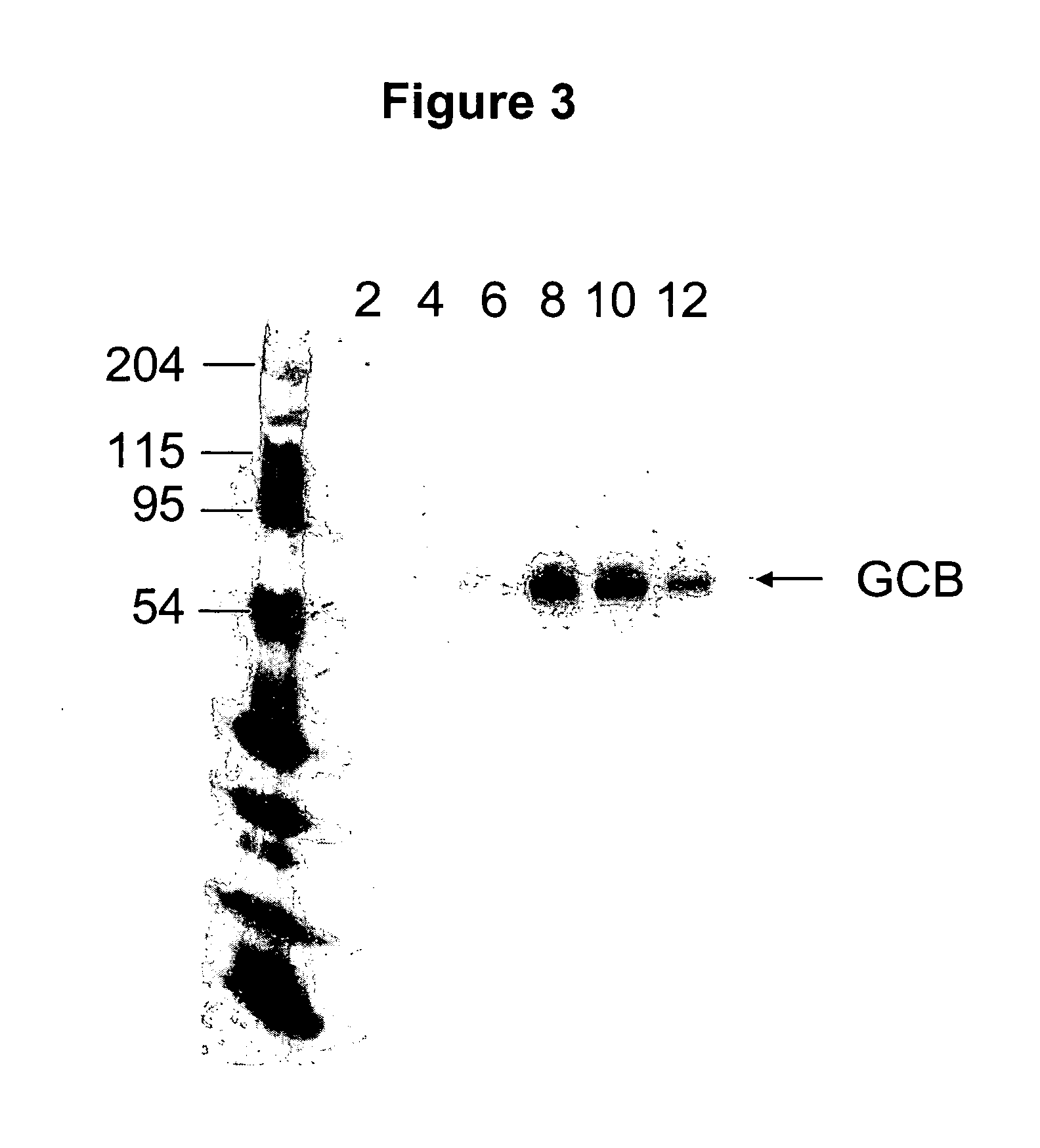
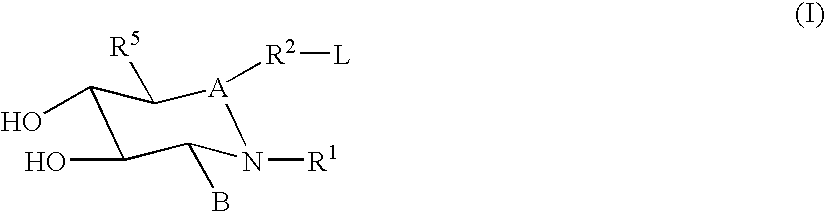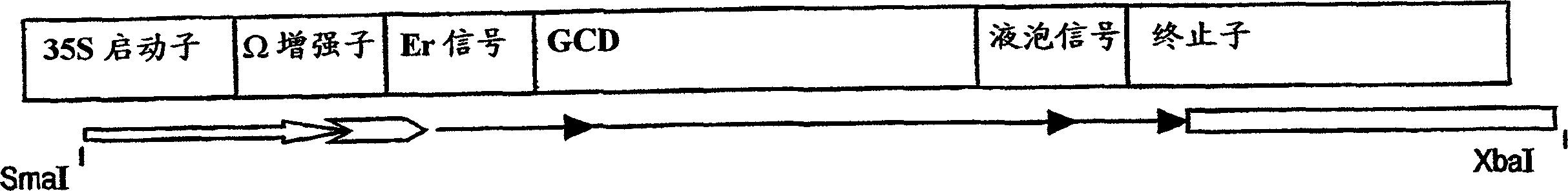Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Glucocerebrosidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
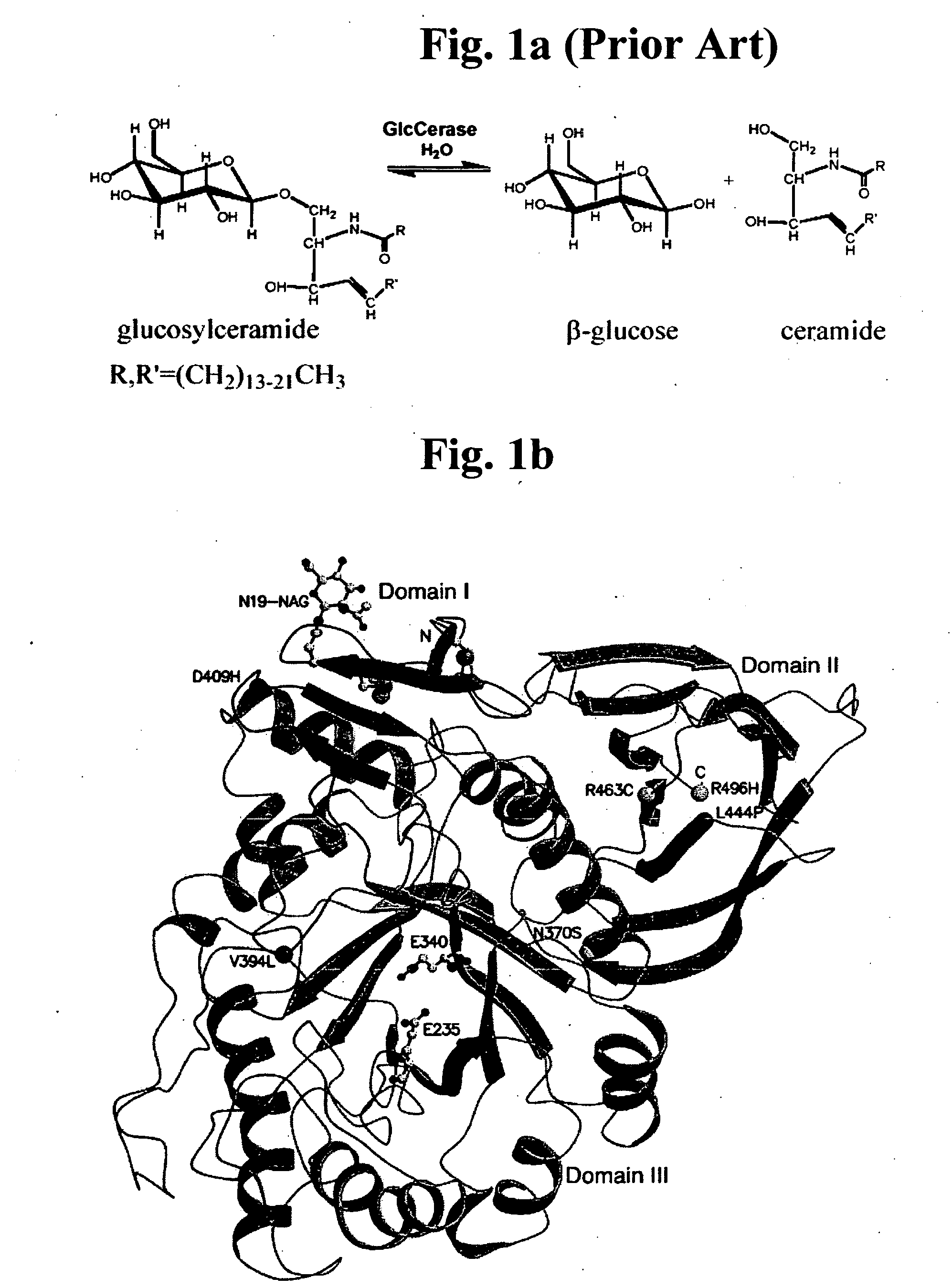
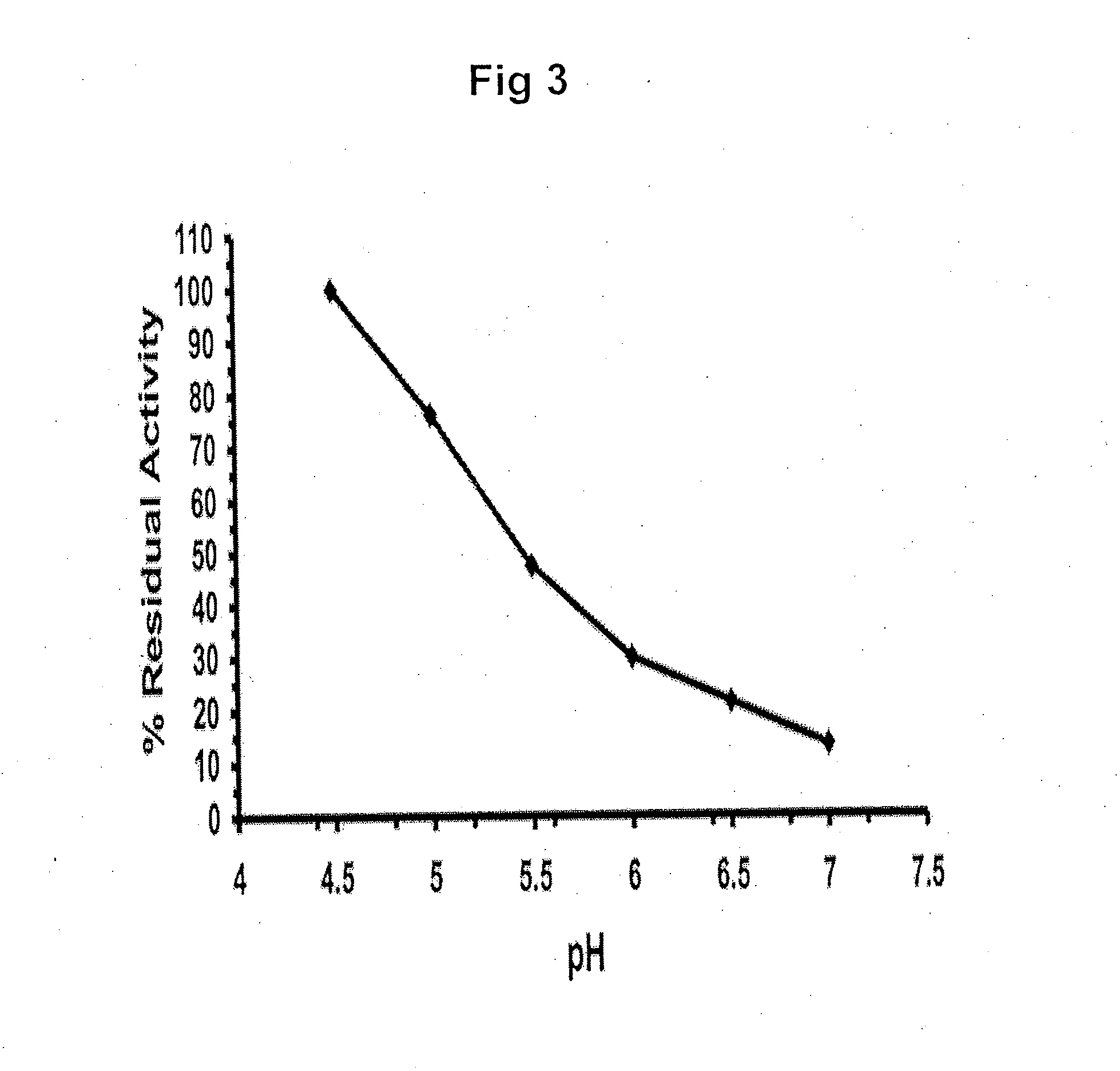
Β-Glucocerebrosidase (also called acid β-glucosidase, D-glucosyl-N-acylsphingosine glucohydrolase, or GCase) is an enzyme with glucosylceramidase activity (EC 3.2.1.45) that is needed to cleave, by hydrolysis, the beta-glucosidic linkage of the chemical glucocerebroside, an intermediate in glycolipid metabolism that is abundant in cell membranes (particularly skin cells). It is localized in the lysosome, where it remains associated with the lysosomal membrane. β-Glucocerebrosidase is 497 amino acids in length and has a molecular weight of 59,700 Daltons.
