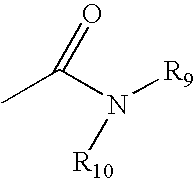
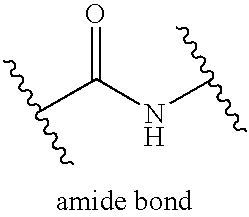
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Apolipoprotein A1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
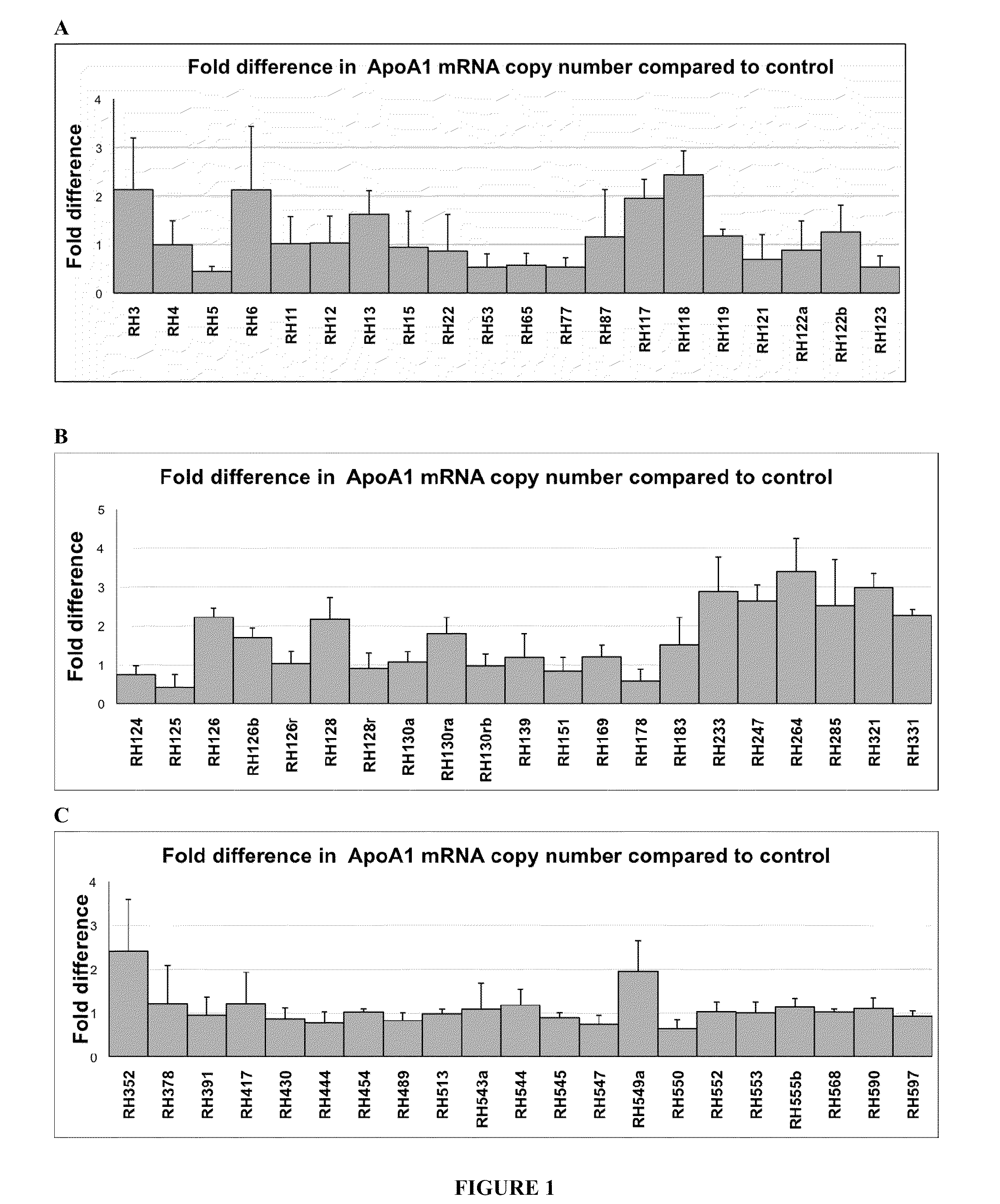
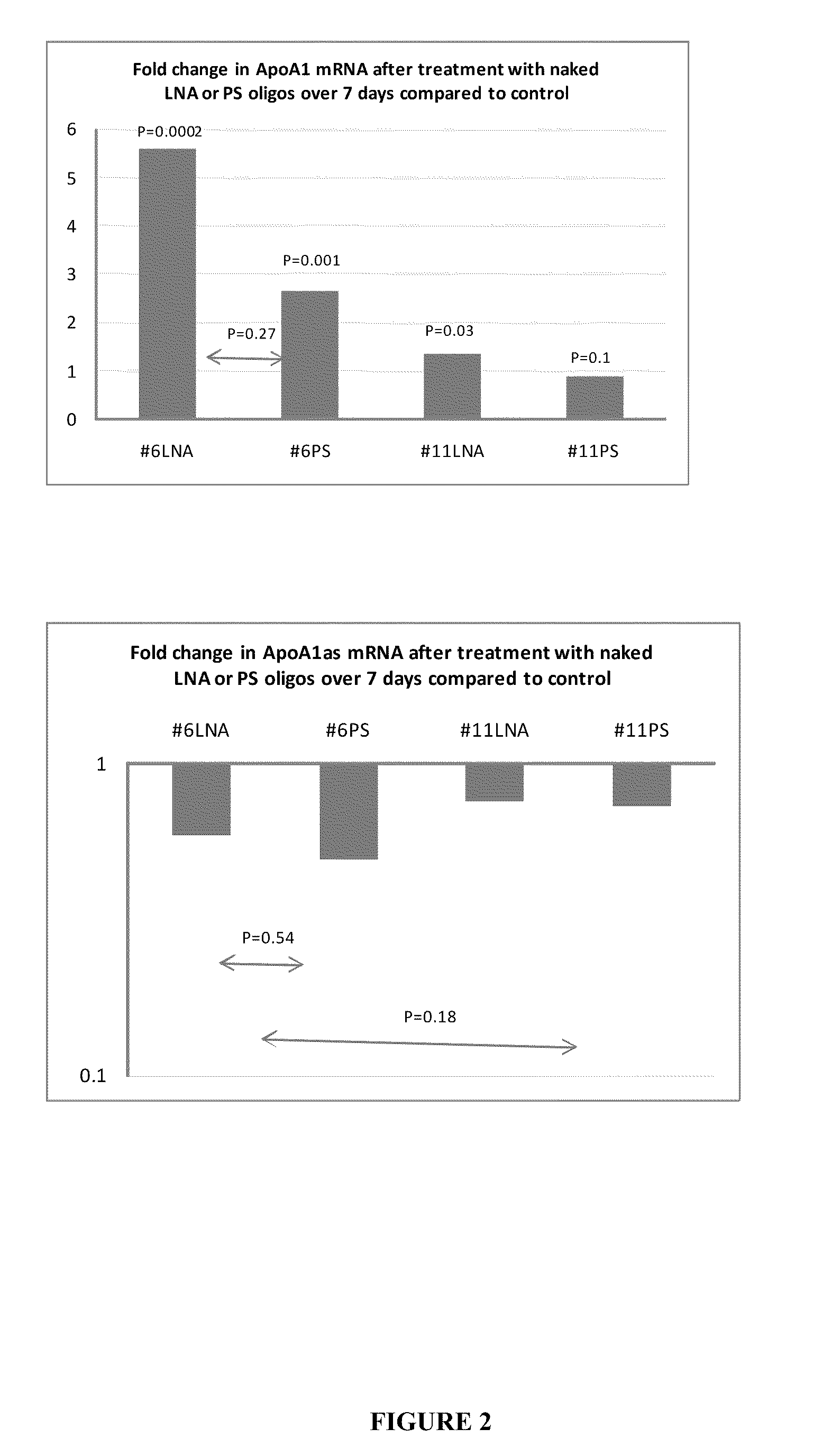
