Gene therapies for lysosomal disorders
A gene and vector technology, applied in the field of gene therapy for lysosomal disorders, can solve problems such as difficult treatment of Gaucher's disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1: rAAV vector
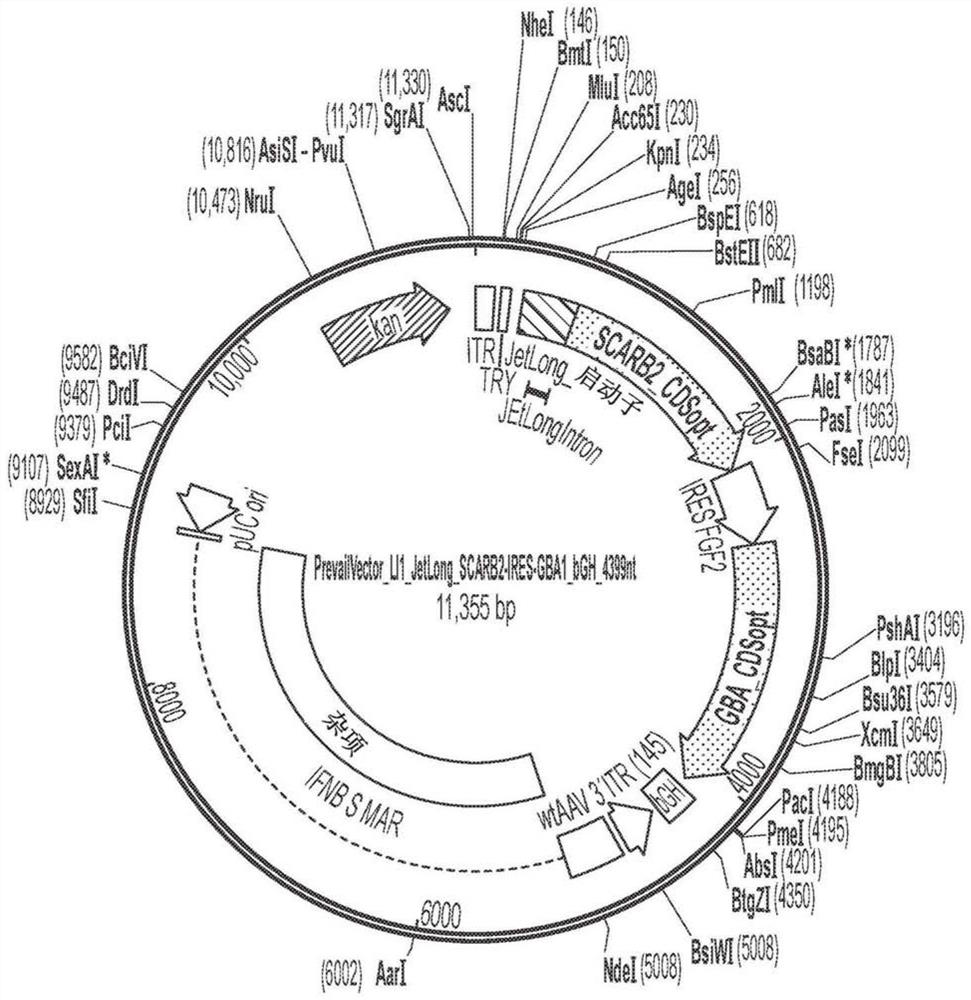
[0135] AAV vectors are produced using cells such as HEK293 cells for triple plasmid transfection. ITR sequences flank expression constructs containing promoter / enhancer elements, 3' polyA signals, and post-translational signals such as WPRE elements for each transgene of interest. Multiple gene products such as GBA1 and LIMP2 and / or prosaposin can be expressed simultaneously by fusion of protein sequences, or using 2 peptides with added amino acids due to prevention of peptide bond formation 2A peptide linkers of fragments such as T2A or P2A are achieved either using an IRES element or by expression with 2 separate expression cassettes. The presence of highly spliced short intronic sequences upstream of the expressed gene can increase expression levels. shRNA and other regulatory RNAs can potentially be included in these sequences. Examples of expression constructs described in this disclosure are shown in Figure 1-8 and 21-35 and in Tabl...
Embodiment 2
[0139] Example 2: Cell-Based Assay for Viral Transduction in GBA Deficient Cells
[0140] GBA1-deficient cells are obtained as eg fibroblasts, monocytes or hES cells from GD patients or patient-derived induced pluripotent stem cells (iPSCs). These cells accumulate substrates such as glucosylceramide and glucosphingosine (GlcCer and GlcSph). Treatment of wild-type or mutant cultured cell lines with Gcase inhibitors, such as CBE, has also been used to obtain GBA-deficient cells.
[0141] Using these cell models, lysosomal defects are quantified by accumulation of protein aggregates such as α-synuclein aggregates, using antibodies against this protein or phosphorylated αSyn, and then by imaging using fluorescence microscopy. Lysosomal abnormalities have also been imaged by ICC against protein markers such as LAMP1, LAMP2, LIMP1, LIMP2 or using dyes such as Lysotracker, or by uptake of fluorescent dextran or other markers through endocytic compartments. Accumulation of autophagy...
Embodiment 3
[0143] Example 3: In vivo assay using mutant mice
[0144] This example describes an in vivo assay of AAV vectors using mutant mice. In vivo studies of AAV vectors in mutant mice as described above, using, for example, Liou et al. (2006) J. Biol. Chem. 281(7):4242-4253, Sun et al. 46:2102–2113 and the assay described by Farfel-Becker et al., (2011) Dis. Model Mech.4(6):746–752.
[0145] Medium control and AAV vector (for example, in 2×10 11 Intrathecal or intracerebroventricular delivery of vg / mouse) is performed using concentrated AAV stocks, for example, with injection volumes between 5-10 μL. Intraparenchymal delivery is by convective enhanced delivery.
[0146] Treatment begins before or after symptoms appear. Endpoints measured were substrate accumulation in the CNS and CSF, accumulation of Gcase enzymes as determined by ELISA and enzymatic activity, motor and cognitive endpoints, lysosomal dysfunction, and α-synuclein monomers, fibrils Accumulation of filaments or f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com