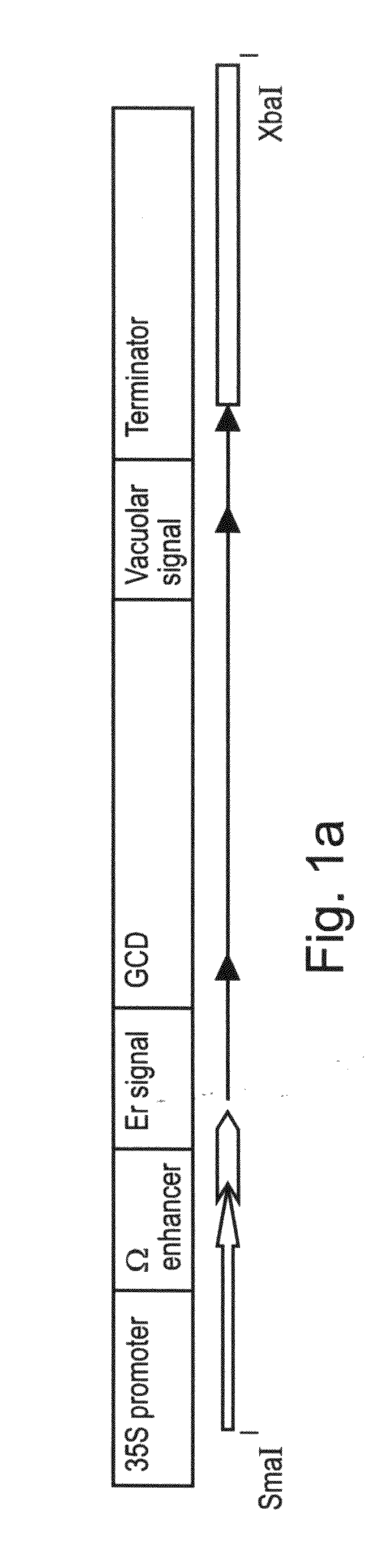
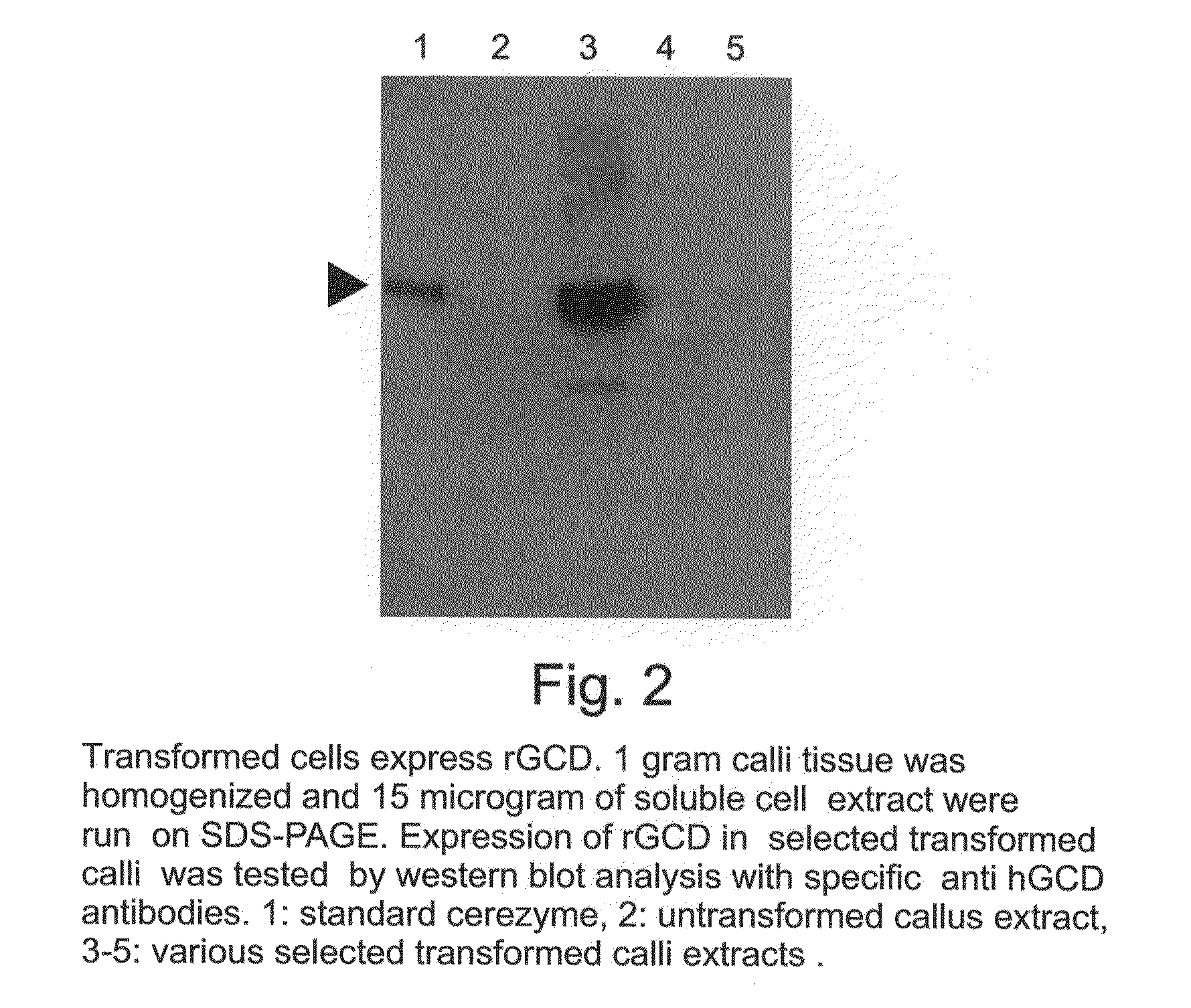
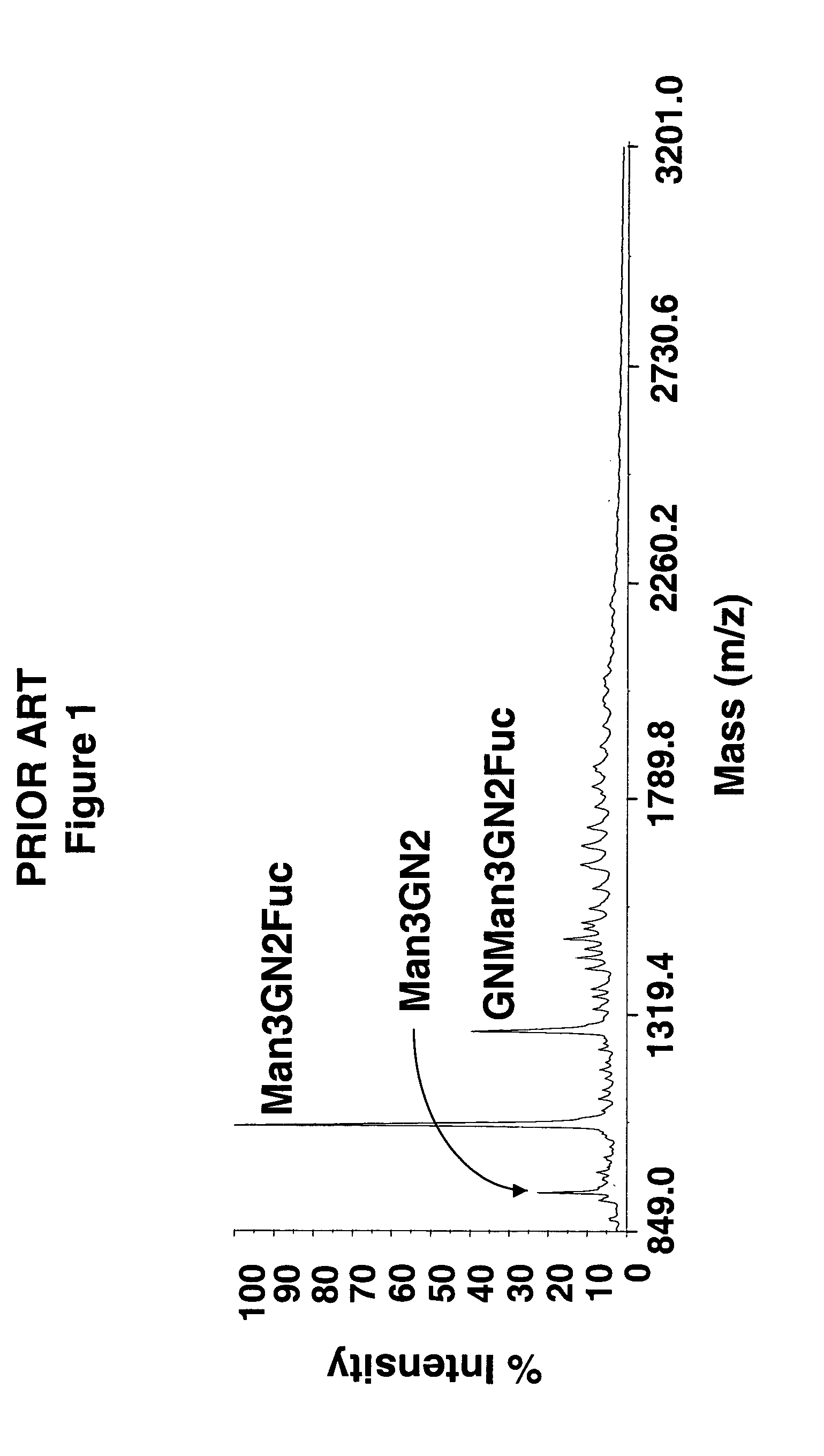
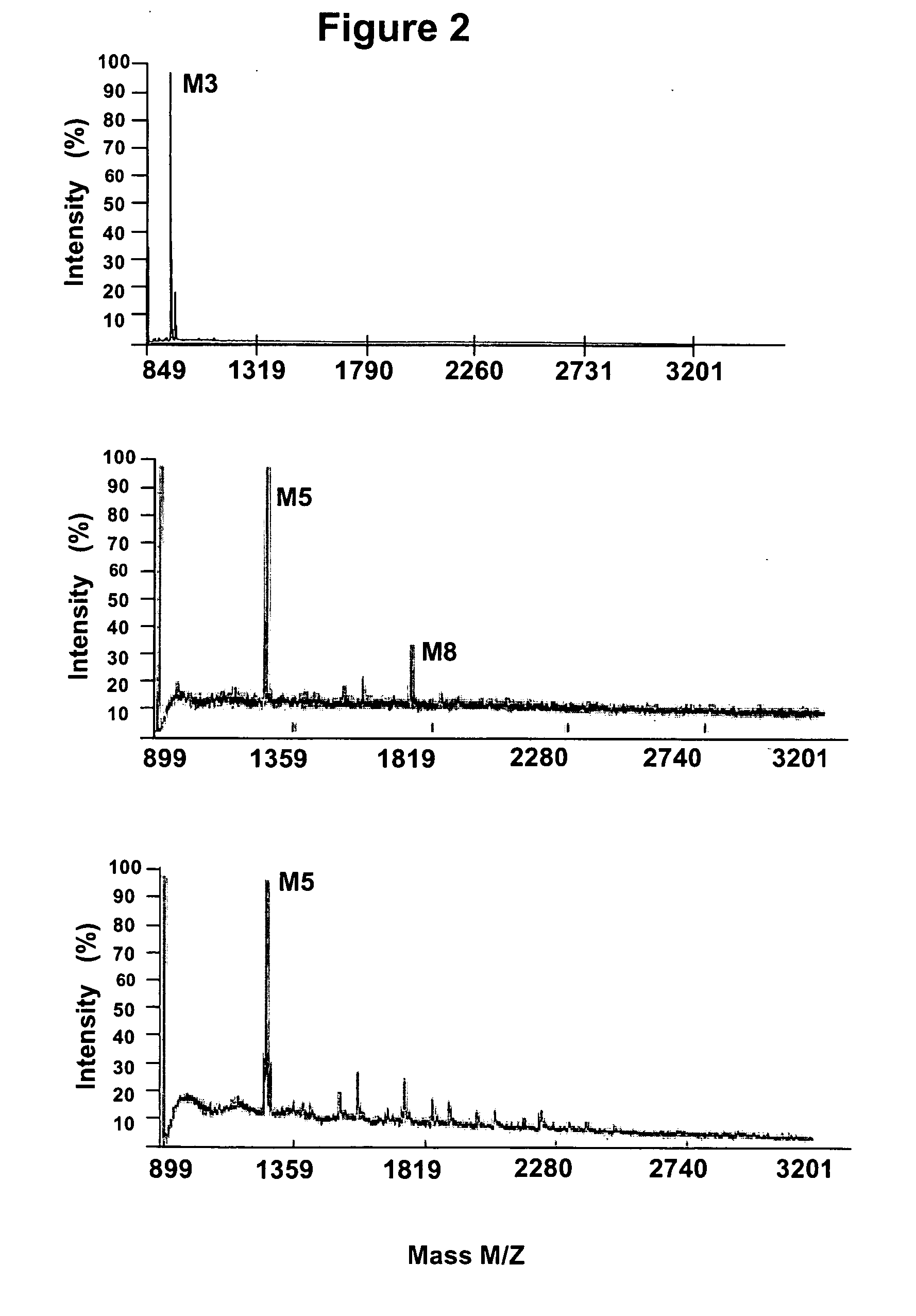
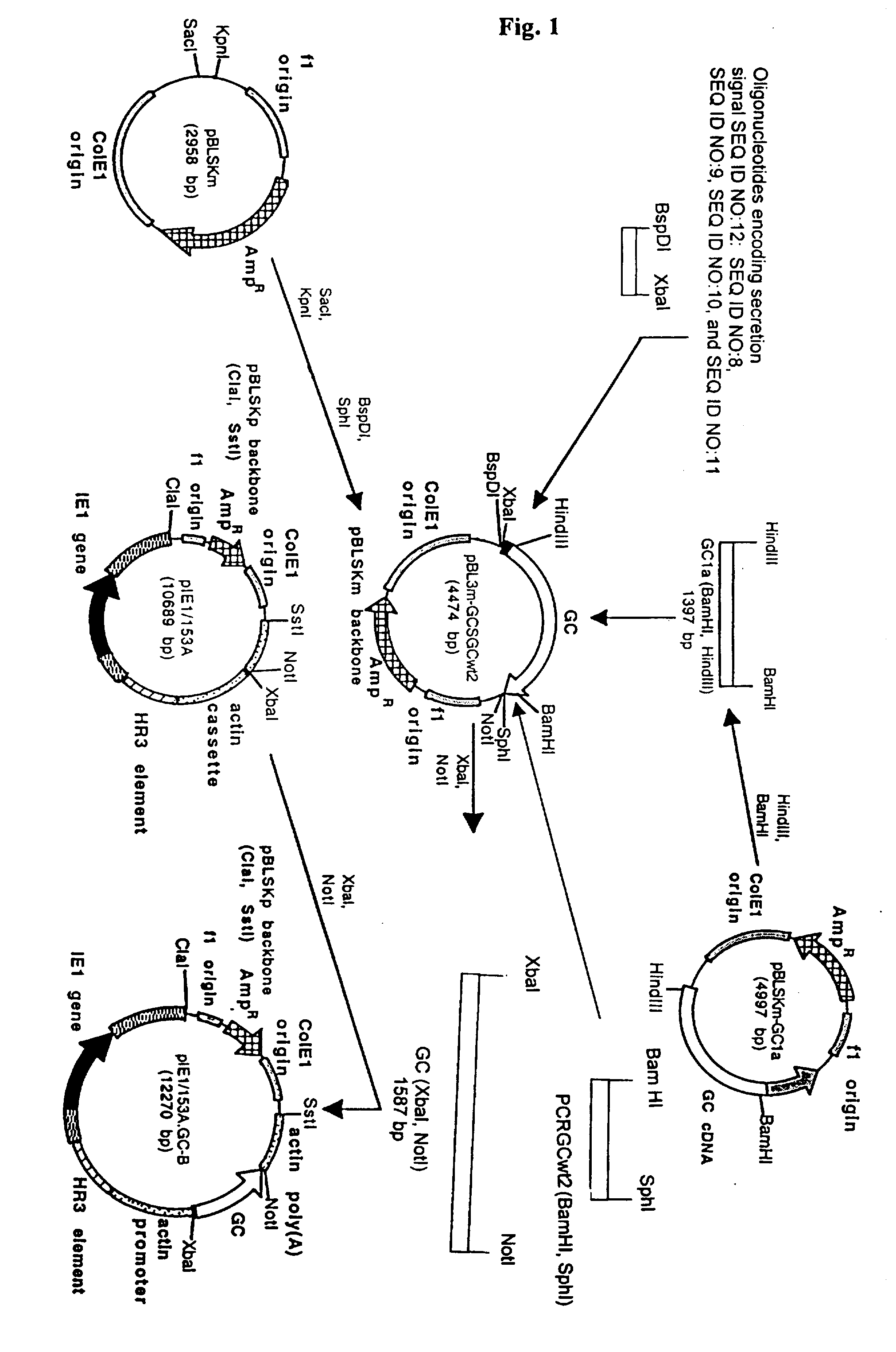
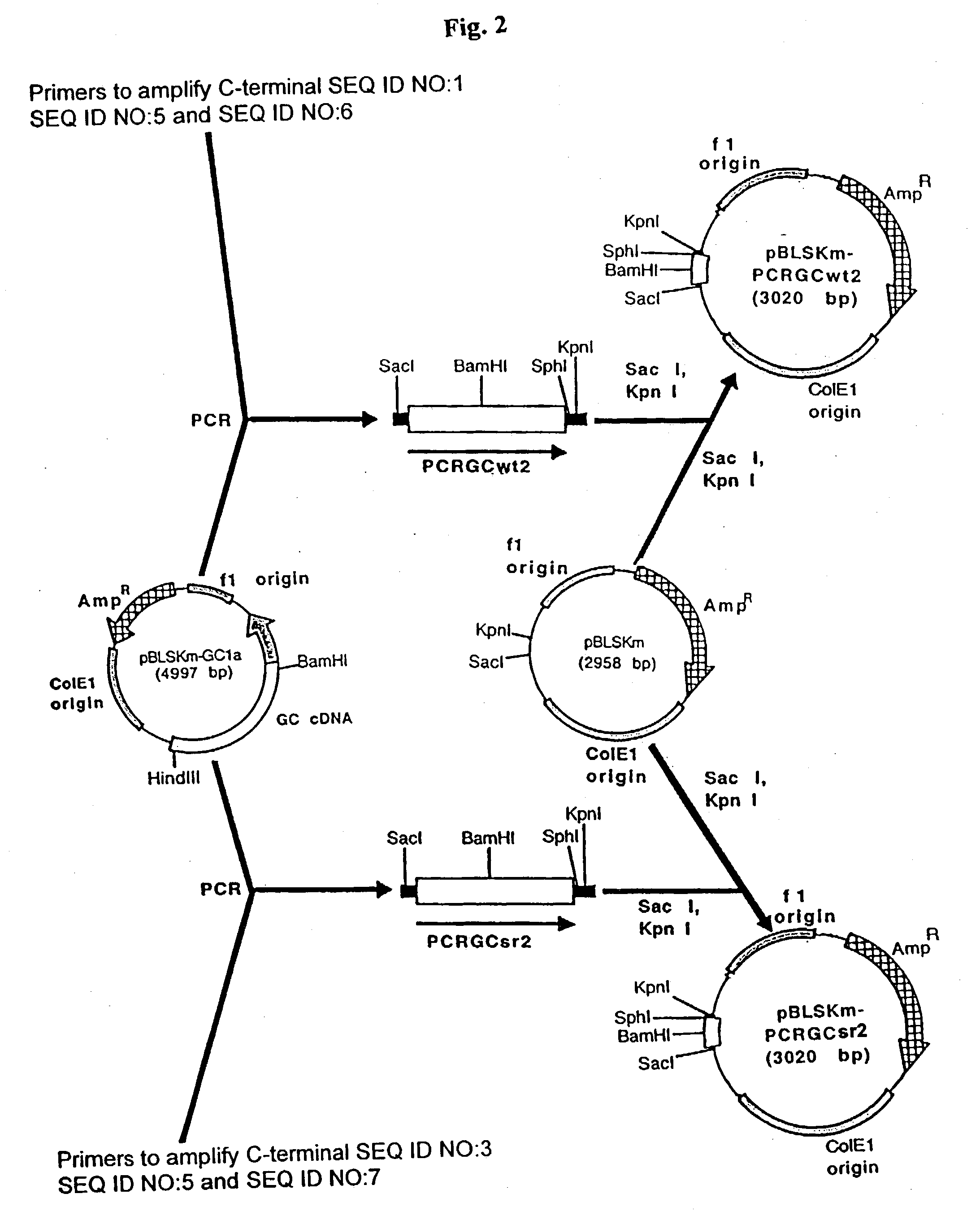
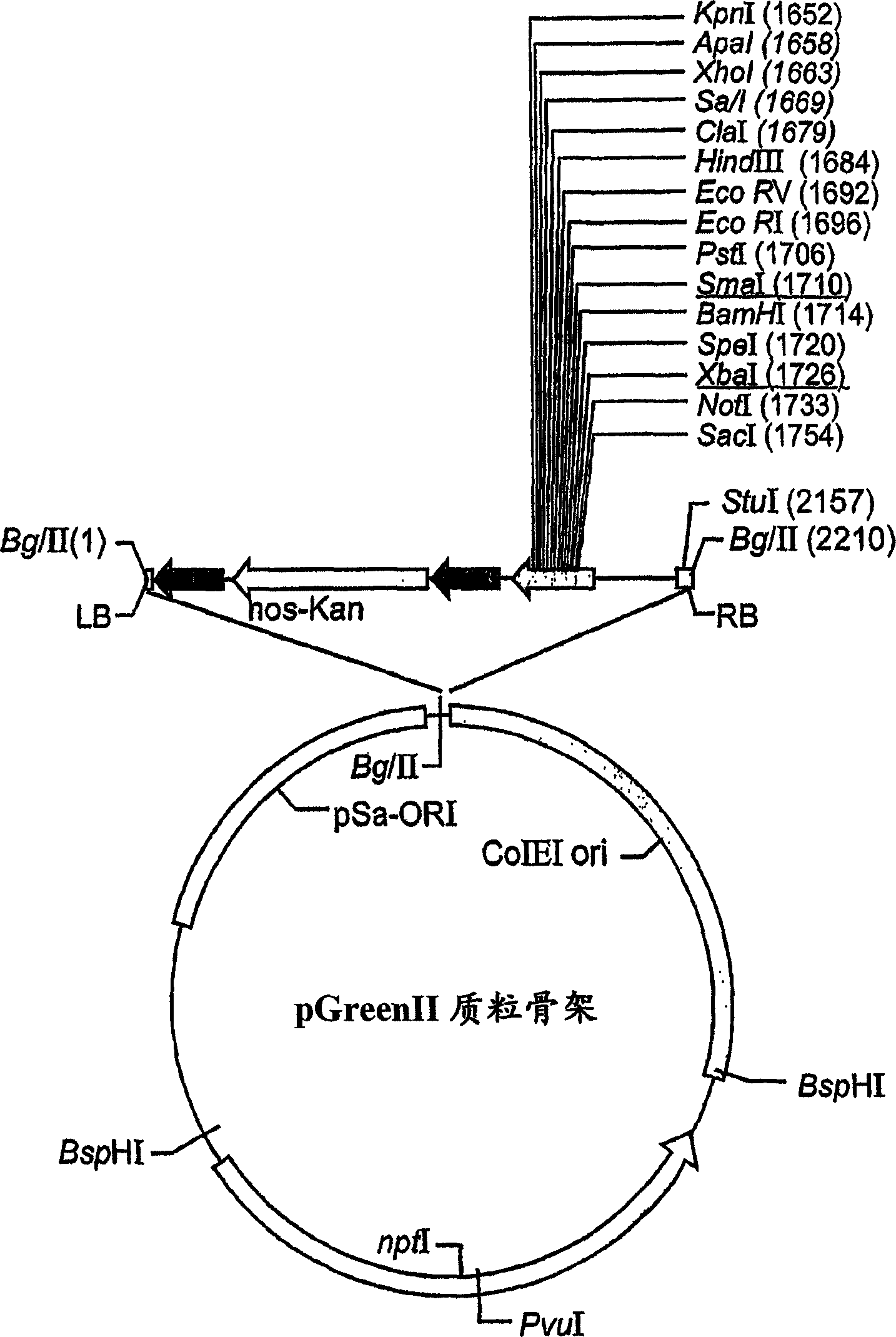
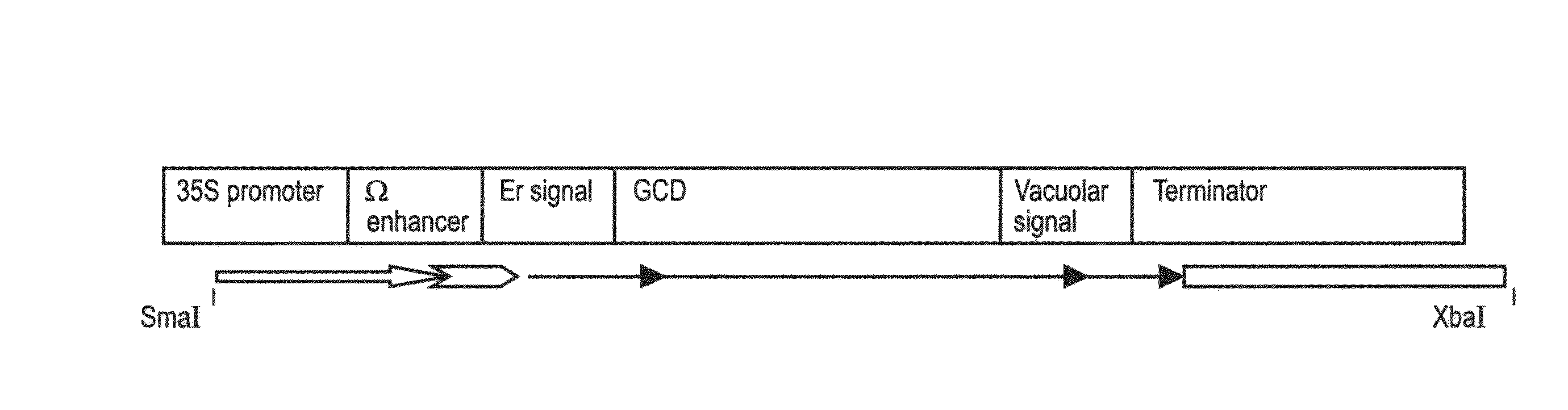
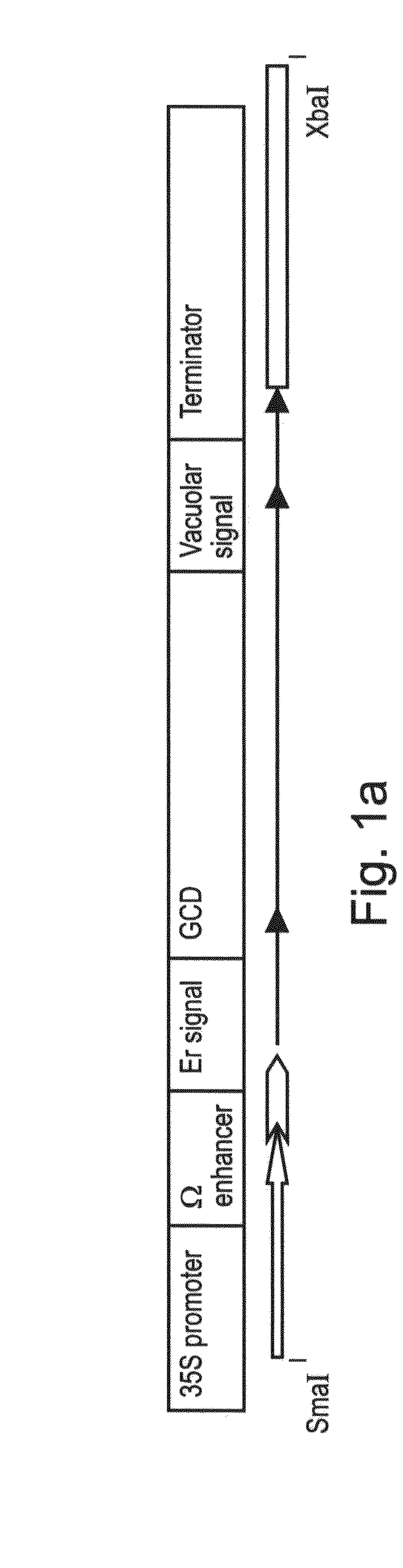
Production of high mannose proteins in plant culture
ActiveUS20090208477A1High glycosylationEfficient targetingBioreactor/fermenter combinationsBiological substance pretreatmentsHigh mannosePlant roots
A device, system and method for producing glycosylated proteins in plant culture, particularly proteins having a high mannose glycosylation, while targeting such proteins with an ER signal and / or by-passing the Golgi. The invention further relates to vectors and methods for expression and production of enzymatically active high mannose lysosomal enzymes using transgenic plant root, particularly carrot cells. More particularly, the invention relates to host cells, particularly transgenic suspended carrot cells, vectors and methods for high yield expression and production of biologically active high mannose Glucocerebrosidase (GCD). The invention further provides for compositions and methods for the treatment of lysosomal storage diseases.