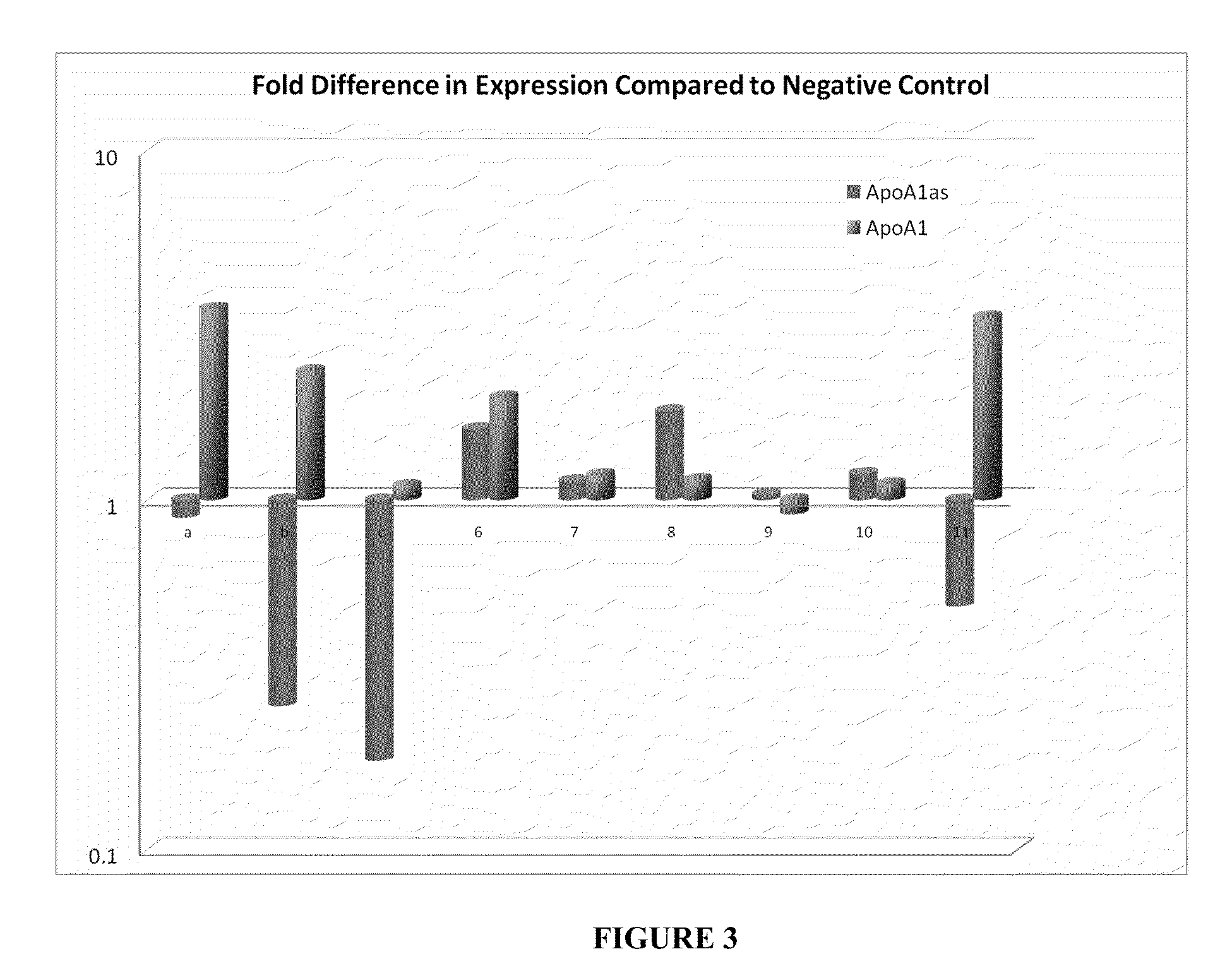
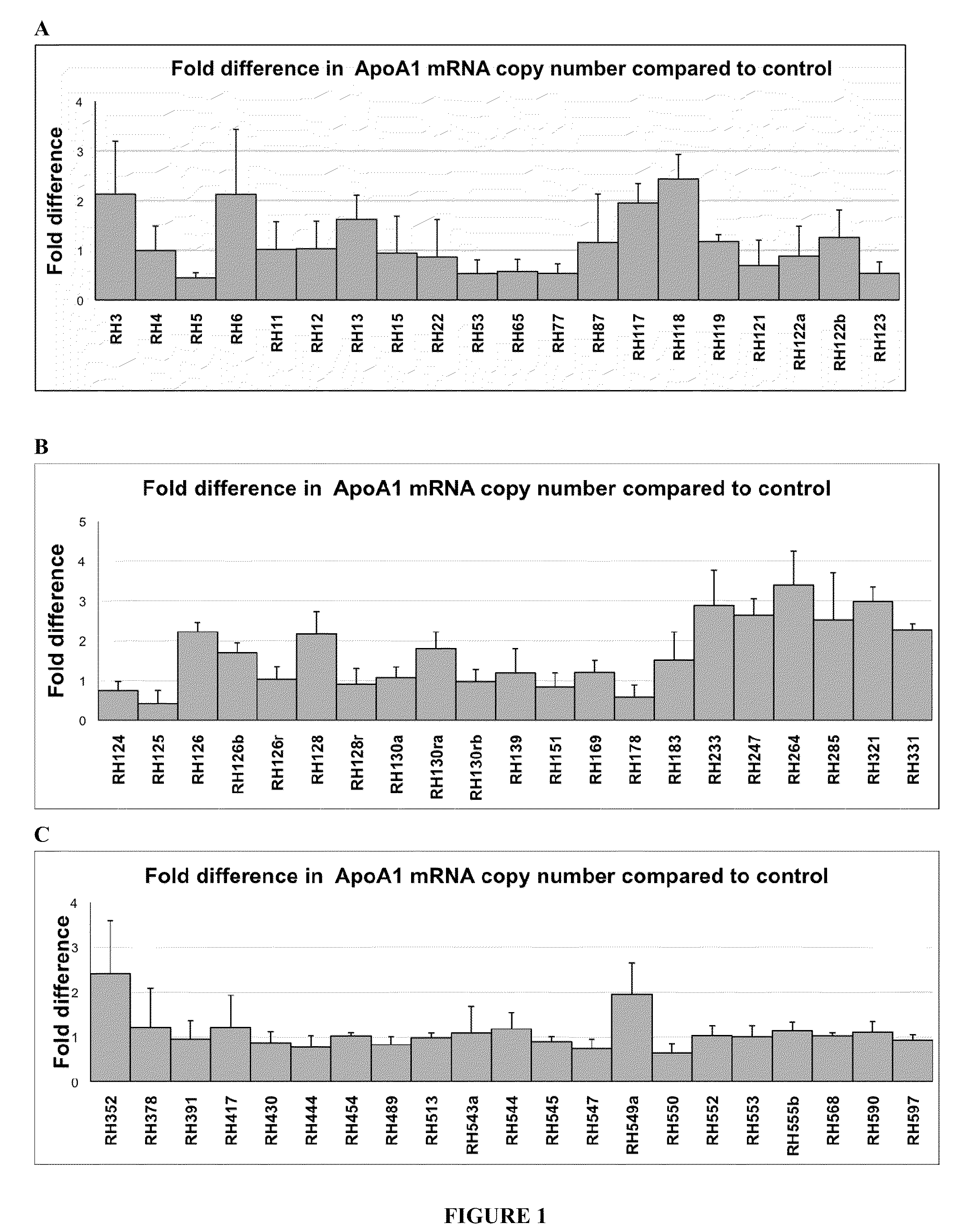
Apolipoprotein A1 is a protein that in humans is encoded by the APOA1 gene. It has a specific role in lipid metabolism. The text in a recent report suggested that APOA1 mRNA is regulated by endogenously expressed antisense RNA.
