently attached to a polyethylene glycol (PEG) derivative via a succinic acid bridge ( O'O-bis(2-aminopropyl) polyethylene glycol 1900) (Jeffamine), and C-terminal amidation to stabilize the native sequence.
Embodiment 5
[0174] The peptide of Example 5 was purchased from Alta Bioscience, Birmingham, UK and synthesized by standard techniques using a peptide synthesizer. These peptides are non-pegylated, free of L-D conversion and C-terminal amidation.
[0175] In addition, a peptide corresponding to the peptide in 1.1 above with the same L-D conversion but without PEGylation was also tested for stability (see 5.2.3 below). This peptide is synthesized by standard techniques using a peptide synthesizer. The resulting product was purified by HPLC and analyzed by MALDI-MS.
[0176] 1.3 Peptides of Example 7
[0177] 1.3.1 Peptides of Example 7.1
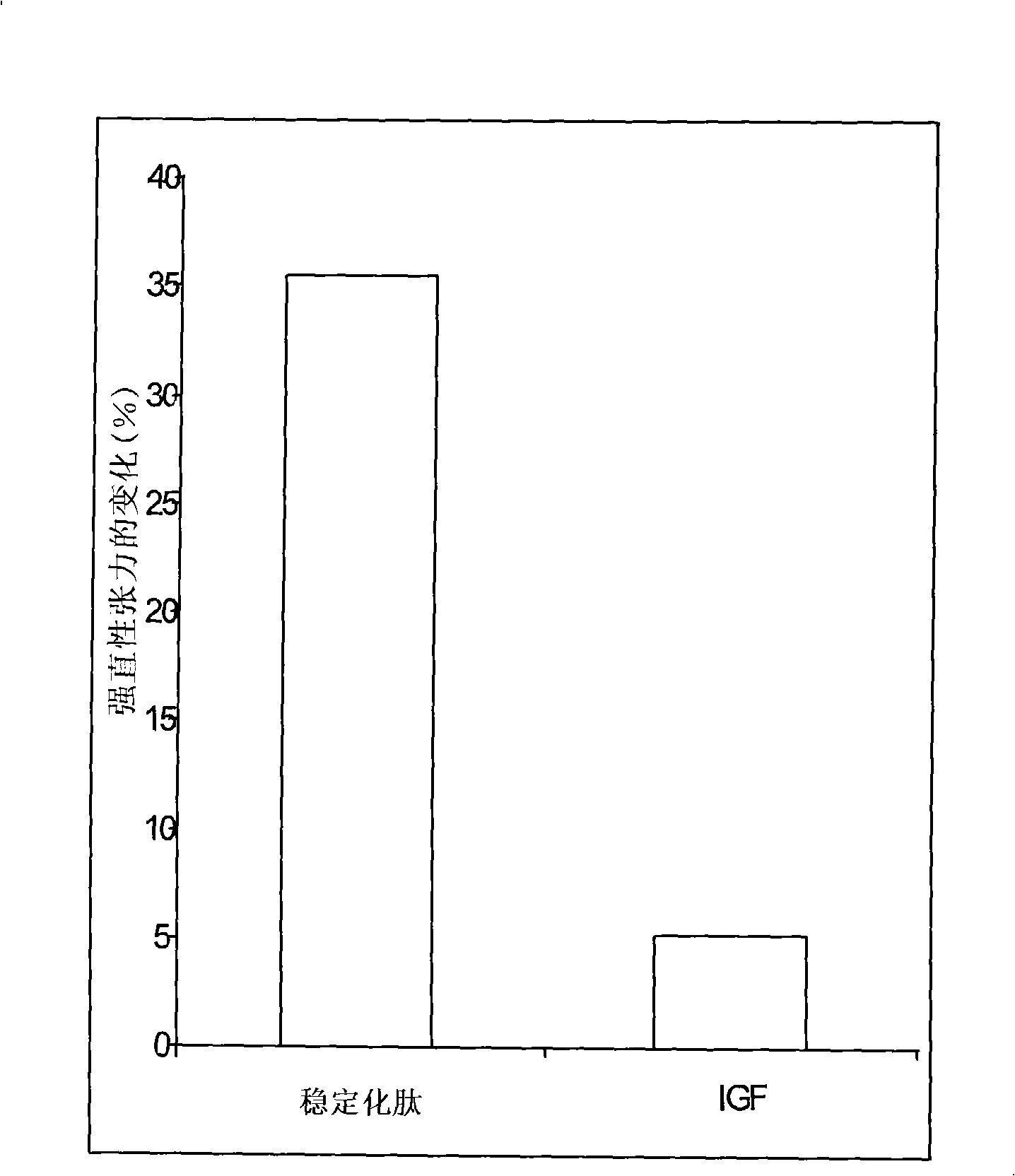
[0178] The peptide of Example 7.1 has an 8 amino acid sequence, Gly-Ser-Thr-Phe-Glu-Glu-His-Lys (SEQ ID NO: 33), with modifications to improve stability. exist Figure 8 In the peptide called DMGF in , stabilization can be achieved by N-terminal PEGylation in 1.1 above. exist Figure 8 In a peptide called CMGF in , stabilization can be achieved by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com