Owner:PROTALIX
High mannose proteins and methods of making high mannose proteins
InactiveUS7138262B1Prevent removalInhibitory activitySenses disorderNervous disorderHigh mannoseOligosaccharide
The invention features a method of producing a high mannose glucocerebrosidase (hmGCB) which includes: providing a cell which is capable of expressing glucocerebrosidase (GCB), and allowing production of GCB having a precursor oligosaccharide under conditions which prevent the removal of at least one mannose residue distal to the pentasaccharide core of the precursor oligosaccharide of GCB, to thereby produce an hmGCB preparation. Preferably, the condition which prevents the removal of at least one mannose residue distal to the pentasaccharide core is inhibition of a class 1 processing mannosidase and / or a class 2 processing mannosidase. The invention also features an hmGCB preparation and methods of using an hmGCB preparation.
Owner:SHIRE HUMAN GENETIC THERAPIES INC
Treatment of CNS disorders associated with mutations in genes encoding lysosomal enzymes
Described is a method for treating an individual having a neurological disorder with an associated mutation or mutations in a gene encoding a lysosomal enzyme. Specifically, the individual is administered a specific pharmacological chaperone for the lysosomal enzyme which increases trafficking of the protein from the ER to the lysosome in neural cells, with or without concomitantly increasing enzyme activity in neural cells. Restoration of trafficking relieves cell stress and other toxicities associated with accumulation of mutant proteins. Restoration of enzyme activity relieves substrate accumulation and pathologies associated with lipid accumulation. In a specific embodiment, the neurological disorder is Parkinson's disease or parkinsonism which is associated with mutations in glucocerebrosidase.
Owner:AMICUS THERAPEUTICS INC
Glycosylated glucocerebrosidase expression in fungal hosts
InactiveUS20050265988A1Improve effectivenessEfficiently takenFungiSugar derivativesBiotechnologyGlucocerebrosidase
A recombinant fungal host cell producing recombinant glucocerebrosidase is provided. A functional recombinant glucocerebrosidase produced in recombinant fungal host cells is also provided. Methods for producing and isolating functional recombinant glucocerebrosidase from fungal hosts are also provided.
Owner:GLYCOFI
Glucoimidazole and polyhydroxycyclohexenyl amine derivatives to treat gaucher disease
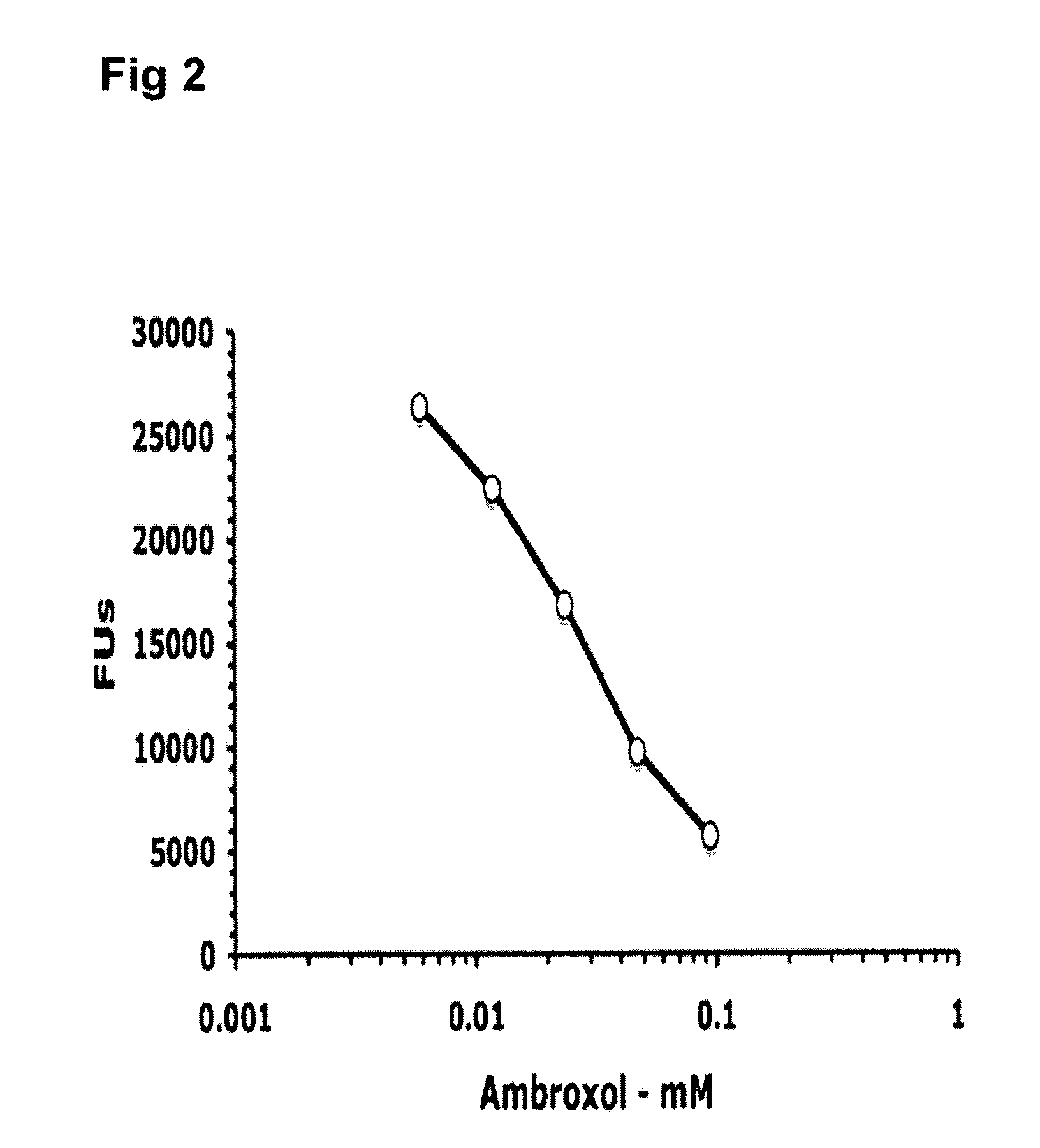
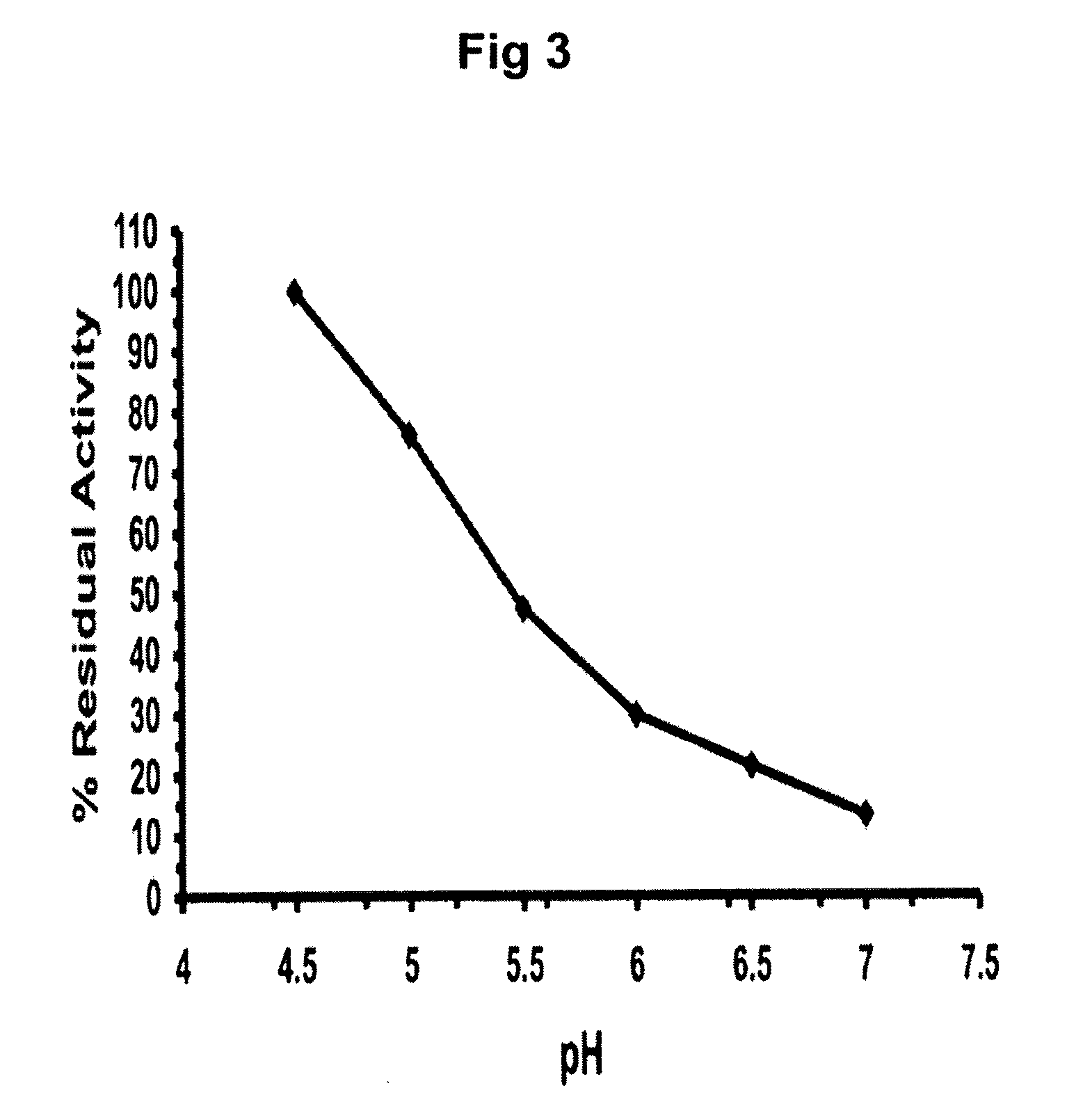
InactiveUS20050137223A1Stabilize GCaseEffective and stableBiocideNervous disorderPyranoseActive site
Owner:AMICUS THERAPEUTICS INC
Hydroxy piperidine derivatives to treat gaucher disease
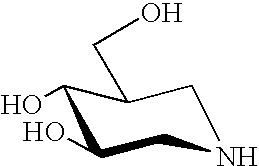
The present invention provides novel hydroxy piperidine (HP) derivatives having (i) a positive charge at the position corresponding to the anomeric position of a pyranose ring; (ii) a short, flexible linker emanating from the corresponding position of the ring oxygen in a pyranose; and (iii) a lipophilic moiety connected to the linker and pharmaceutically acceptable salts thereof. The linker can be absent if the lipophilic moiety corresponds to a hydrocarbon chain with a linear length of 6 or more carbons. The present invention further provides a method for treating individuals having Gaucher disease by administering the novel HP derivative as “active-site specific chaperones” for the mutant glucocerebrosidase associated with the disease.
Owner:AMICUS THERAPEUTICS INC
Treatment of CNS disorders associated with mutations in genes encoding lysosomal enzymes
Described is a method for treating an individual having a neurological disorder with an associated mutation or mutations in a gene encoding a lysosomal enzyme. Specifically, the individual is administered a specific pharmacological chaperone for the lysosomal enzyme which increases trafficking of the protein from the ER to the lysosome in neural cells, with or without concomitantly increasing enzyme activity in neural cells. Restoration of trafficking relieves cell stress and other toxicities associated with accumulation of mutant proteins. Restoration of enzyme activity relieves substrate accumulation and pathologies associated with lipid accumulation. In a specific embodiment, the neurological disorder is Parkinson's disease or parkinsonism which is associated with mutations in glucocerebrosidase.
Owner:AMICUS THERAPEUTICS INC
Expression system for effeiciently producing clinically effective lysosomal enzymes (glucocerebrosidase)
The invention as described herein relates to the efficient production of recombinant, clinically effective lysosomal enzymes using a transformed insect cell expression system. For example, to create the expression system of the invention, any insect cell can be transfected with a plasmid comprised of a gene encoding the human glucocerebrosidase gene and genetic elements that enhance its expression. The insect cell transfected with the plasmid encoding glucocerebrosidase secretes synthesized glucocere-brosidase into its growth media. The recombinantly produced clinically effective glucocerebrosidase produced by the insect cell expression system can be used to treat Gaucher's disease.
Owner:EXEGENICS
High mannose proteins and methods of making high mannose proteins
InactiveUS20070031945A1Efficiently target mannose receptorPrecise deliveryBiocideOrganic active ingredientsHigh mannoseOligosaccharide
The invention features a method of producing a high mannose glucocerebrosidase (hmGCB) which includes: providing a cell which is capable of expressing glucocerebrosidase (GCB), and allowing production of GCB having a precursor oligosaccharide under conditions which prevent the removal of at least one mannose residue distal to the pentasaccharide core of the precursor oligosaccharide of GCB, to thereby produce an hmGCB preparation. Preferably, the condition which prevents the removal of at least one mannose residue distal to the.pentasaccharide core is inhibition of a class 1 processing mannosidase and / or a class 2 processing mannosidase. The invention also features an hmGCB preparation and methods of using an hmGCB preparation.