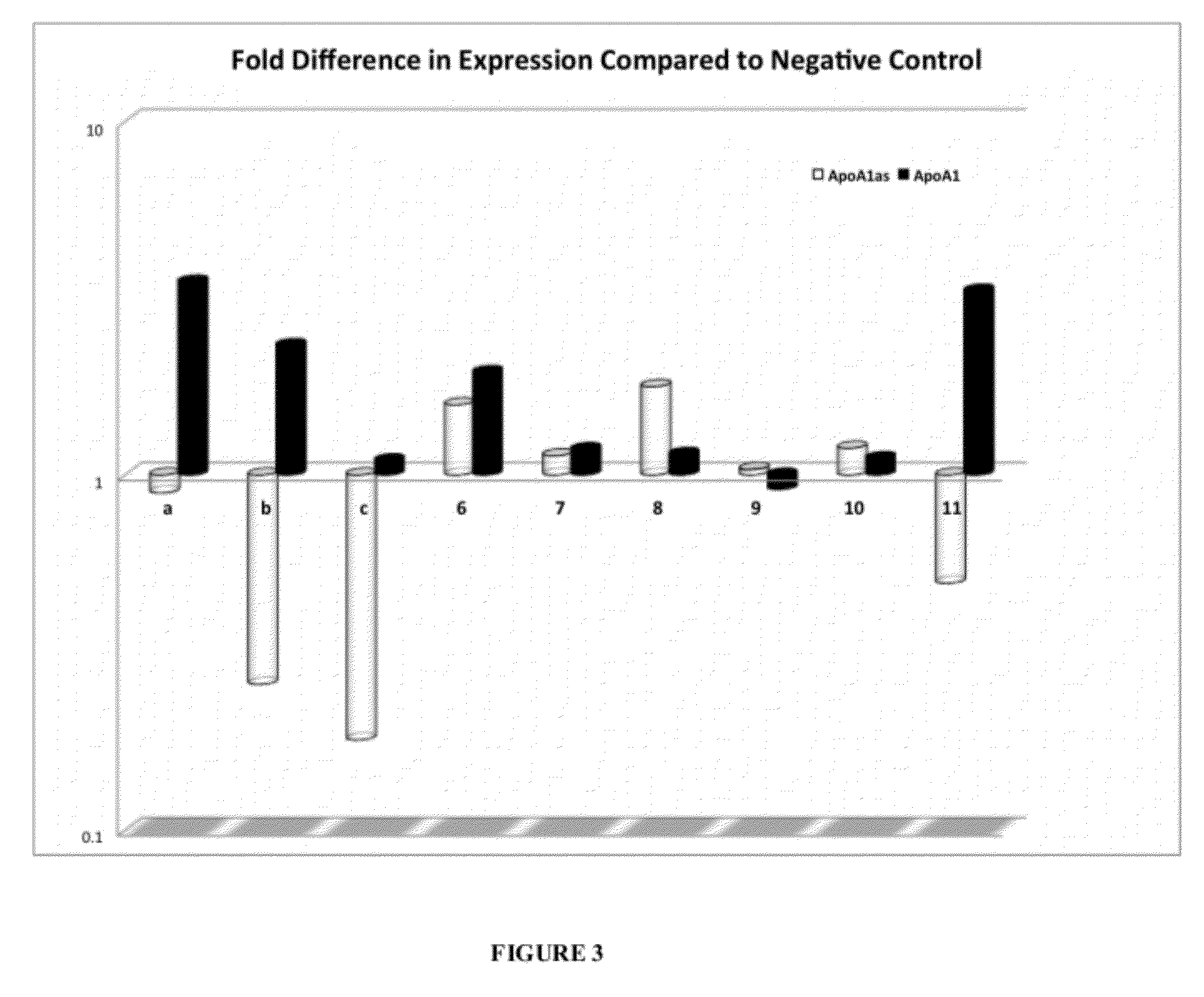
Treatment of Apolipoprotein-A1 Related Diseases by Inhibition of Natural Antisense Transcript to Apolipoprotein-A1
ActiveUS20100105760A1Modulate its functionNervous disorderAntipyreticApolipoproteins EPolynucleotide
Oligonucleotide compounds modulate expression and / or function of an apolipoprotein (ApoA1) polynucleotides and encoded products thereof. Methods for treating diseases associated with apolipoprotein-A1 (ApoA1) comprise administering one or more Oligonucleotide compounds designed to inhibit the Apo-A1 natural antisense transcript to patients.
Owner:CURNA INC
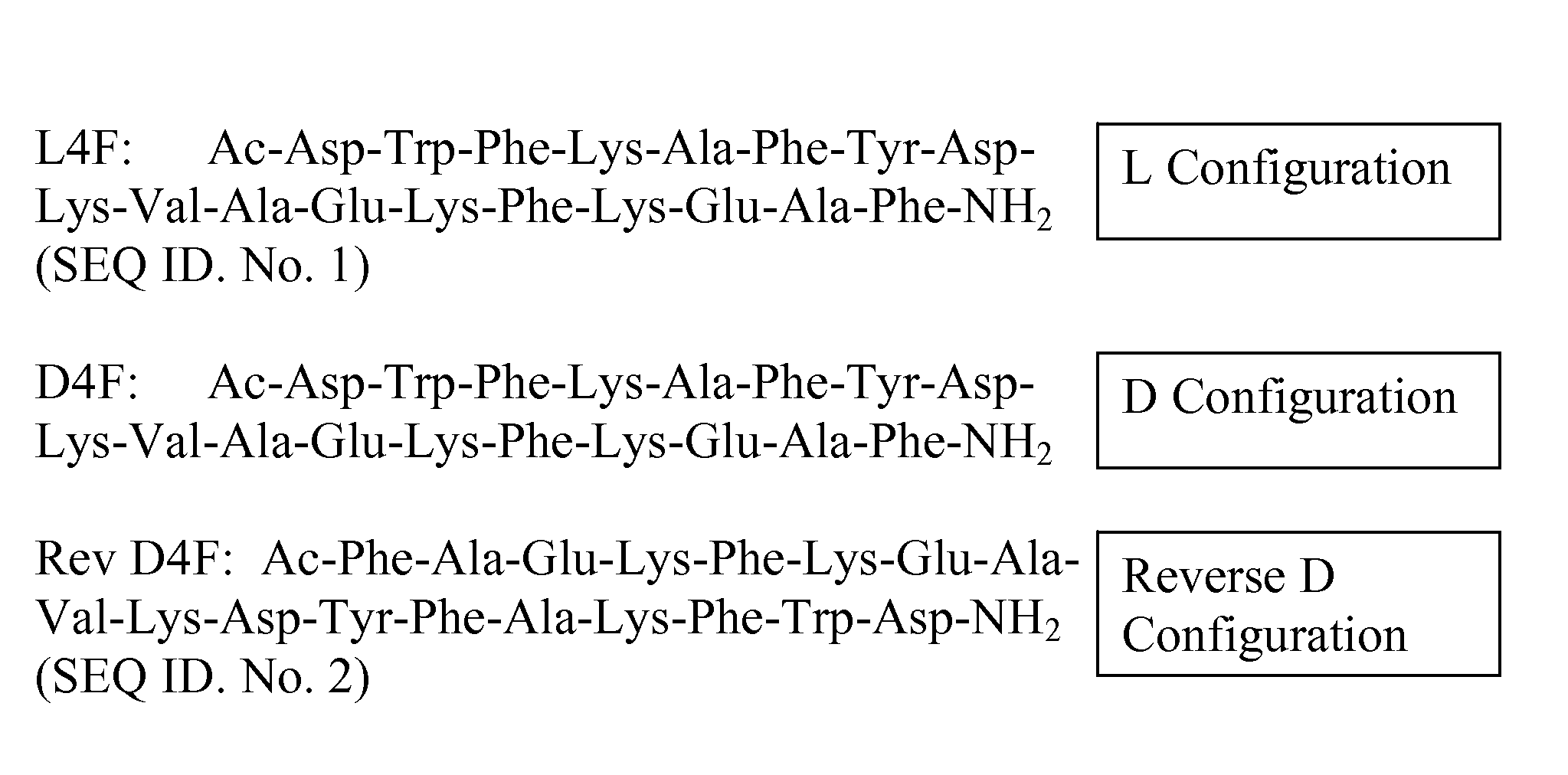
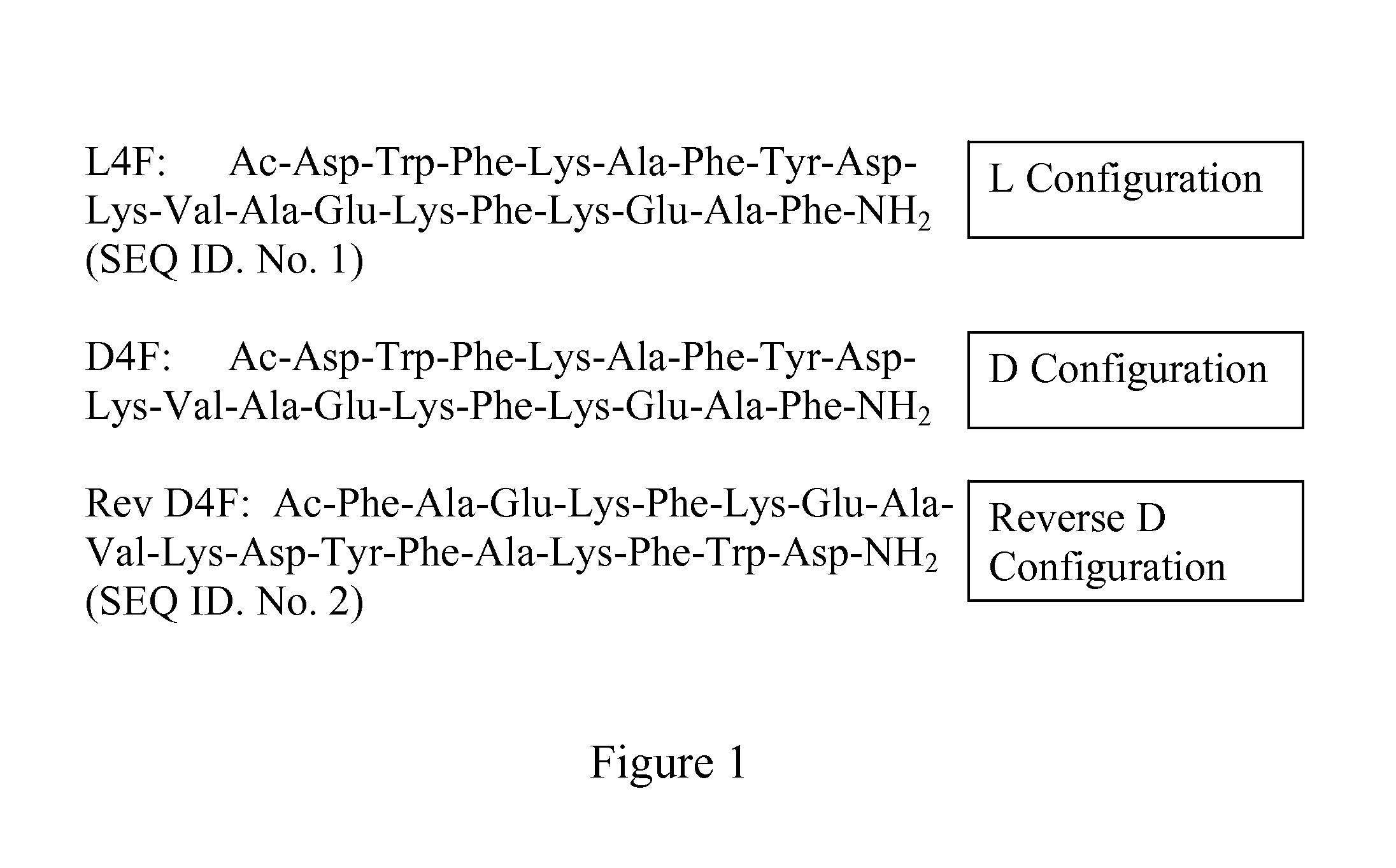
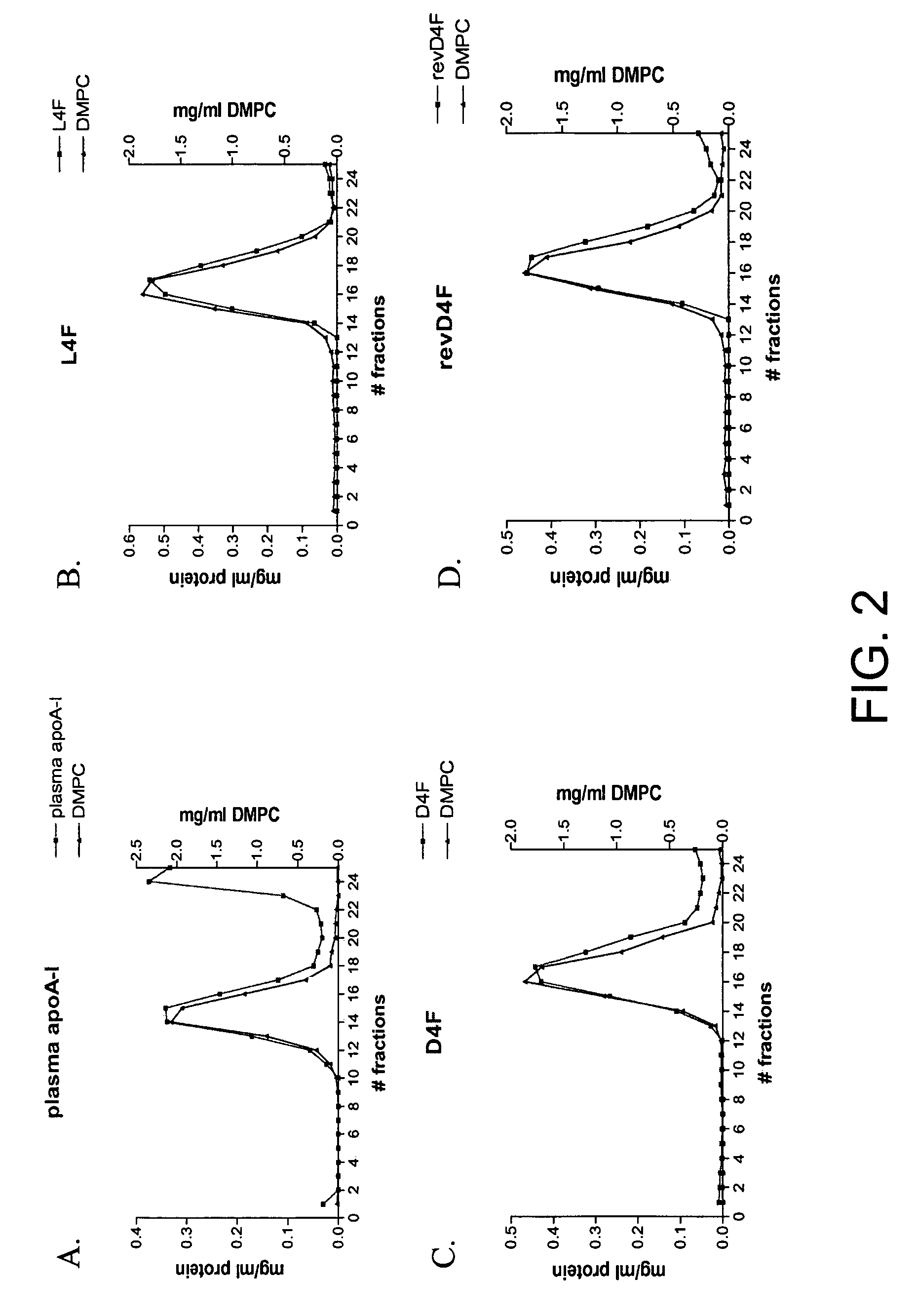
Apolipoprotein A1 mimetics and uses thereof
The present invention provides peptidomimetics derived from Apolipoprotein A-I, which is useful for beneficially influencing lipid parameters and / or plasma cholesterol levels. The invention also provides pharmaceutical compositions and methods of treatment for elevated levels of plasma cholesterol.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Human blood fat (serum/plasma) quality management reference kit and preparation method
ActiveCN103472240ANo medium effect interferenceSolve medium effect interferenceBiological testingAntigenLow density lipoprotein cholesterol
The invention provides a human blood fat (serum / plasma) quality management reference kit. The kit takes human serum (plasma) as a matrix; a human blood fat component (human apolipoprotein A1 antigen, apolipoprotein B antigen, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL) and the like), a stable system and an anticorrosion system are added to the matrix; the stable system is any combination of a surfactant of Triton series, Tween series, EDTA (ethylene diamine tetraacetic acid) series and the like, a polymer of polyvinyl alcohol series, polyvinylpyrrolidone series, polyethylene glycol series and the like, and a phosphate buffer solution; and the anticorrosion system is any combination of sodium azide, gentamicin, amphomycin and ProClin series. The invention further provides a preparation method of the kit. The kit is free from medium effect interference, is used for quality control and evaluation between the detection systems, and more really reflects actual quality conditions of human serum (plasma) samples, which are detected by detection systems.