Owner:SHIRE HUMAN GENETIC THERAPIES INC
Production of high mannose proteins in plant culture
Owner:普罗塔里克斯有限公司
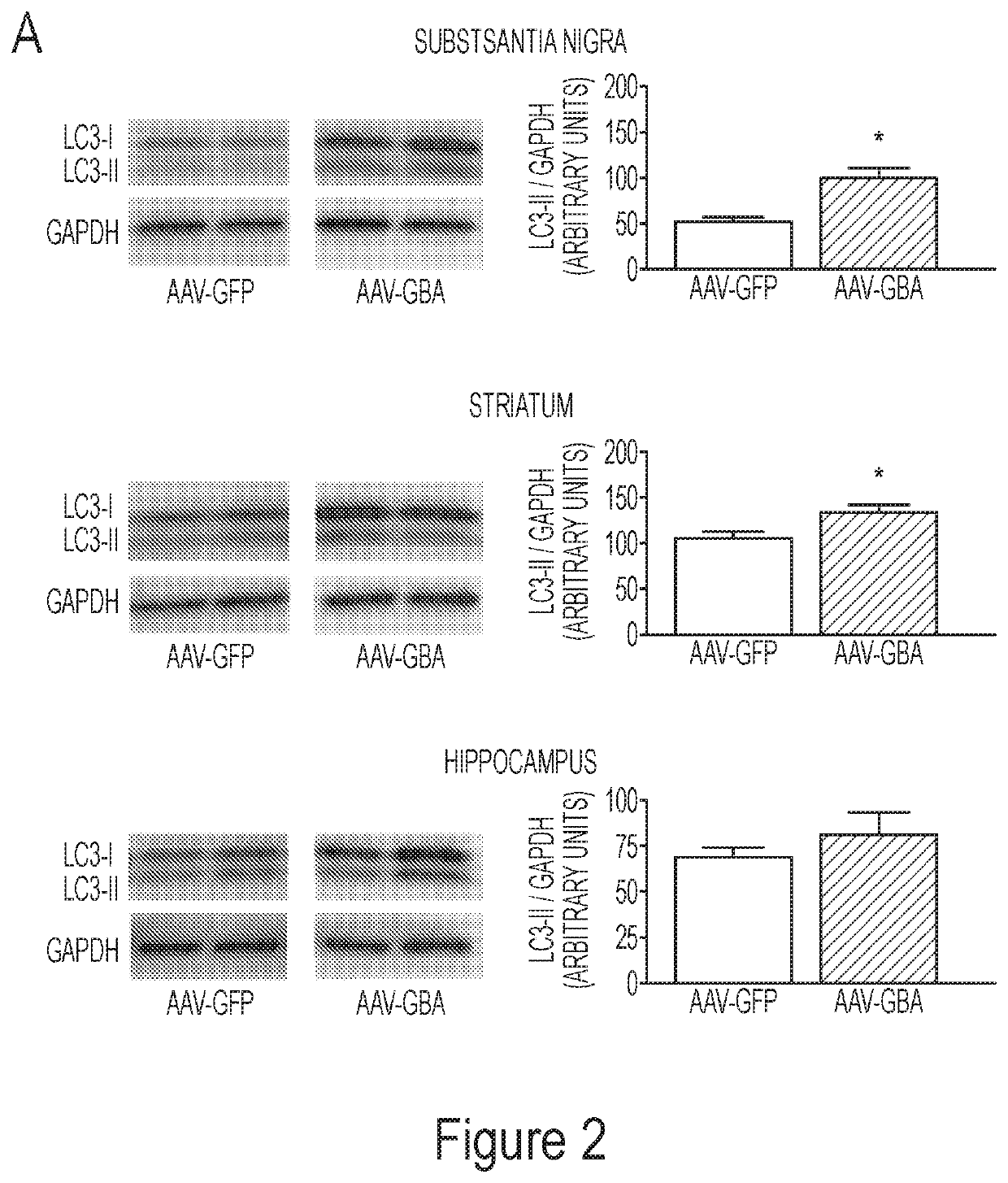
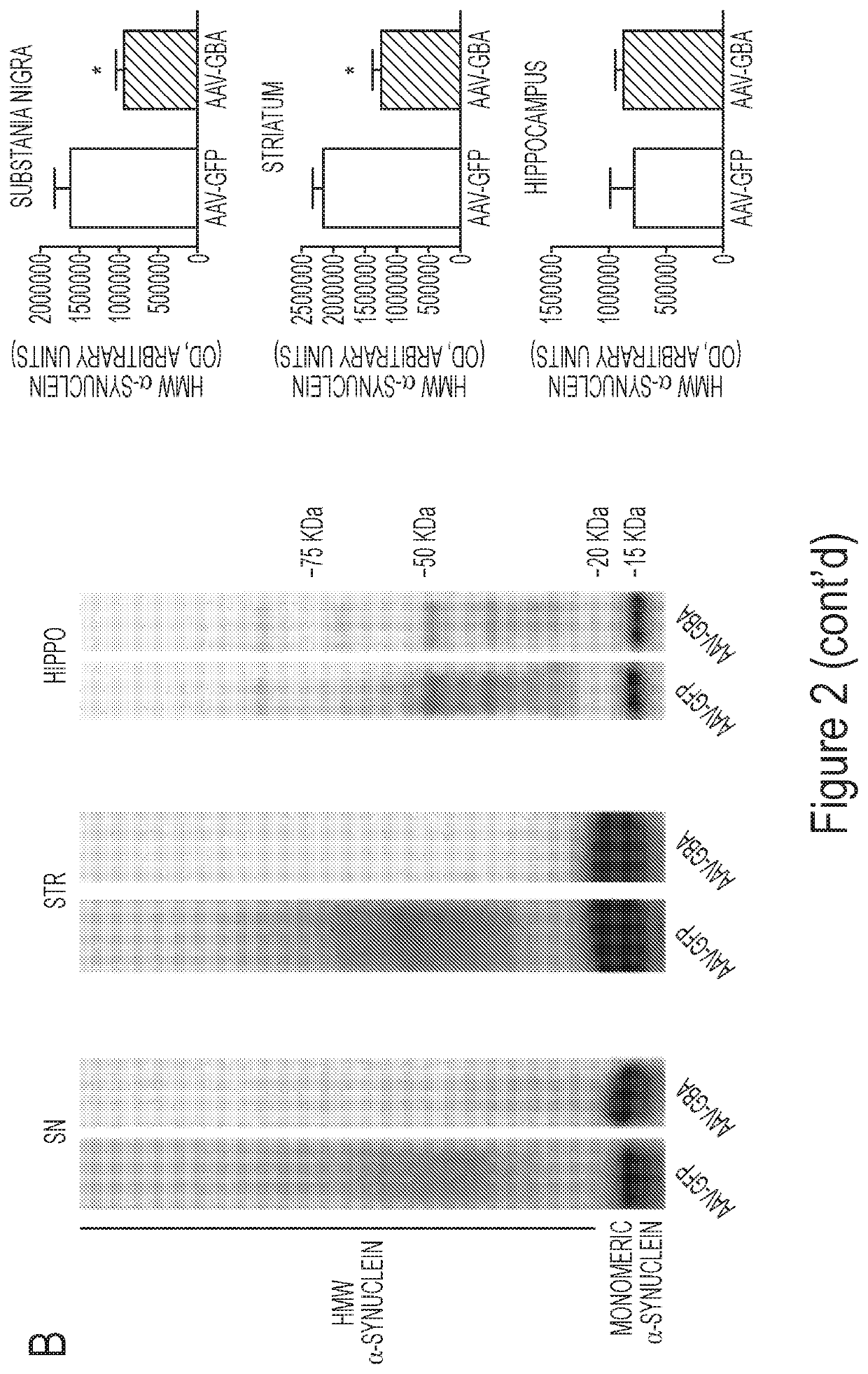
Glucocerebrosidase gene therapy for parkinson's disease
ActiveUS20180147300A1Small sizeReduce the amount requiredNervous disorderPeptide/protein ingredientsDiseaseMedicine
The present invention is directed, in part, to the treatment of a subject having a neurodegenerative disorder, such as Parkinson's disease (PD), by providing glucocerebrosidase enzyme. The enzyme may be provided, e.g., through gene therapy or by administration of a glucocerebrosidase protein. Accordingly, the present invention encompasses glucocerebrosidase nucleic acids or proteins for use in the treatment of PD or other neurodegenerative disorders.
Owner:THE MCLEAN HOSPITAL CORP +1
Gaucher disease drugs and methods of identifying same
InactiveUS20070166813A1High activitySubstantial glucocerebrosidase activityCompound screeningNervous disorderAspartic acid residueCrystallography
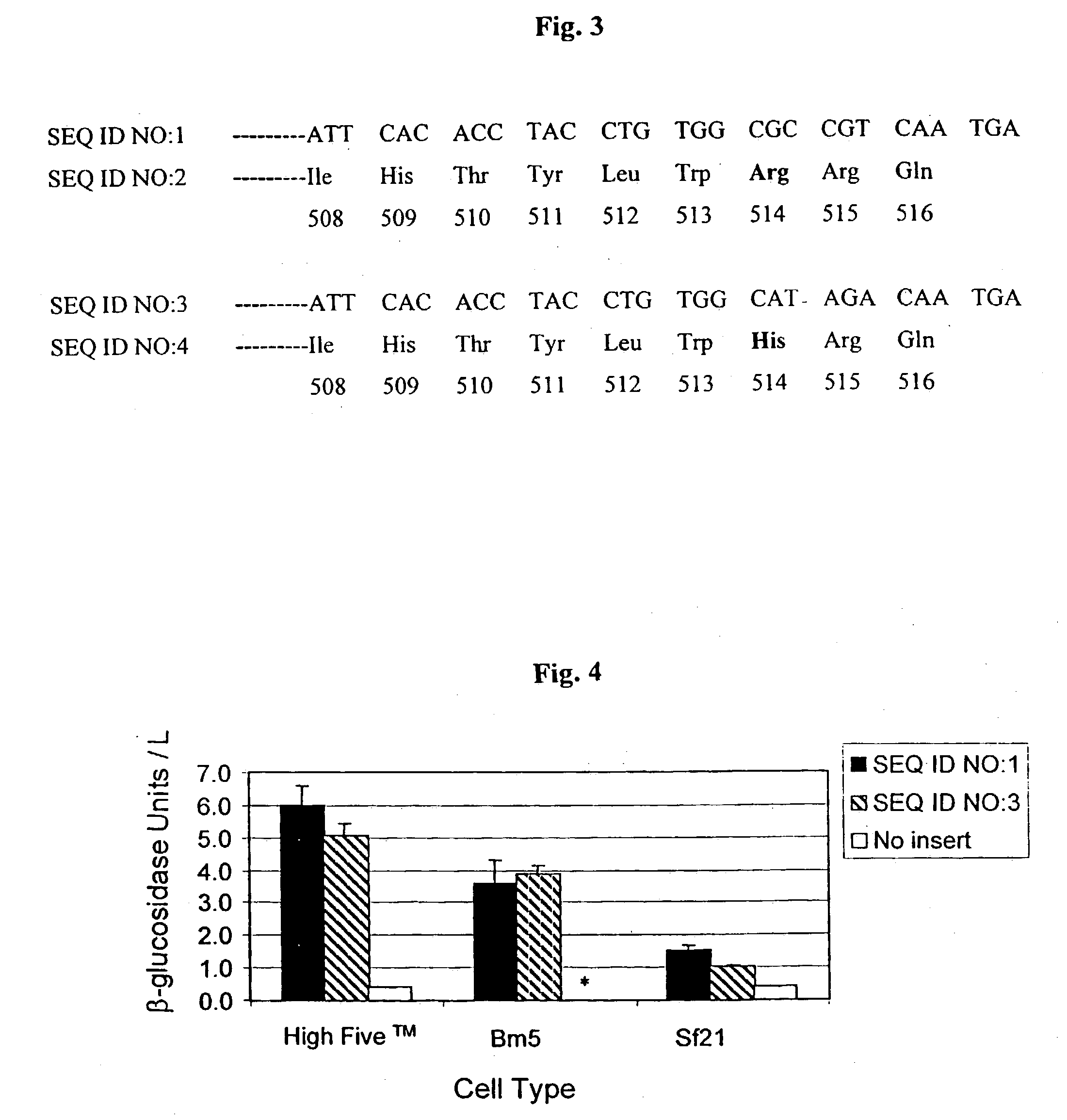
A method of identifying a compound capable of correcting an impaired enzymatic activity of a mutant glucocerebrosidase molecule, the method comprising: (a) obtaining a first set of structure coordinates, the first set of structure coordinates defining a 3D structure of a glucocerebrosidase molecule capable of displaying normal enzymatic activity or a portion thereof; (b) computationally generating using the first set of structure coordinates a second set of structure coordinates, the second set of structure coordinates defining a predicted 3D structure of the mutant glucocerebrosidase molecule or a portion thereof; and (c) computationally identifying, using the second set of structure coordinates, a compound capable of interacting with the mutant glucocerebrosidase molecule in such a way as to correct the impaired enzymatic activity thereof, thereby identifying the compound capable of correcting the impaired enzymatic activity of the mutant glucocerebrosidase molecule. A glucocerebrosidase preparation comprising a population of glucocerebrosidase molecules, wherein substantially each of said glucocerebrosidase molecules: (i) has an amino acid sequence at least 95 percent homologous to an amino acid sequence set forth by SEQ ID NO: 1 or 8; (ii) is glycosylated at, or has an aspartatic acid residue at, glycosylation residue 1 of said amino acid sequence; and (iii) is independently unglycosylated at one or more glycosylation residues selected from the group consisting of glycosylation residues 2, 3 and 4 of said amino acid sequence.
Owner:YEDA RES & DEV CO LTD
Expression of soluble therapeutic proteins
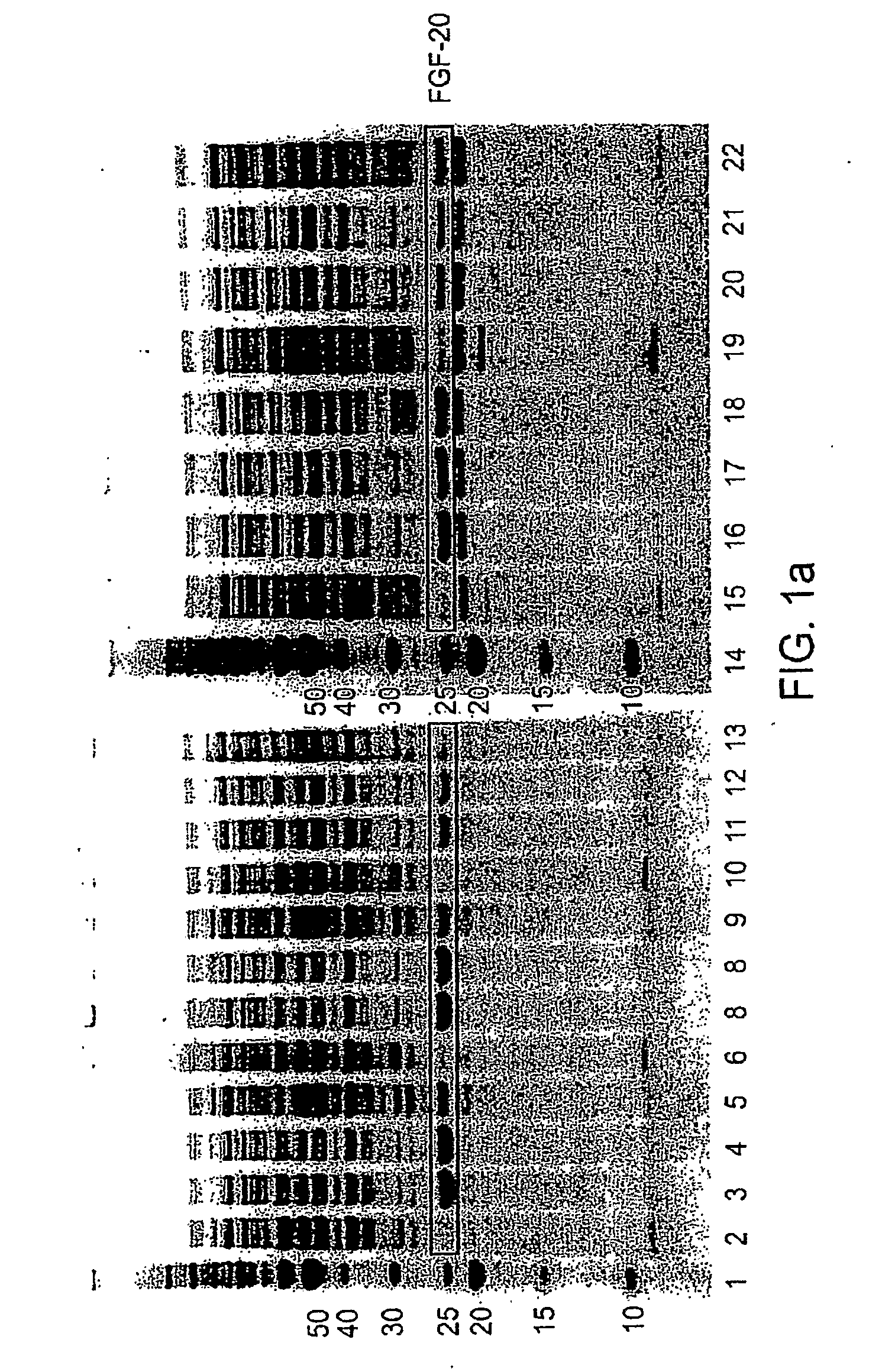
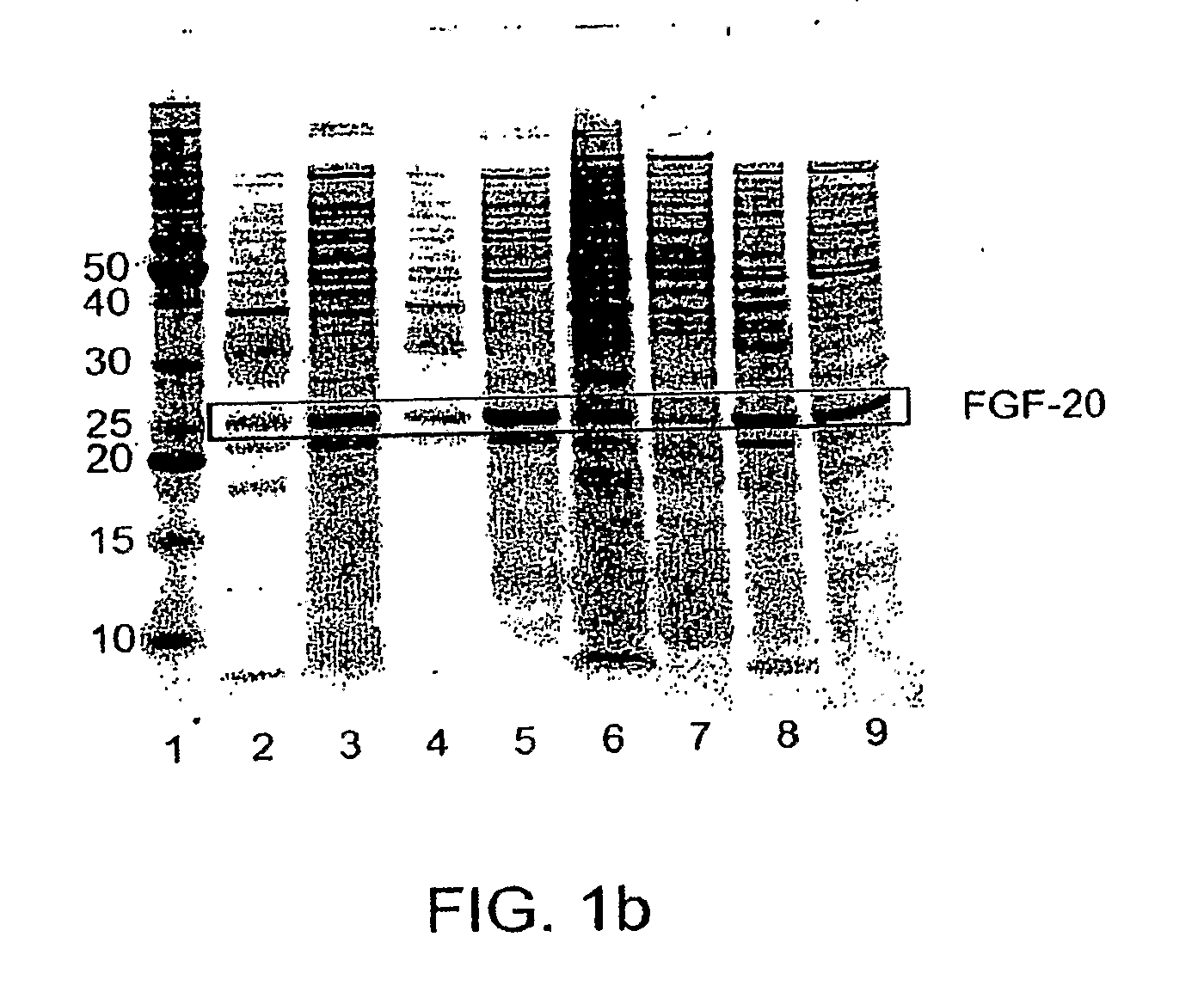
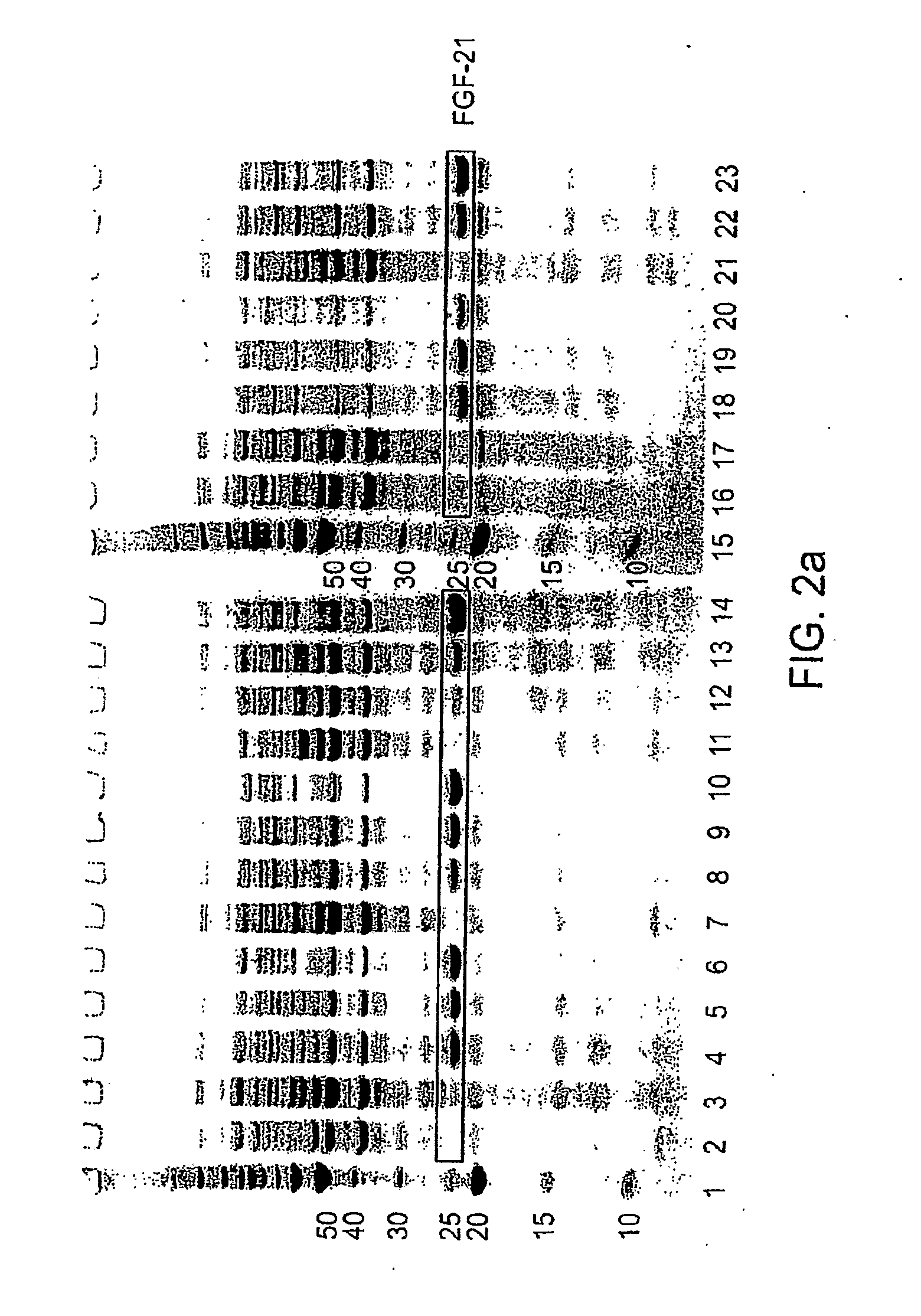
ActiveUS20090298121A1Improve solubilityFactor VIIPeptide/protein ingredientsMicroorganismNeurotrophin-3
The present invention provides enhanced methods of producing soluble, active fibroblast growth factor-20 (FGF-20), FGF-21, neurotrophin-3 (NT-3), growth hormone (GH), granulocyte colony stimulating factor (G-CSF), or glucocerebrosidase proteins in microorganisms that have an oxidizing environment.
Owner:NOVO NORDISK AS
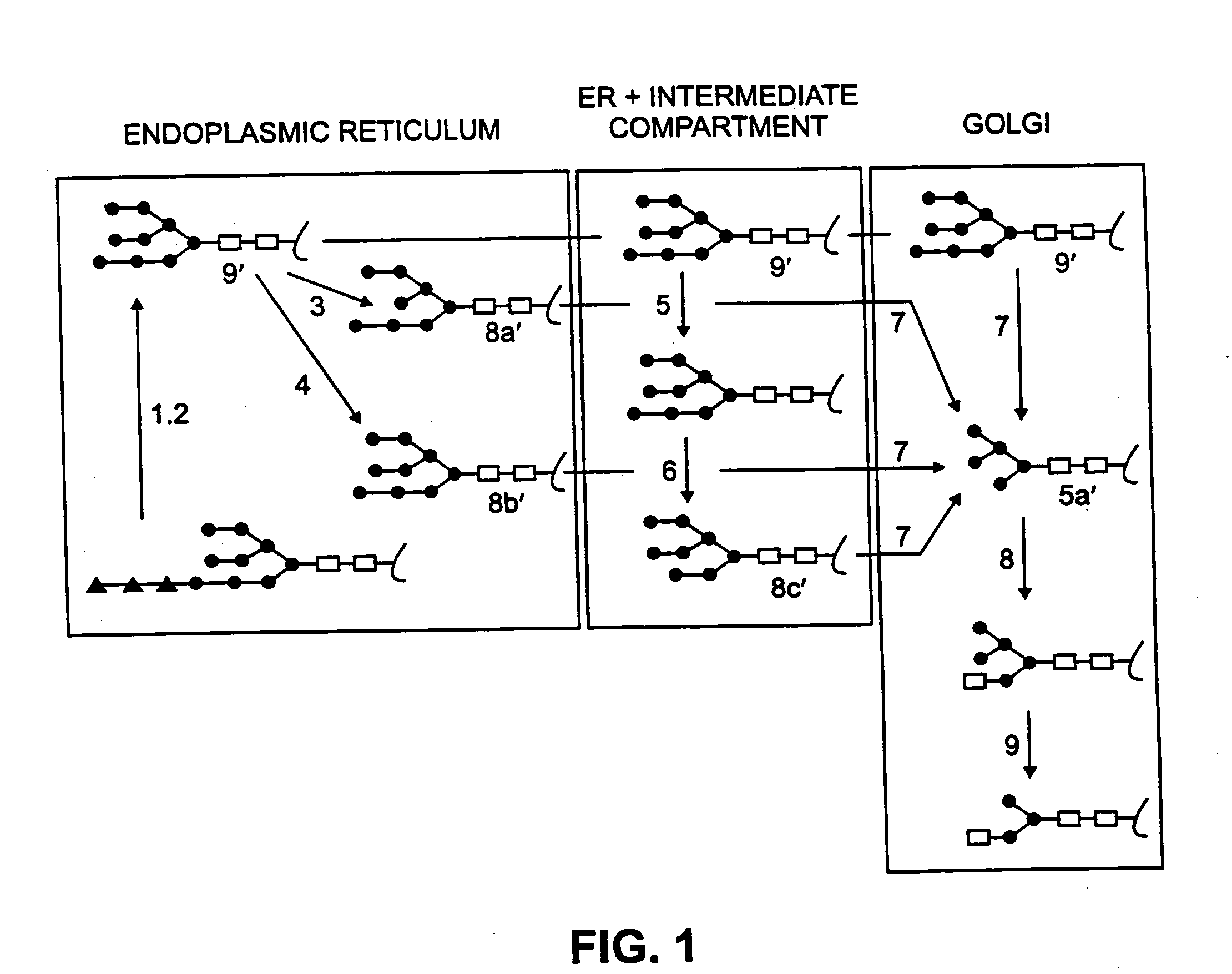
Production of lysosomal enzymes in plants by transient expression
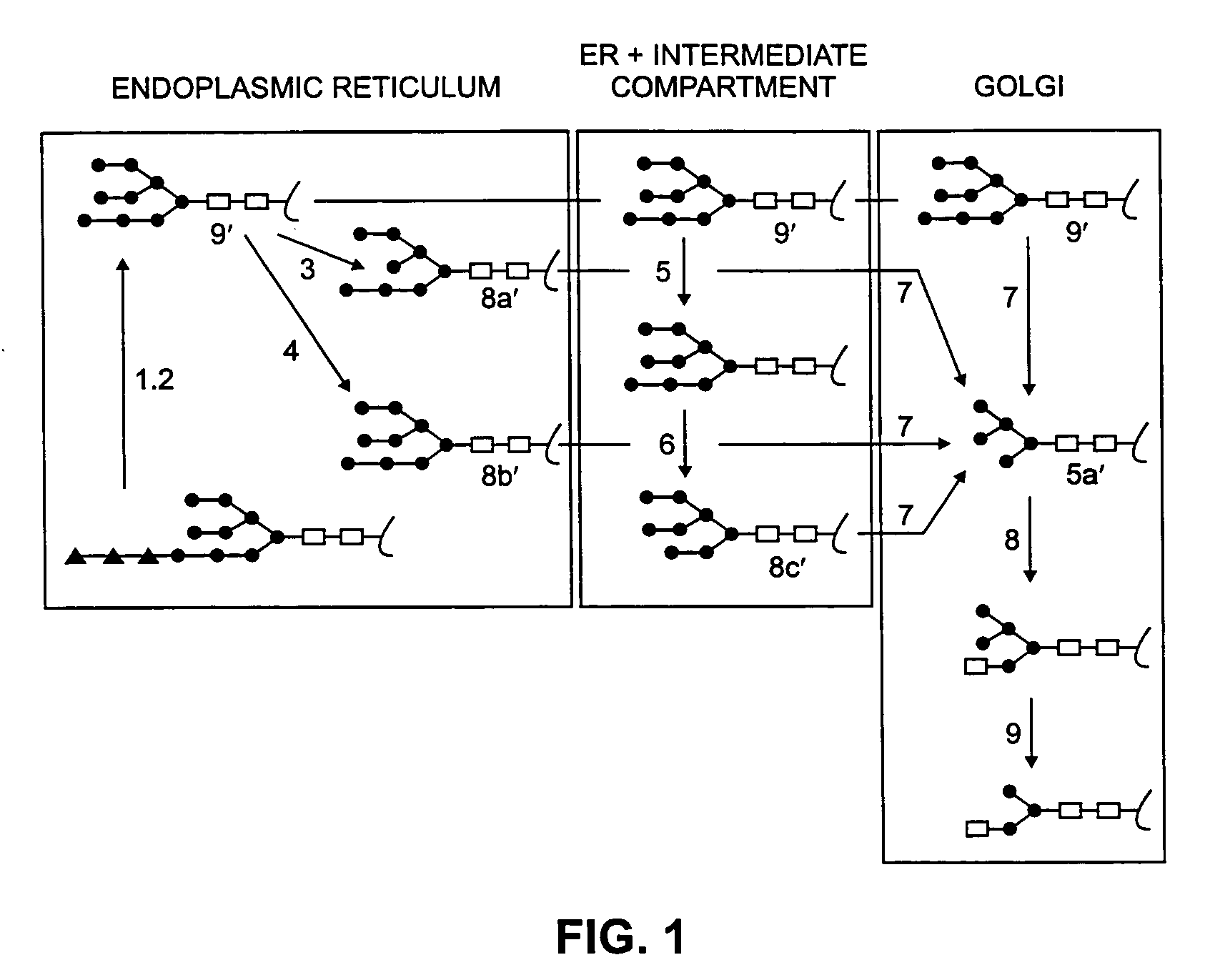
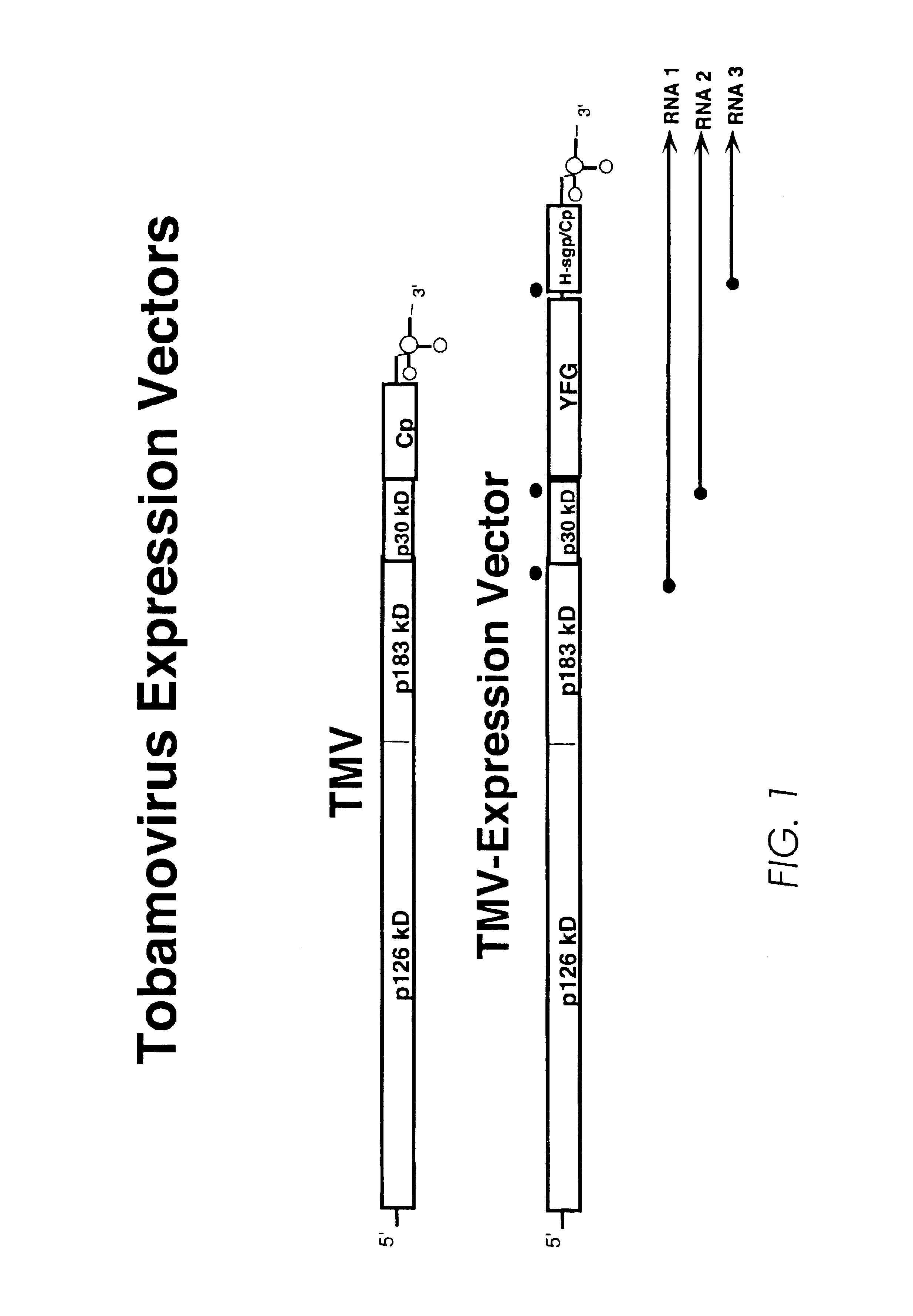
InactiveUS6887696B2High specific activitySsRNA viruses positive-senseAntibody mimetics/scaffoldsNicotiana tabacumNucleotide
The invention relates to α-galactosidase truncated at the carboxy terminus and the production of enzymatically active recombinant human and animal lysosomal enzymes involving construction and expression of recombinant expression constructs comprising coding sequences of human or animal lysosomal enzymes in a plant expression system. The plant expression system provides for post-translational modification and processing to produce a recombinant gene product exhibiting enzymatic activity. The invention is demonstrated by working examples in which transgenic tobacco plants express recombinant expression constructs comprising human glucocerebrosidase nucleotide sequences. The invention is also demonstrated by working examples in which transfected tobacco plants express recombinant viral expression constructs comprising human α galactosidase nucleotide sequences. The recombinant lysosomal enzymes produced in accordance with the invention may be used for a variety of purposes, including but not limited to enzyme replacement therapy for the therapeutic treatment of human and animal lysosomal storage diseases.