Owner:上海北加生化试剂有限公司
Treatment of apolipoprotein-A1 related diseases by inhibition of natural antisense transcript to apolipoprotein-A1
Oligonucleotide compounds modulate expression and / or function of an apolipoprotein (ApoA1) polynucleotides and encoded products thereof. Methods for treating diseases associated with apolipoprotein-A1 (ApoA1) comprise administering one or more Oligonucleotide compounds designed to inhibit the Apo-A1 natural antisense transcript to patients.
Owner:CURNA INC
Methods and kits for the diagnosis of acute coronary syndrome
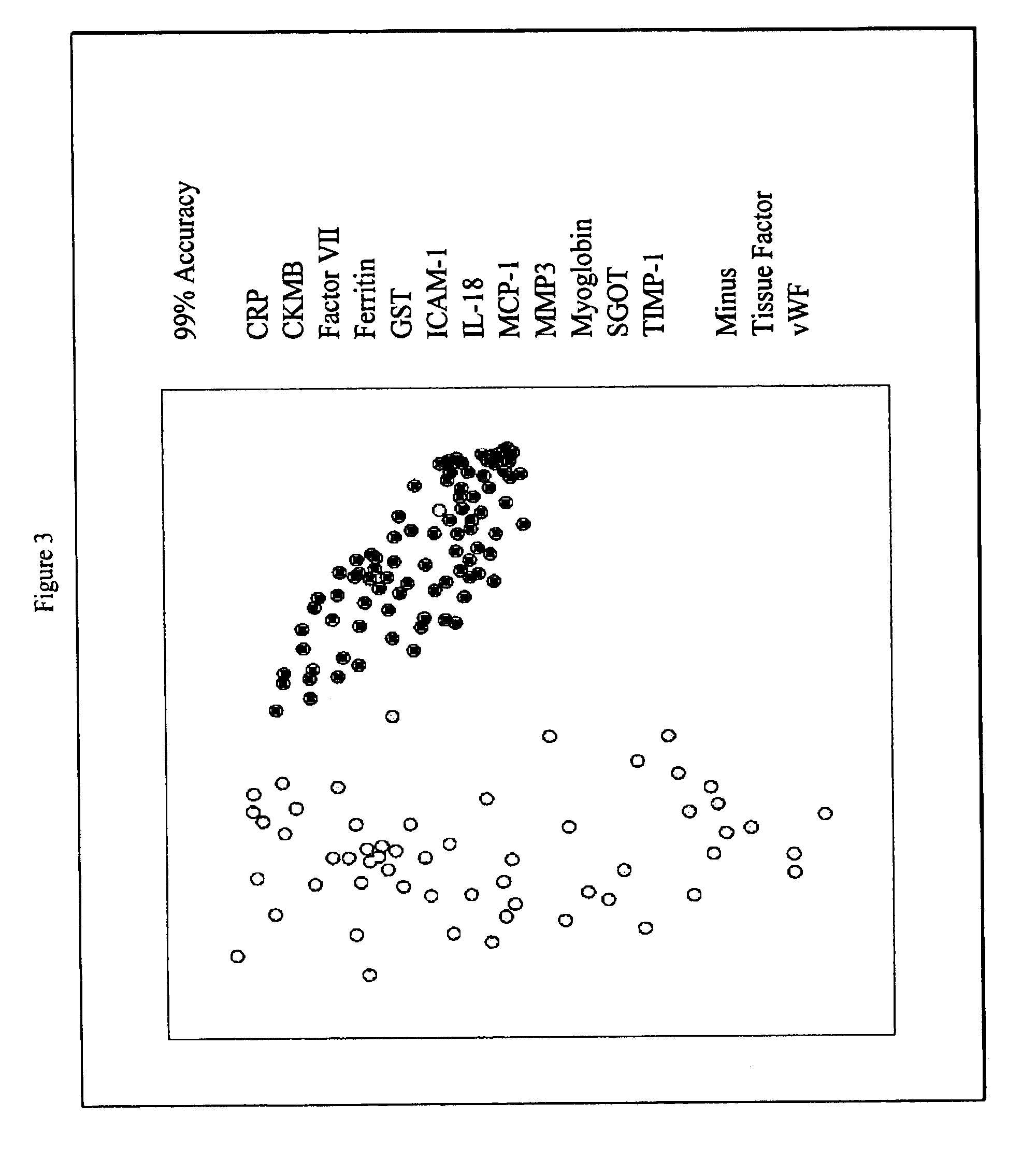
InactiveUS20070003981A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
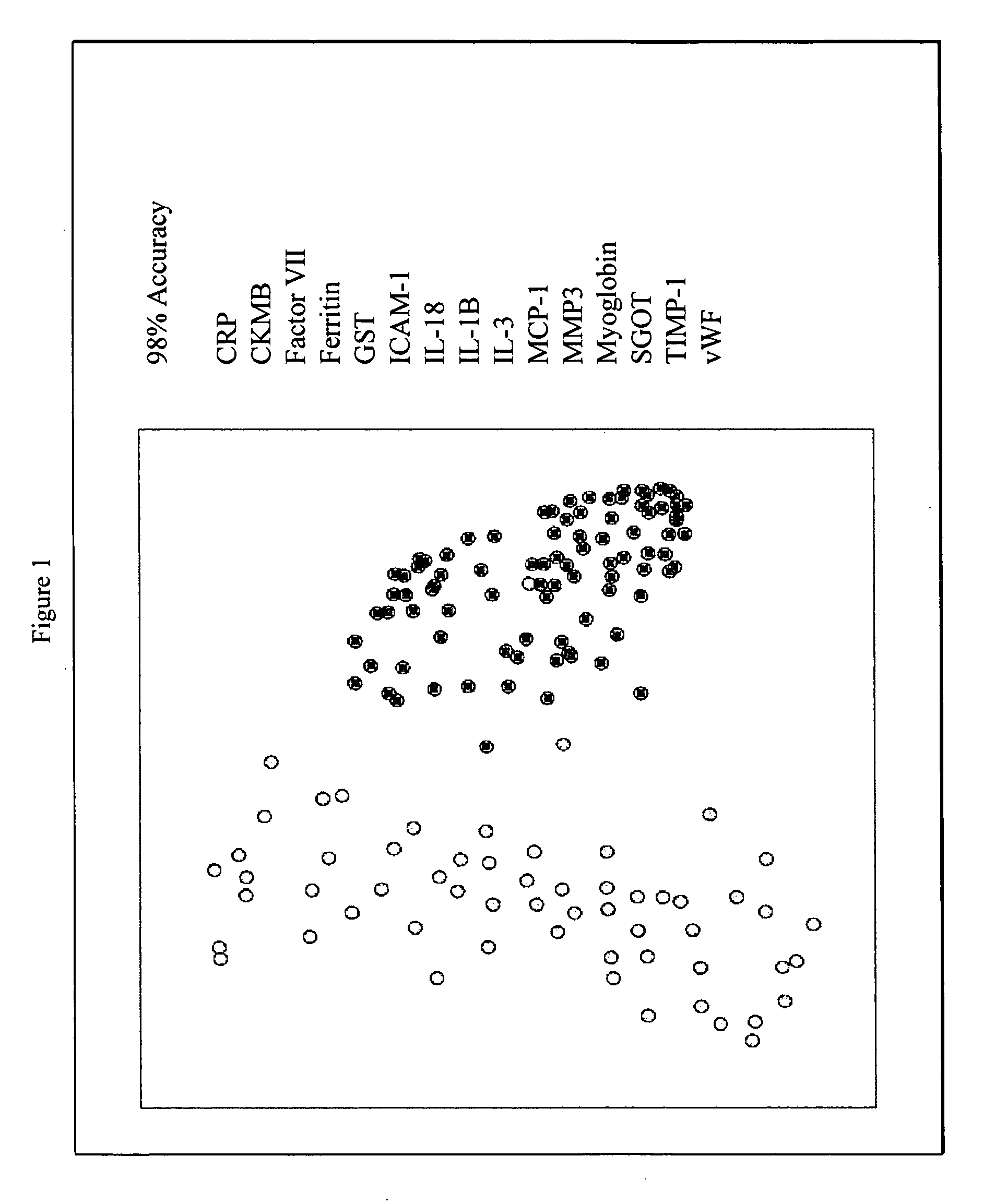
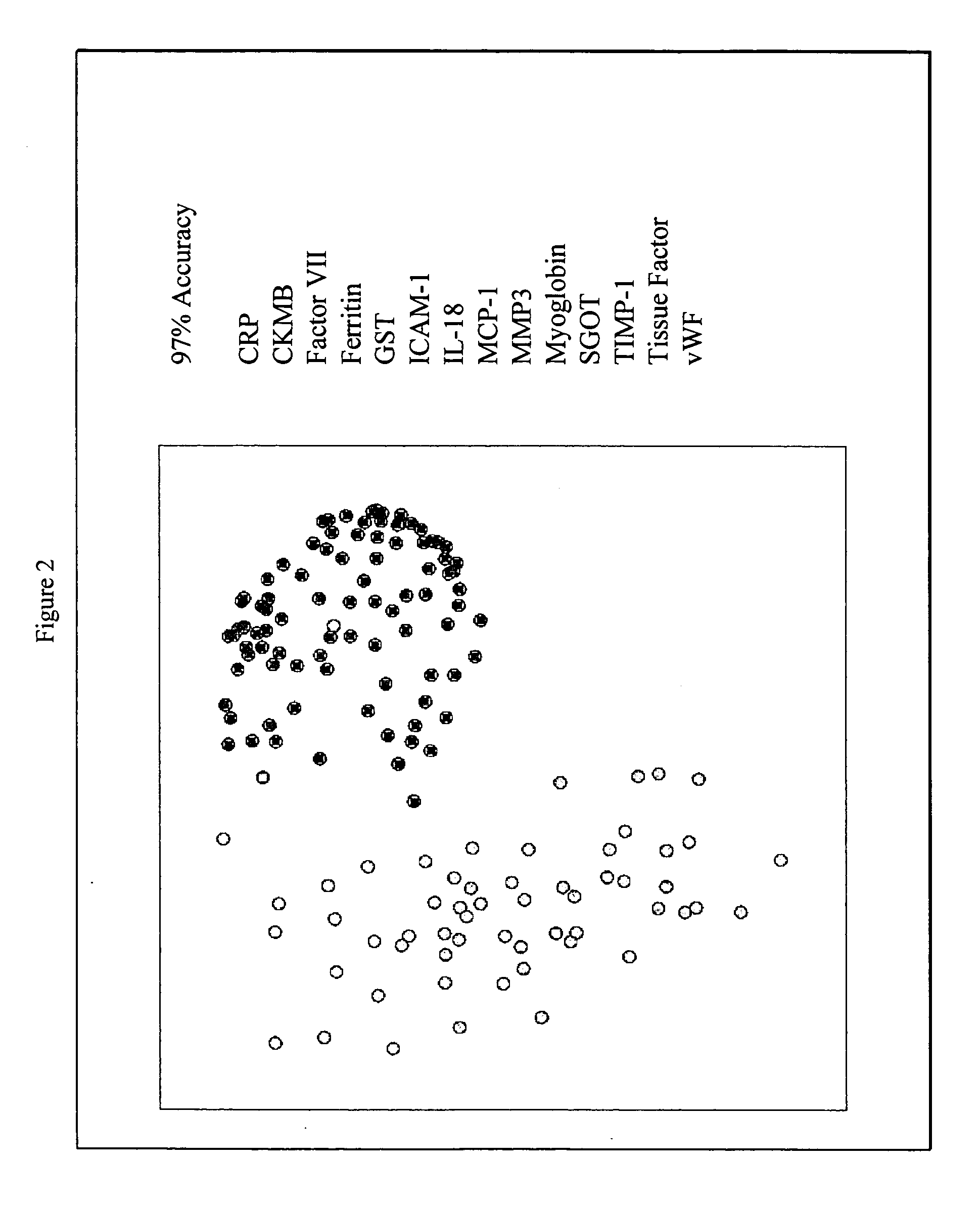
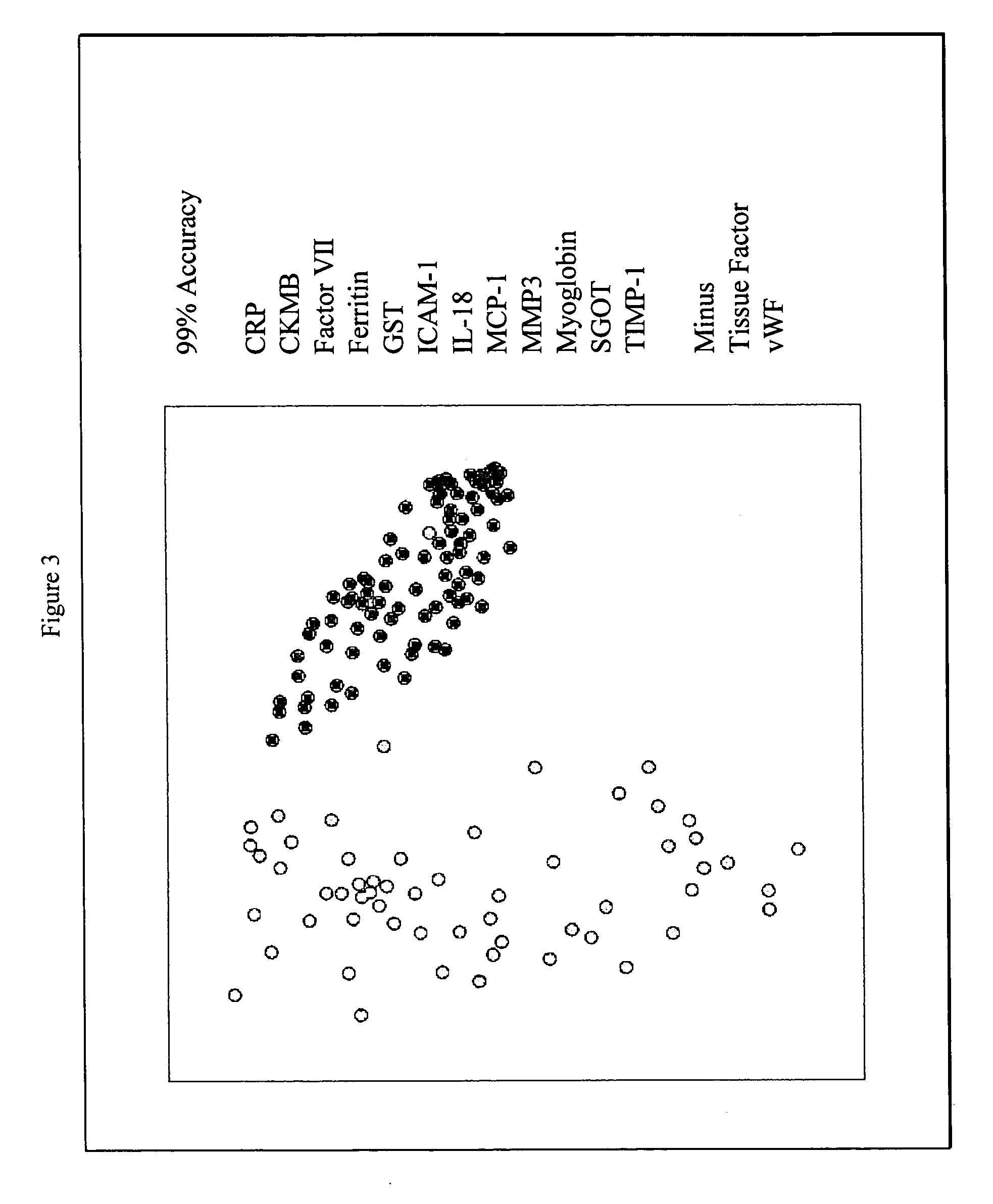
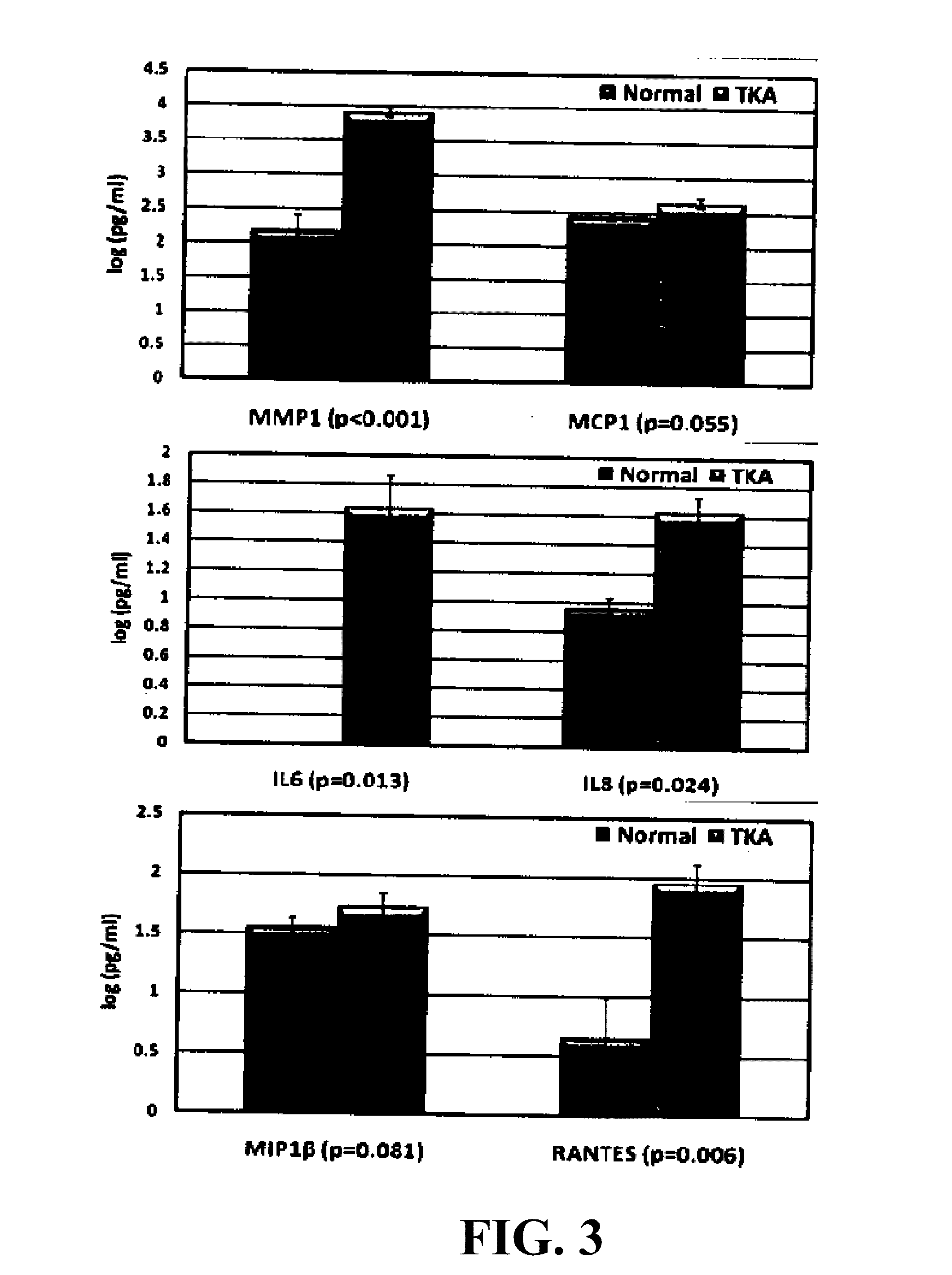