Owner:KENTUCKY BIOPROCESSING
Production of high mannose proteins in plant culture
InactiveUS20100196345A1Efficient productionSufficient quantityPolypeptide with localisation/targeting motifSugar derivativesPlant rootsHigh mannose
A device, system and method for producing glycosylated proteins in plant culture, particularly proteins having a high mannose glycosylation, while targeting such proteins with an ER signal and / or by-passing the Golgi. The invention further relates to vectors and methods for expression and production of enzymatically active high mannose lysosomal enzymes using transgenic plant root, particularly carrot cells. More particularly, the invention relates to host cells, particularly transgenic suspended carrot cells, vectors and methods for high yield expression and production of biologically active high mannose Glucocerebrosidase (GCD). The invention further provides for compositions and methods for the treatment of lysosomal storage diseases.
Owner:PROTALIX
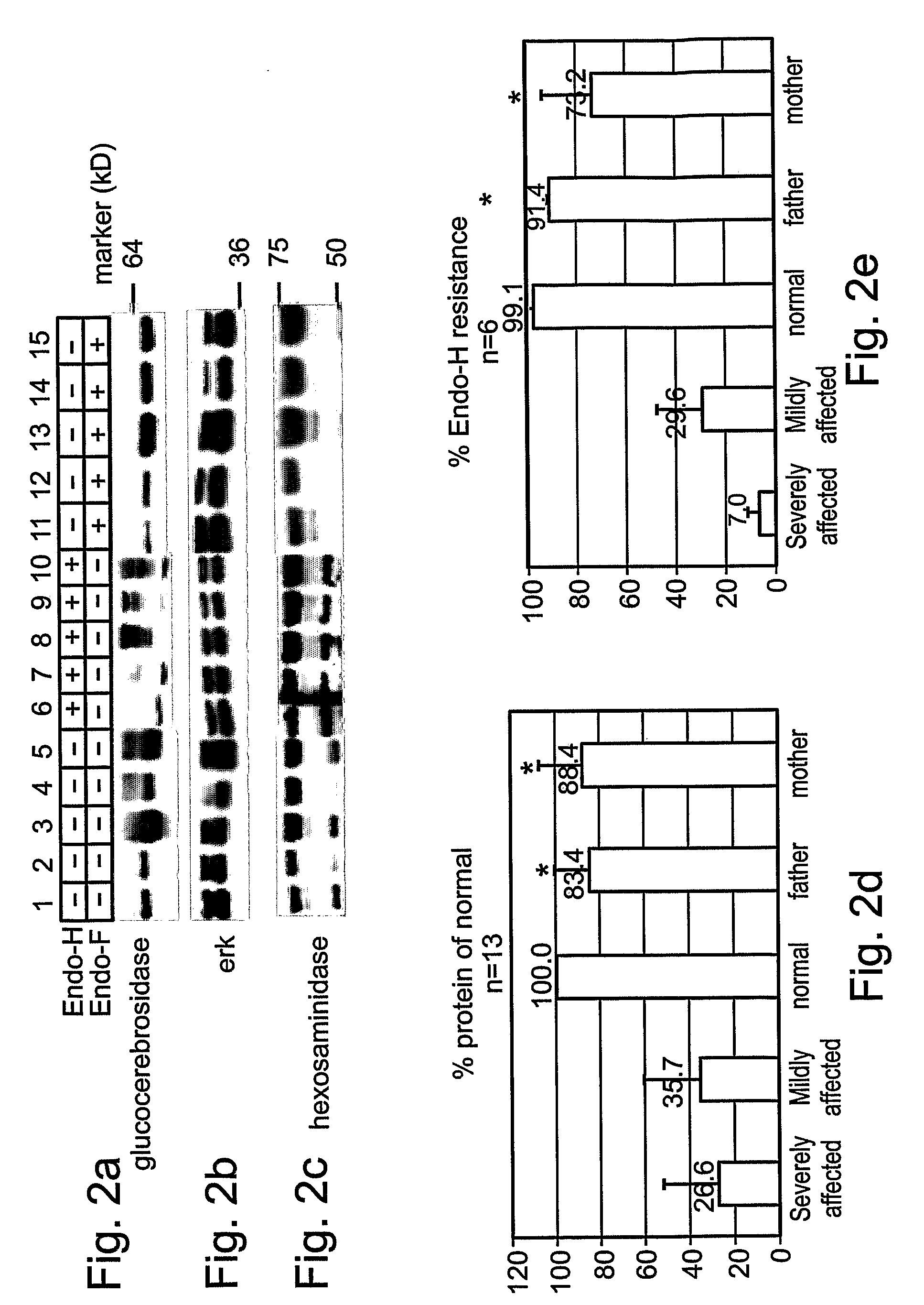
Methods and kits for diagnosing and/or assessing severity and treating gaucher disease
InactiveUS20090239807A1Improve the level ofMicrobiological testing/measurementTetrapeptide ingredientsLysosomeTreatment hypertension
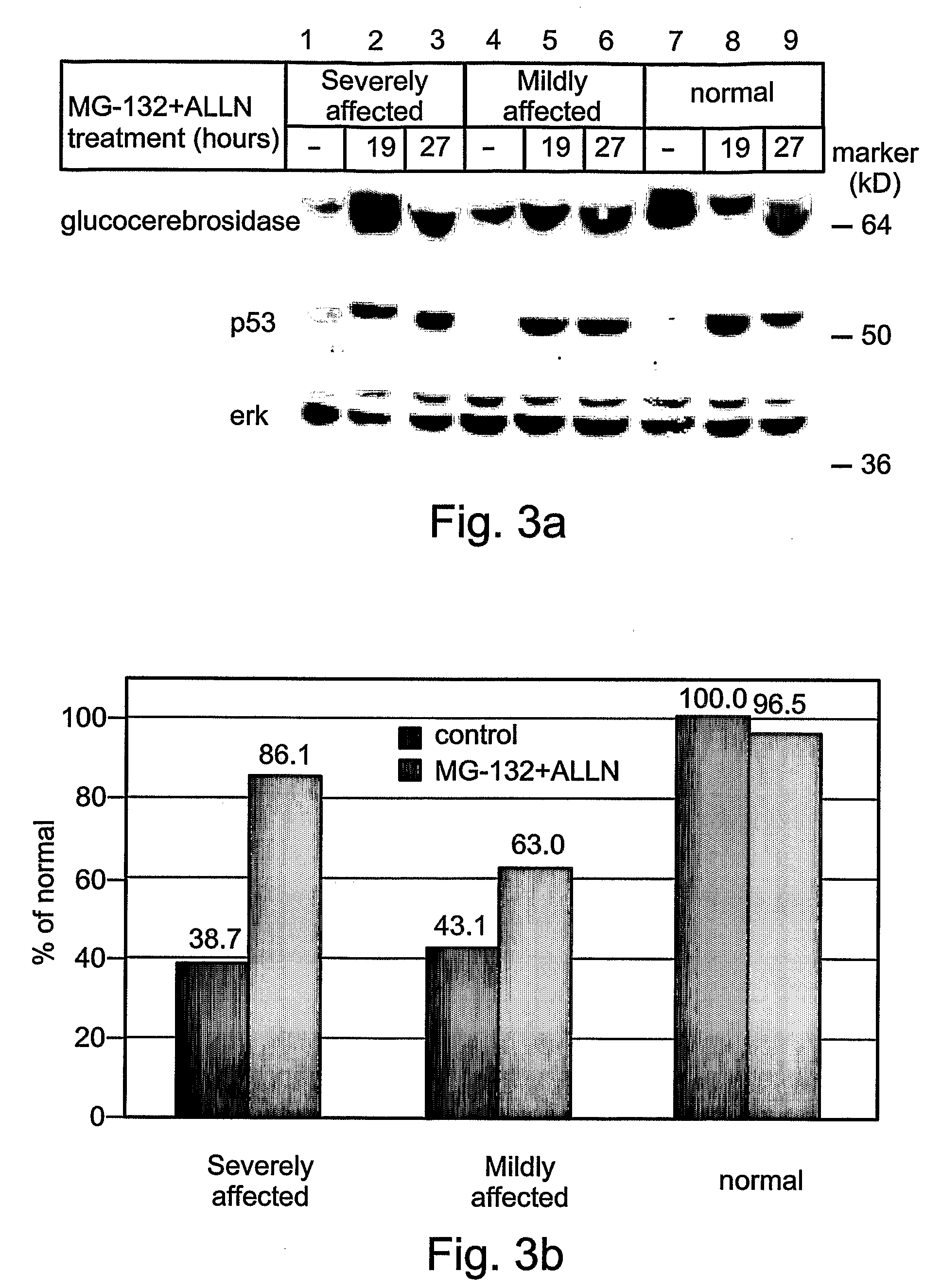
Methods and kits for treating Gaucher disease are provided. The methods are based on using agents capable of inhibiting proteasomal degradation of glucocerebrosidase and / or elevating a level of mis-folded yet active glucocerebrosidase in cell lysosomes. Also provided are methods and kits for diagnosing and / or assessing a severity and determining prognosis of Gaucher disease or other diseases associated with abnormally folded proteins which are retained in the ER.
Owner:RAMOT AT TEL AVIV UNIV LTD
Glucocerebrosidase multimers and uses thereof
InactiveUS20120328589A1Improved and lasting activityHigh activitySenses disorderPeptide/protein ingredientsSerum igeDisease
Multimeric protein structures comprising at least two glucocerebrosidase molecules being covalently linked to one another via a linking moiety are disclosed herein, as well a process for preparing same, and uses thereof in the treatment of Gaucher disease. The multimeric protein structures are characterized by longer-lasting activity as compared to native glucocerebrosidase both in serum and in lysosomes.
Owner:PROTALIX
Production of lysosomal enzymes in plants by transient expression
The invention relates to α-galactosidase truncated at the carboxy terminus and the production of enzymatically active recombinant human and animal lysosomal enzymes involving construction and expression of recombinant expression constructs comprising coding sequences of human or animal lysosomal enzymes in a plant expression system. The plant expression system provides for post-translational modification and processing to produce a recombinant gene product exhibiting enzymatic activity. The invention is demonstrated by working examples in which transgenic tobacco plants express recombinant expression constructs comprising human glucocerebrosidase nucleotide sequences. The invention is also demonstrated by working examples in which transfected tobacco plants express recombinant viral expression constructs comprising human α galactosidase nucleotide sequences. The recombinant lysosomal enzymes produced in accordance with the invention may be used for a variety of purposes, including but not limited to enzyme replacement therapy for the therapeutic treatment of human and animal lysosomal storage diseases.
Owner:KENTUCKY BIOPROCESSING
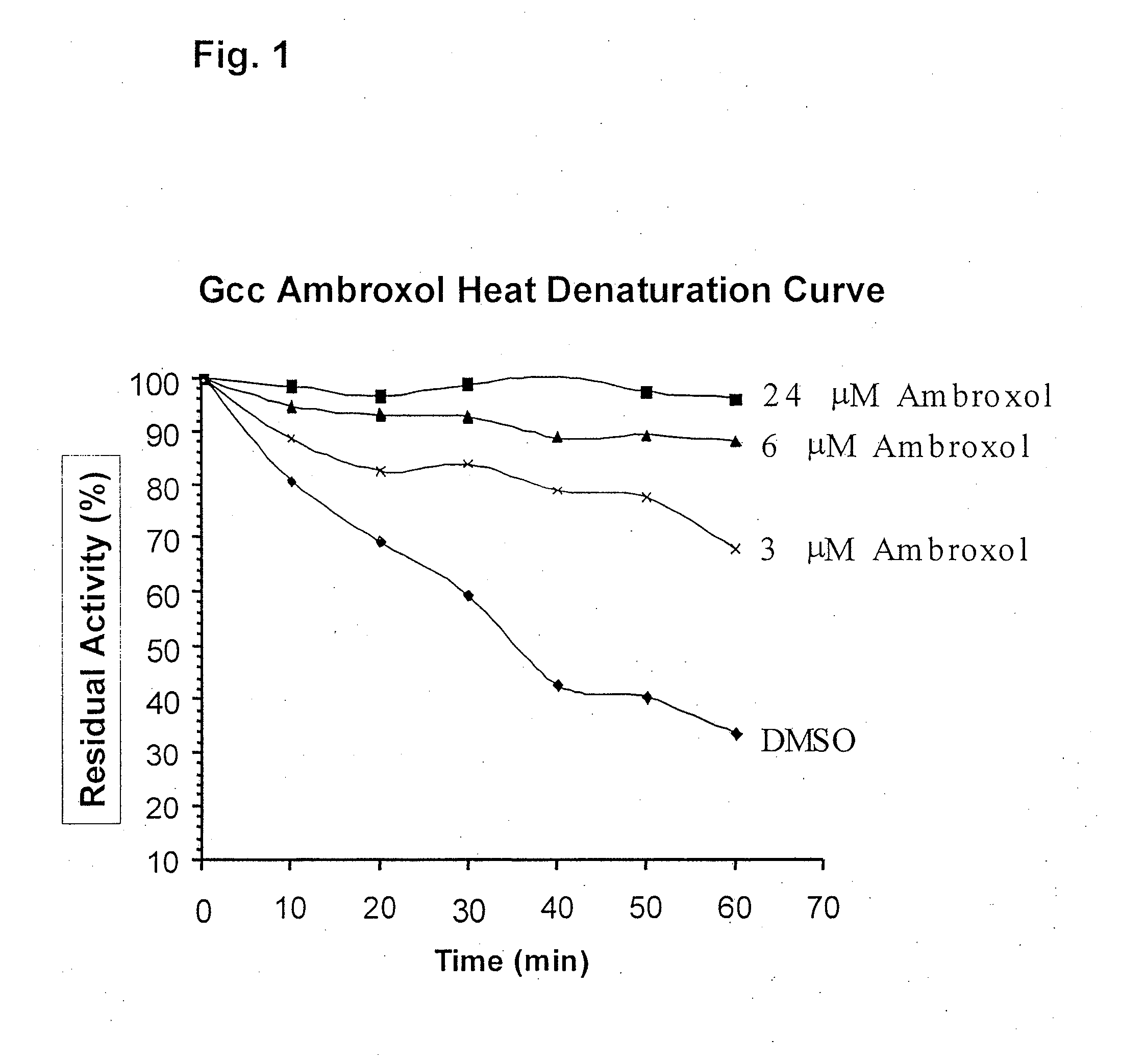
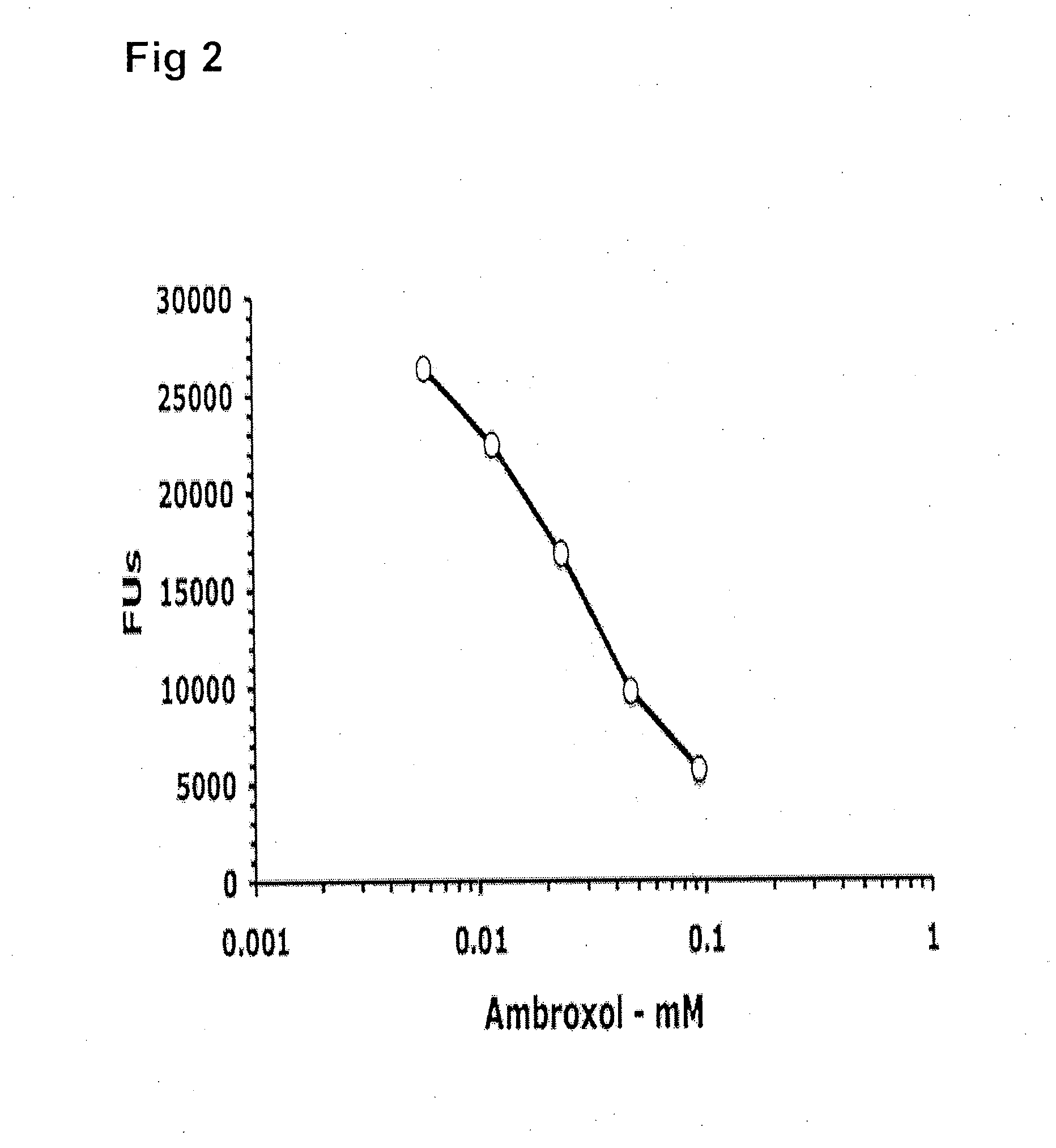
Method of treating gaucher disease
ActiveUS20090075960A1Optimal pharmacological chaperoningOptimal enzyme enhancementBiocidePeptide/protein ingredientsLate onsetPharmacological chaperone
Therapeutic compositions and methods for treatment of late-onset Gaucher disease are described herein. The compositions comprise compounds having activity as pharmacological chaperones for mutant forms of the beta-glucocerebrosidase. Methods of treatment involve providing therapeutically effective amounts of such compositions to subjects in need thereof.
Owner:HOSPITAL FOR SICK CHILDREN +1
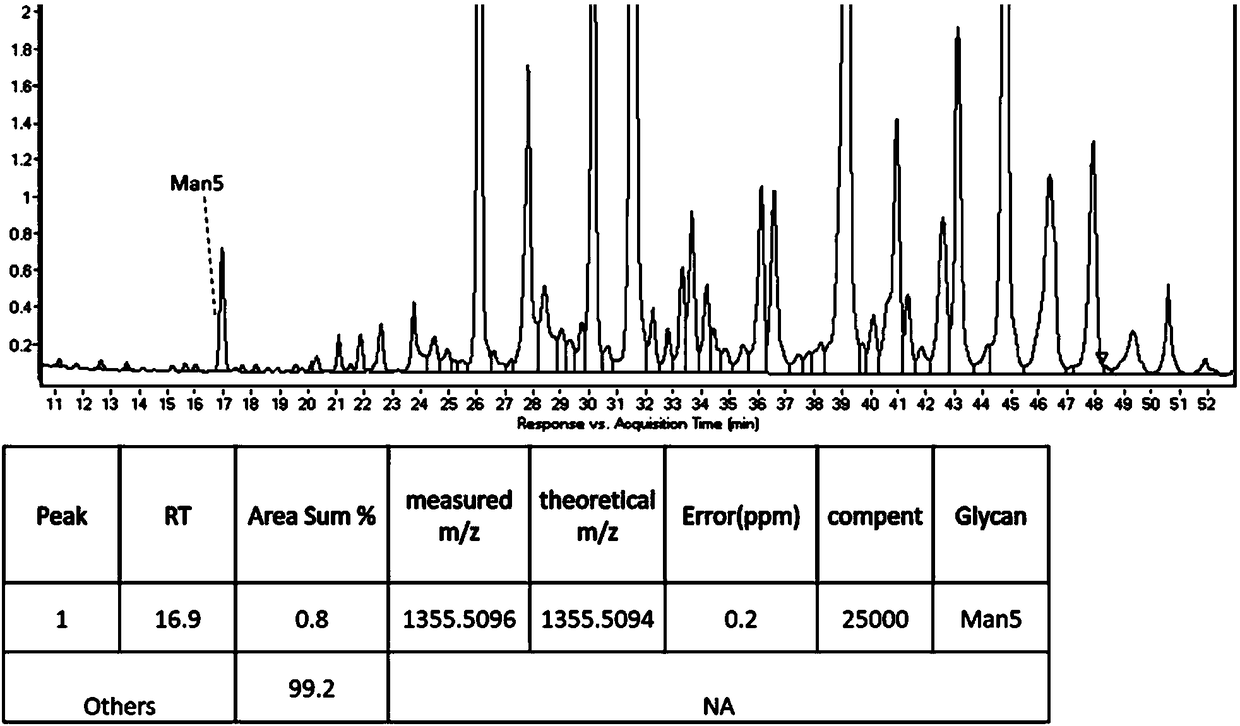
Cell strain capable of expressing glucocerebrosidase with high mannose content as well as preparation method and applications of cell strain
PendingCN108588127AIncrease enzyme activityHigh mannose glycoform contentPeptide/protein ingredientsMetabolism disorderHigh mannoseEukaryotic plasmids
The invention discloses a preparation method of a cell strain capable of expressing glucocerebrosidase with high mannose content. The preparation method comprises the following steps: (1) carrying outlipofection transfection, namely, aiming at the sequence of a GNT1 gene, designing a sgRNA sequence guiding cutting of endonuclease, recombining the sgRNA sequence into a knock-out vector, thus obtaining synthetic plasmids with coexpression of sgRNA and endonuclease, and transfecting a cell pool stably expressing glucocerebrosidase by adopting the synthetic plasmids through a liposome transfection method; (2) carrying out cloning by adopting a limiting dilution method, namely, screening out monoclonal cells, and carrying out enlarged culturing; (3) carrying out mutation cloning screening on the target gene, namely, acquiring monoclone with the GNT1 gene knocked out through cell PCR sequencing, and thus the cell strain capable of expressing glucocerebrosidase with high mannose content is obtained. The cell strain can stably express glucocerebrosidase, meanwhile, the content of the mannose is obviously improved, the binding capacity with mannose receptor is effectively improved, the enzyme activity capacity is improved, and the purpose of increasing the efficacies is achieved.
Owner:WUXI BIOLOGICS IRELAND LTD
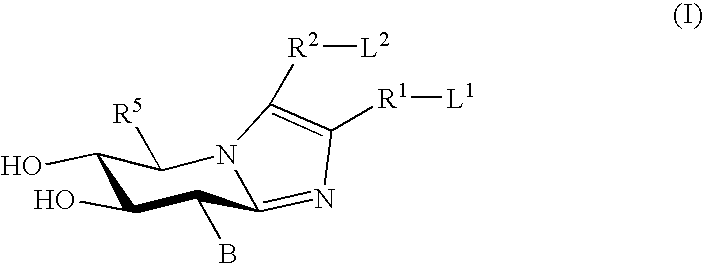
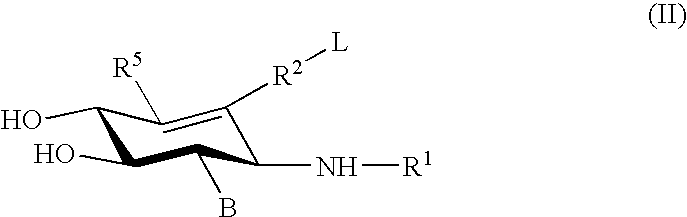
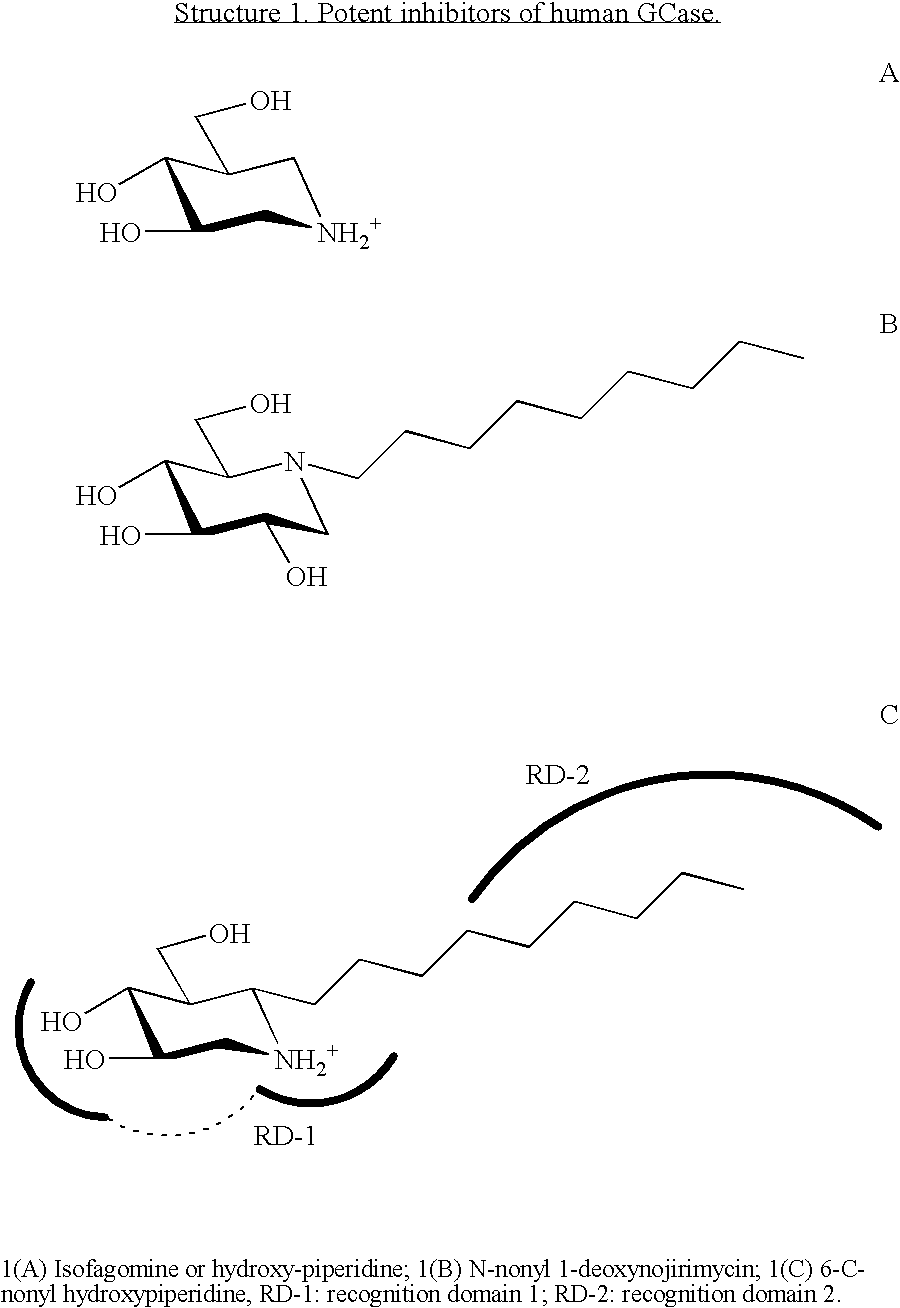
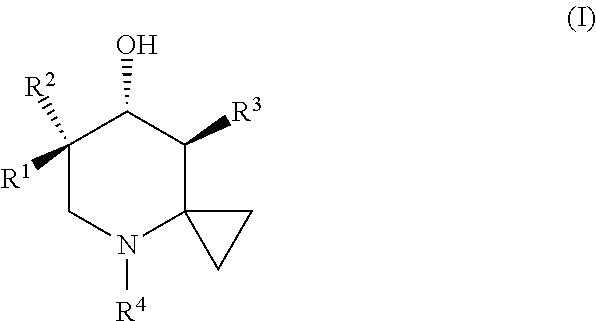
Glucocerebrosidase modulators and uses thereof
ActiveUS20170029379A1Boost protein levelsIncrease activity levelSenses disorderNervous disorderLysosomal enzyme defectStructural formula
The invention provides isofogamine analogs of structural formula (I) below that modulate and stabilize glucocerebrosidases and enhance their enzymatic activity in vivo. Such compounds, prodrugs and compositions thereof are useful in treating synucleinopathy, lysosomal storage disease and relevant neurodegenerative disease.
Owner:ALECTOS THERAPEUTICS
Production of lysosomal enzymes in plants by transient expression
InactiveUS20040093646A1Extended half-lifeLow costSsRNA viruses positive-senseAntibody mimetics/scaffoldsNicotiana tabacumNucleotide
The invention relates to the production of enzymatically active recombinant human and animal lysosomal enzymes involving construction and expression of recombinant expression constructs comprising coding sequences of human or animal lysosomal enzymes in a plant expression system. The plant expression system provides for post-translational modification and processing to produce a recombinant gene product exhibiting enzymatic activity. The invention is demonstrated by working examples in which transgenic tobacco plants express recombinant expression constructs comprising human glucocerebrosidase nucleotide sequences. The invention is also demonstrated by working examples in which transfected tobacco plants express recombinant viral expression constructs comprising human alpha galactosidase nucleotide sequences. The recombinant lysosomal enzymes produced in accordance with the invention may be used for a variety of purposes, including but not limited to enzyme replacement therapy for the therapeutic treatment of human and animal lysosomal storage diseases.
Owner:ERWIN ROBERT L +4
Methods for treating parkinson's disease
ActiveUS20180135129A1Reducing or preventing leucine rich repeat kinase 2 (LRRK2)-mediated neuronal cell deathReducing or preventing glucocerebrosidase-mediated neuronal cell deathNervous disorderMicrobiological testing/measurementDiseaseLeucine-rich repeat
The present invention provides methods for treating Parkinson's Disease (PD), e.g., PD associated with a genetic mutation in a glucocerebrosidase (GBA) gene or a leucine rich repeat kinase 2 (LRRK2) gene. The methods comprise administering to the subject a modulator, e.g., an inhibitor, of p53-inducible gene 3 (PIG3).
Owner:BPGBIO INC
Glucocerebrosidase modulators and uses thereof
ActiveUS9796680B2Improve breathabilitySenses disorderNervous disorderLysosomal enzyme defectSynuclein
Owner:ALECTOS THERAPEUTICS INC
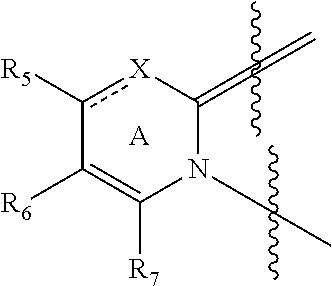
Substituted pyrazolopyrimidines as glucocerebrosidase activators
ActiveUS9974789B2Reduce severityPrevention and reduction of severityOrganic active ingredientsOrganic chemistryLysosomal storage disordersGlucocerebrosidase
Substituted pyrazolopyrimidines and dihydropyrazolopyrimidines and related compounds, their methods of manufacture, compositions containing these compounds, and methods of use of these compounds in treating lysosomal storage disorders such as Gaucher disease are described herein. The compounds are of general Formula (I)in which variables R1-R7 and X are described in the application.
Owner:UNITED STATES OF AMERICA
Methods for treating parkinsons disease and parkinsonism
Described is a method for treating an individual having a neurological disorder with an associated mutation or mutations in a gene encoding a lysosomal enzyme. Specifically, the individual is administered a specific pharmacological chaperone for the lysosomal enzyme which increases trafficking of the protein from the ER to the lysosome in neural cells, with or without concomitantly increasing enzyme activity in neural cells. Restoration of trafficking relieves cell stress and other toxicities associated with accumulation of mutant proteins. Restoration of enzyme activity relieves substrate accumulation and pathologies associated with lipid accumulation. In a specific embodiment, the neurological disorder is Parkinson's disease or parkinsonism which is associated with mutations in glucocerebrosidase.
Owner:AMICUS THERAPEUTICS INC
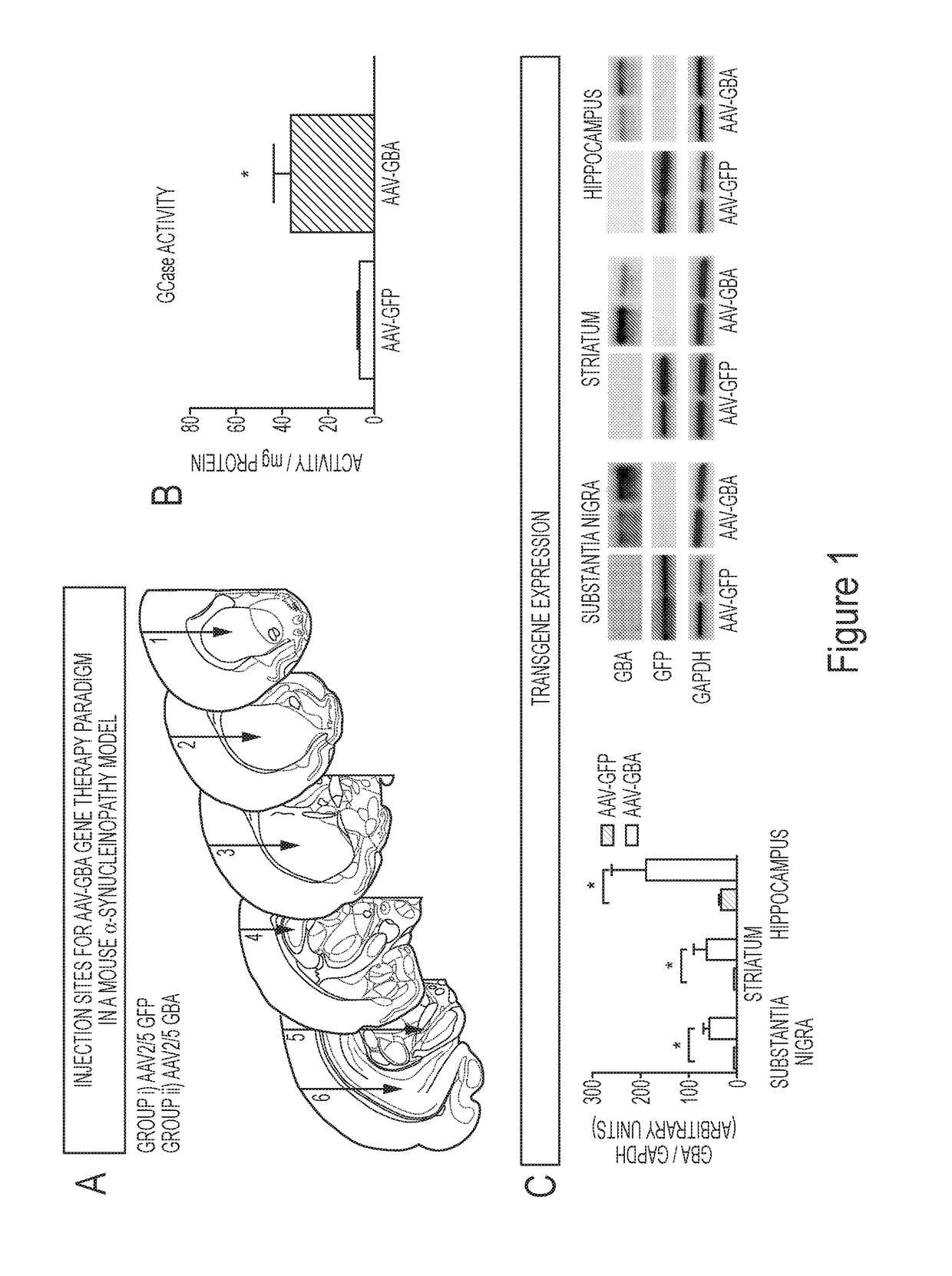
Gene therapies for lysosomal disorders
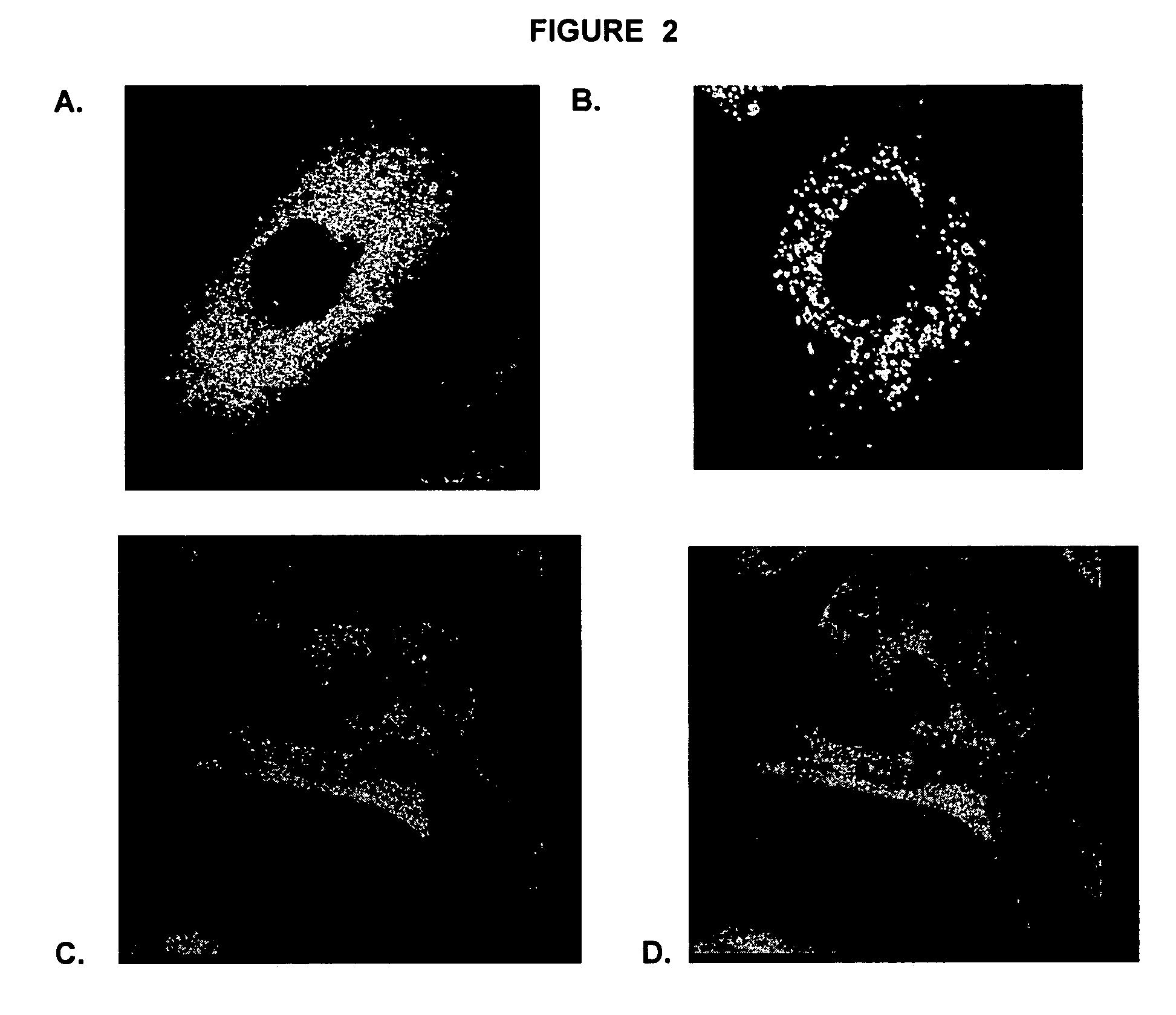
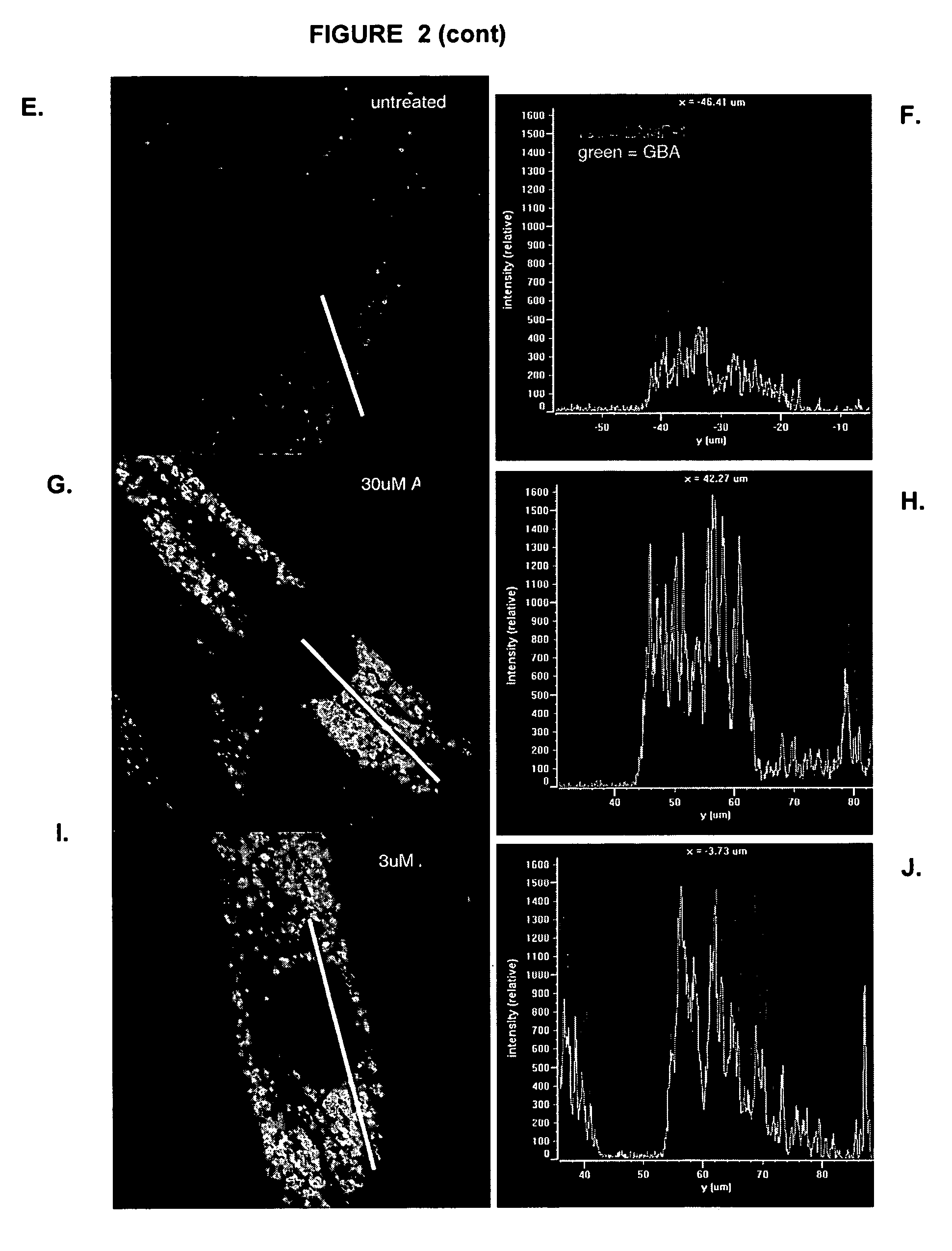
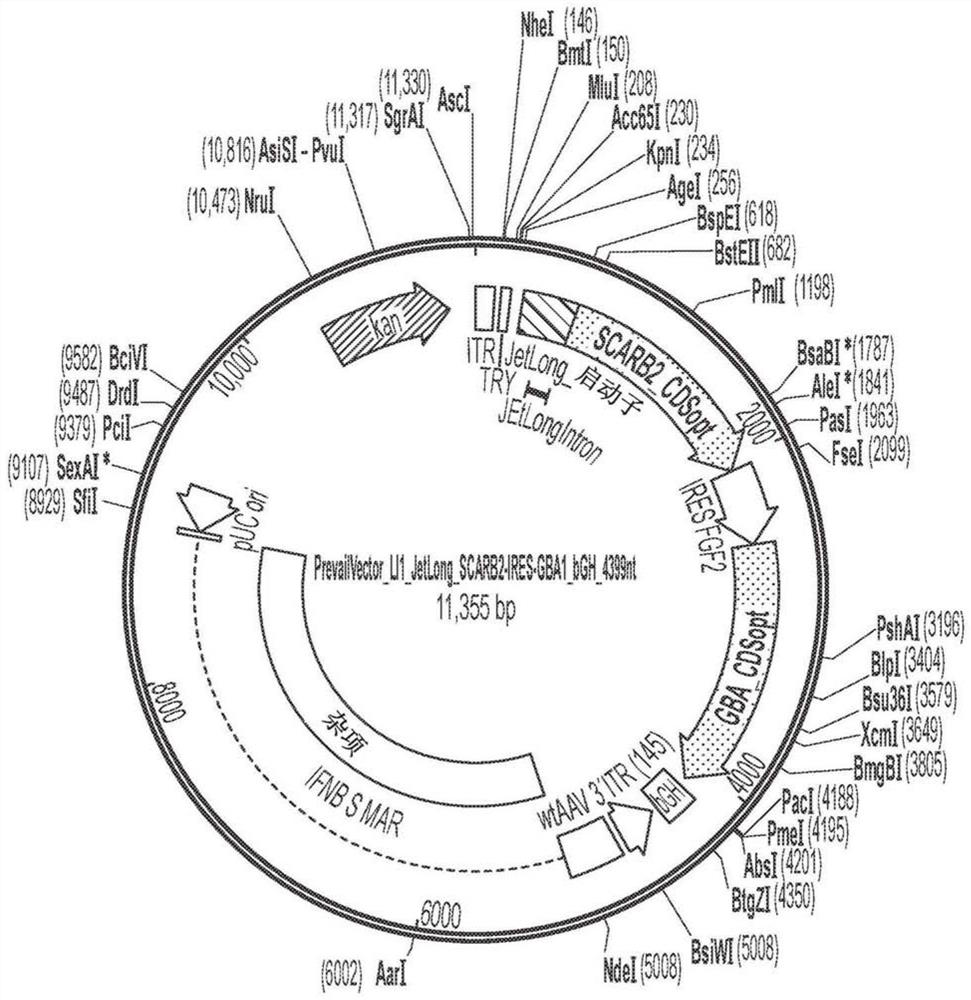
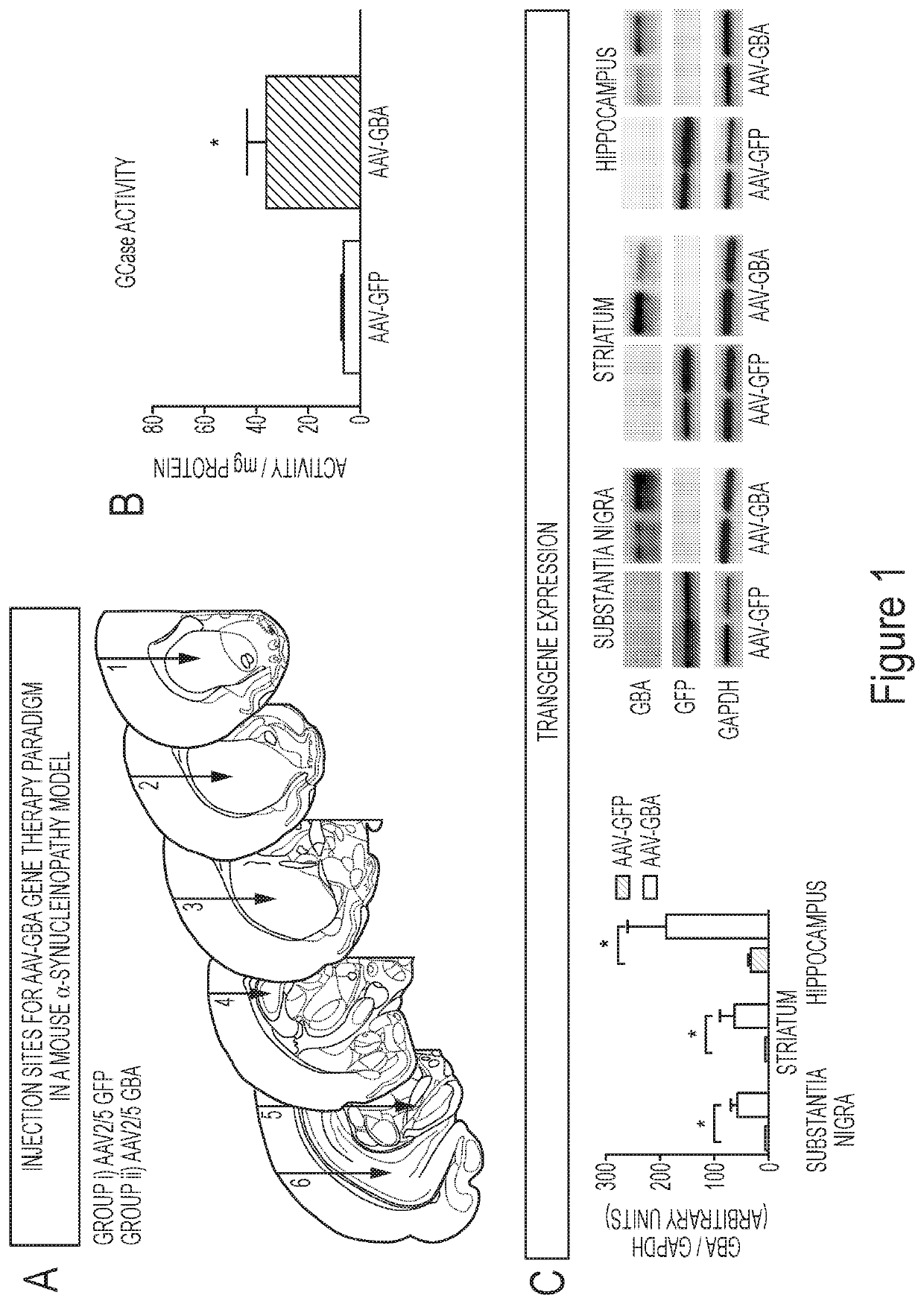
The present invention relates to gene therapies for lysosomal disorders. In particular, the present disclosure relates, in certain aspects, to compositions and methods for treating diseases associatedwith abnormal lysosomal function, such as Parkinson's disease (PD) and Gaucher's disease. In certain embodiments, the present disclosure provides expression constructs comprising a transfection geneencoding beta glucocerebrosidase (GBA), or a portion thereof, alone or in combination with one or more PD-associated genes. In certain embodiments, the present disclosure provides methods of treatingParkinson's disease by administering these expression constructs to a subject in need thereof.
Owner:ПРЕВЕЙЛ ТЕРАПЬЮТИКС ИНК
Glucocerebrosidase gene therapy for Parkinson's disease
ActiveUS10967073B2Small sizeReduce the amount requiredNervous disorderPeptide/protein ingredientsNeuro-degenerative diseaseCerebroside
The present invention is directed, in part, to the treatment of a subject having a neurodegenerative disorder, such as Parkinson's disease (PD), by providing glucocerebrosidase enzyme. The enzyme may be provided, e.g., through gene therapy or by administration of a glucocerebrosidase protein. Accordingly, the present invention encompasses glucocerebrosidase nucleic acids or proteins for use in the treatment of PD or other neurodegenerative disorders.
Owner:THE MCLEAN HOSPITAL CORP +1
Method of treating gaucher disease
Therapeutic compositions and methods for treatment of late-onset Gaucher disease are described herein. The compositions comprise compounds having activity as pharmacological chaperones for mutant forms of the beta-glucocerebrosidase. Methods of treatment involve providing therapeutically effective amounts of such compositions to subjects in need thereof.
Owner:HOSPITAL FOR SICK CHILDREN +1
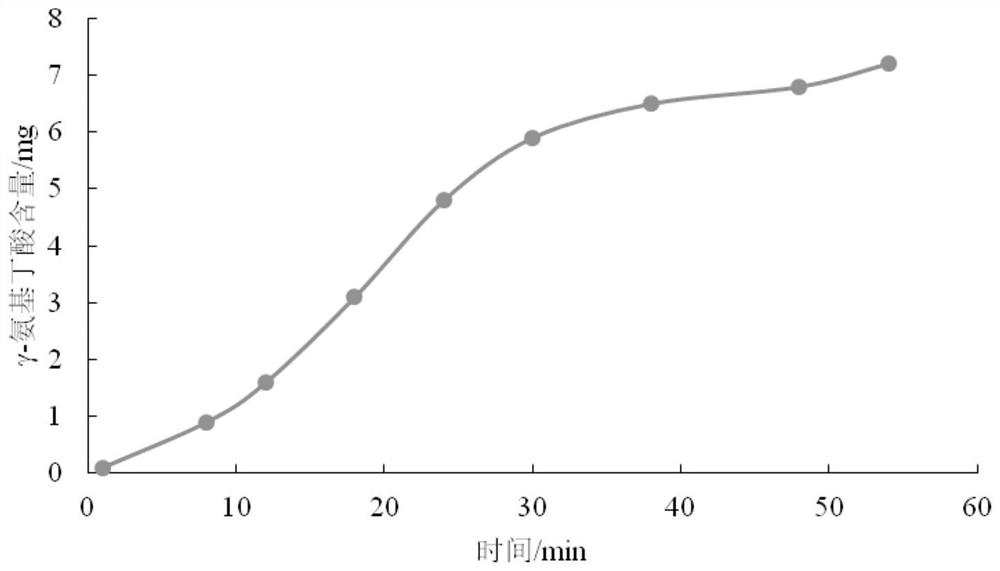
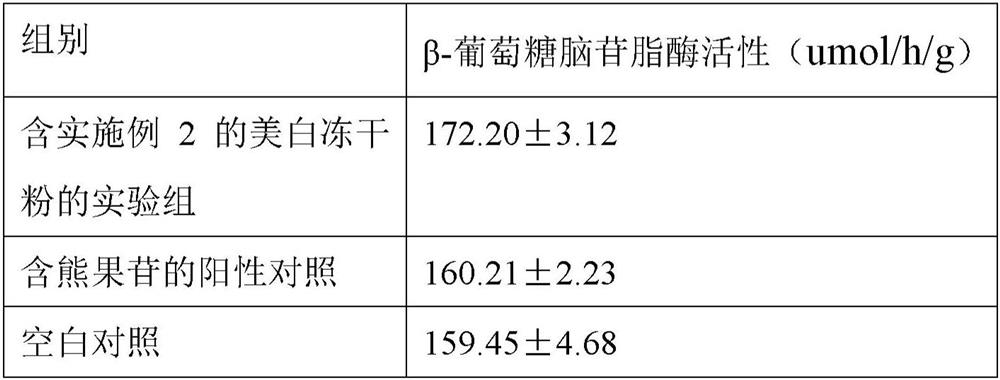
A whitening composition containing coix seed fermented liquid
ActiveCN108078880BWhitening effect is safe and efficientStimulate enzyme activityCosmetic preparationsToilet preparationsBiotechnologyCollagenan
The invention discloses a whitening composition containing a coix seed fermentation liquor, wherein the whitening composition comprises the following effective active components by the weight percentage: 5.0%-50.0% of the coix seed fermentation liquor, 0.1%-5.0% of copper glycyl-histidyl-lysine and 0.1%-6.0% of macromolecular collagen, wherein a preparation method of the coix seed fermentation liquor is described in the patent application having the application number of 201711165203.6 and applied by the inventor. The whitening composition can improve the enzyme activity of beta-glucocerebrosidase in skin, promotes the formation of ceramide of the skin and makes the skin bright and white. Under sustained release of the macromolecular collagen, the small-molecular active substances in the coix seed fermentation liquor and copper glycyl-histidyl-lysine have more lasting effect on the skin and have the effects of whitening, moisturizing and compacting the skin.
Owner:BIOPHARM RES & DEV CENT JINAN +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com