Provided are methods for the detection and diagnosis of acute coronary syndrome or ACS. The methods are based on the discovery that abnormal levels of selected analytes in sample fluid, typically blood samples, of patients who are at risk are supportive of a diagnosis of ACS. At least two new biomarkers for ACS are thus disclosed, MMP-3 and SGOT. Altogether the concentrations of twelve analytes provide a sensitive and selective picture of the patient's condition, namely, whether the patient is suffering a heart attack. Other important biomarkers for ACS are described, including but not limited to IL-18, Factor VII, ICAM-1, Creatine Kinase-MB, MCP-1, Myoglobin, C Reactive Protein, von Willebrand Factor, TIMP-1, Ferritin, Glutathione S-Transferase, Prostate Specific Antigen (free), IL-3, Tissue Factor, alpha-Fetoprotein, Prostatic Acid Phosphatase, Stem Cell Factor, MIP-1-beta, Carcinoembryonic Antigen, IL-13, TNF-alpha, IgE, Fatty Acid Binding Protein, ENA-78, IL-1-beta, Brain-Derived Nerotrophic Factor, Apolipoprotein A1, Serum Amyloid P, Growth Hormone, Beta-2 microglobulin, Lipoprotein (a), MMP-9, Thyroid Stimulating hormone, alpha-2 Macroglobulin, Complement 3, IL-7, Leptin, and IL-6. Kits containing reagents to assist in the analysis of fluid samples are also described.
Owner:RULES BASED MEDICINE
Method of diagnosing non-alcoholic steatohepatitis (nash) using molecular markers
InactiveUS20060135420A1Easy and reliable evaluationEase of evaluationBioreactor/fermenter combinationsBiological substance pretreatmentsAntioxidant proteinSelenium-Binding Proteins
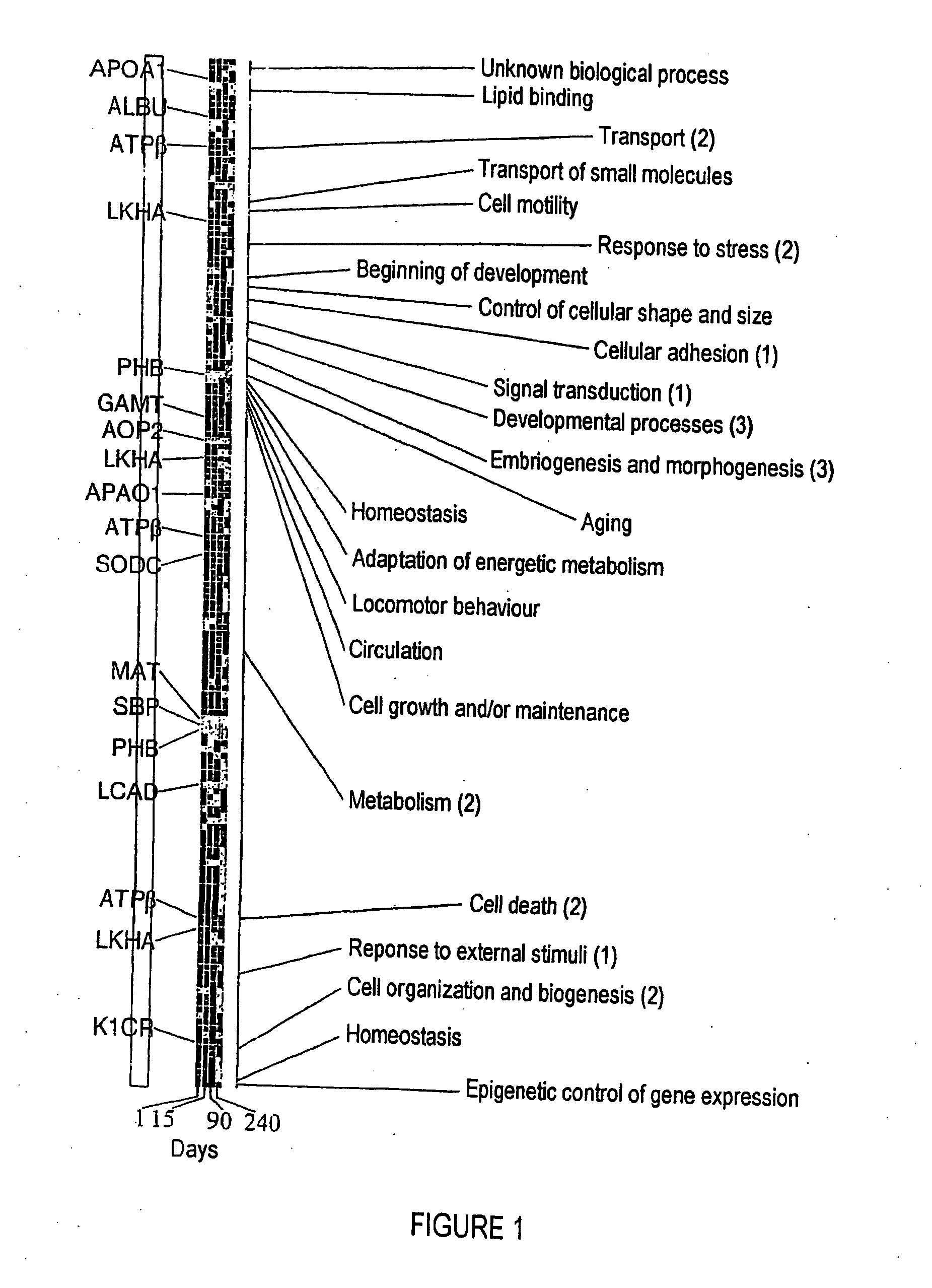
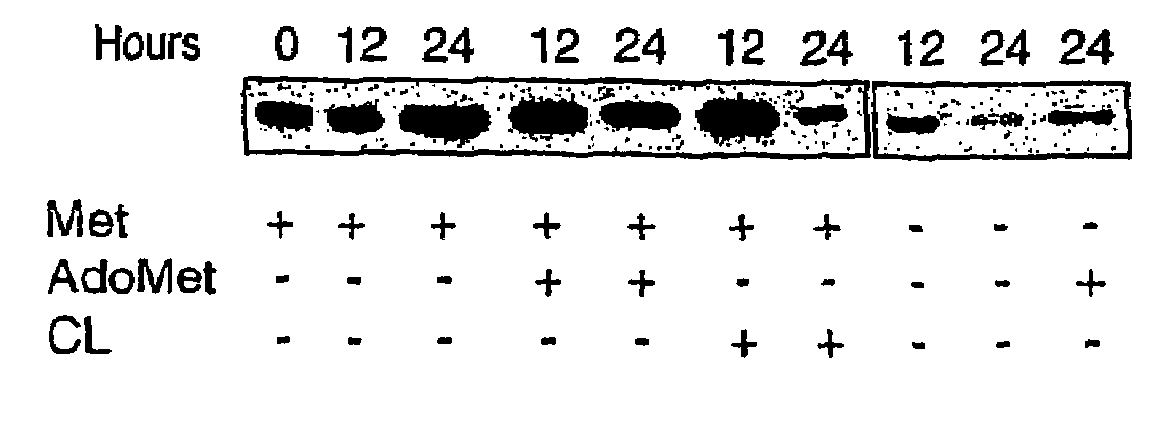
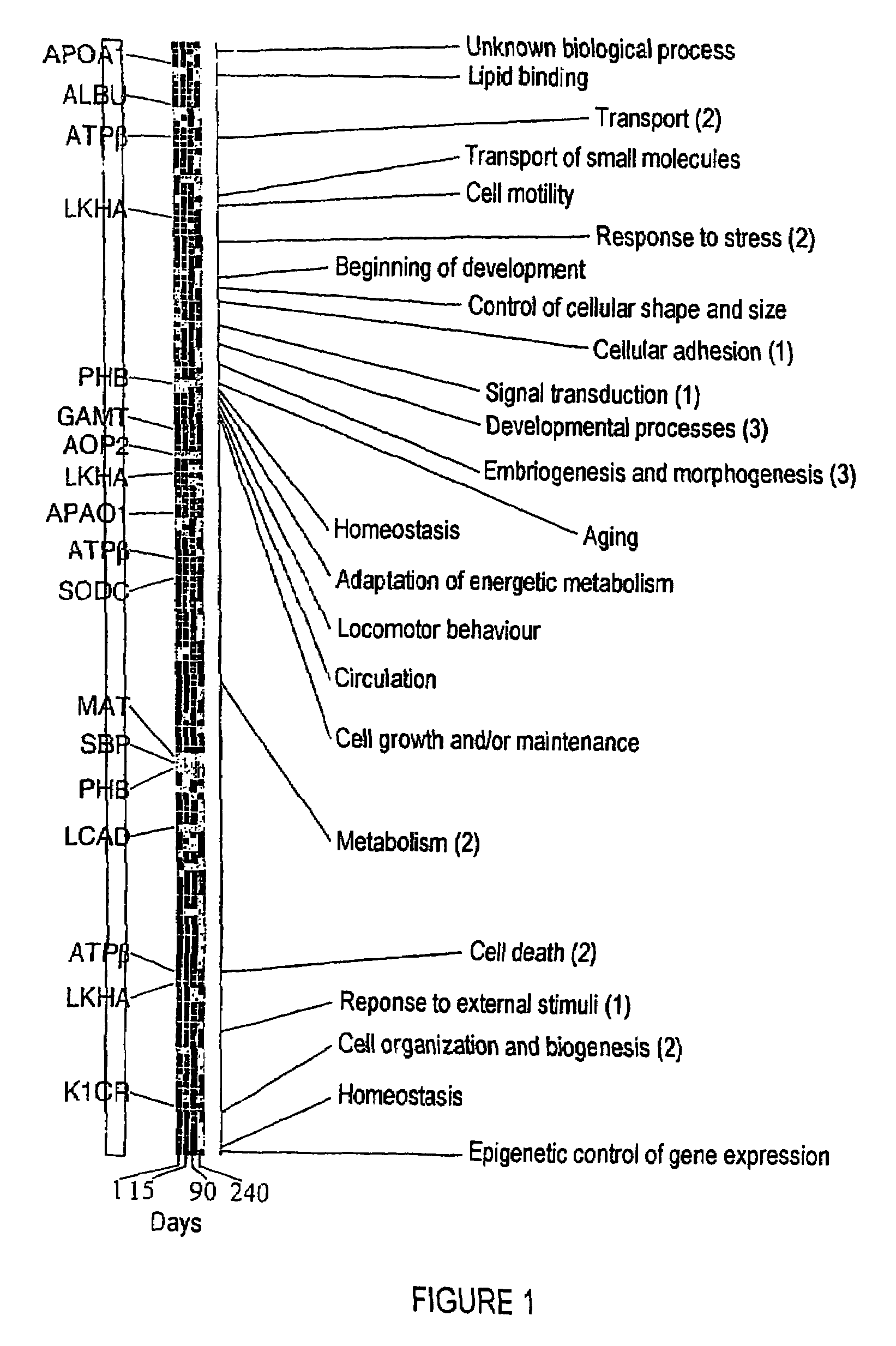
The invention relates to a method of diagnosing non-alcoholic steatohepatitis (NASH) using molecular markers. The inventive method consists in detecting and quantifying, in vitro in a hepatic tissue sample, the levels of a protein which can be used as a NASH molecular marker and which is selected from apolipoprotein A1, sub-unit β of the mitochondrial ATPase, leukotriene A4 hydrolase, keratin 18, guanidine acetate N-methyltransferase, superoxide dismutase, albumin, antioxidant protein 2 (isoform 1), prohibitin 1, methionine adenosyl transferase, long-chain acyl CoA dehydrogenase, selenium binding protein, antioxidant protein 2 (isoform 2), and combinations of same. The invention further consists in comparing the results obtained with the normal values of said proteins in healthy hepatic tissue. Said method can be used to diagnose NASH and / or to assess a patient's potential risk of developing NASH.
Owner:ONE WAY LIVER GENOMICS
Treatment for dark adaptation
InactiveUS7470660B2Increasing reverse cholesterol transportReduce accumulationBiocideSenses disorderReverse cholesterol transportCholesterol
Owner:RGT UNIV OF CALIFORNIA
Biomarkers of osteoarthritis
Owner:UNIVERSITY OF MISSOURI
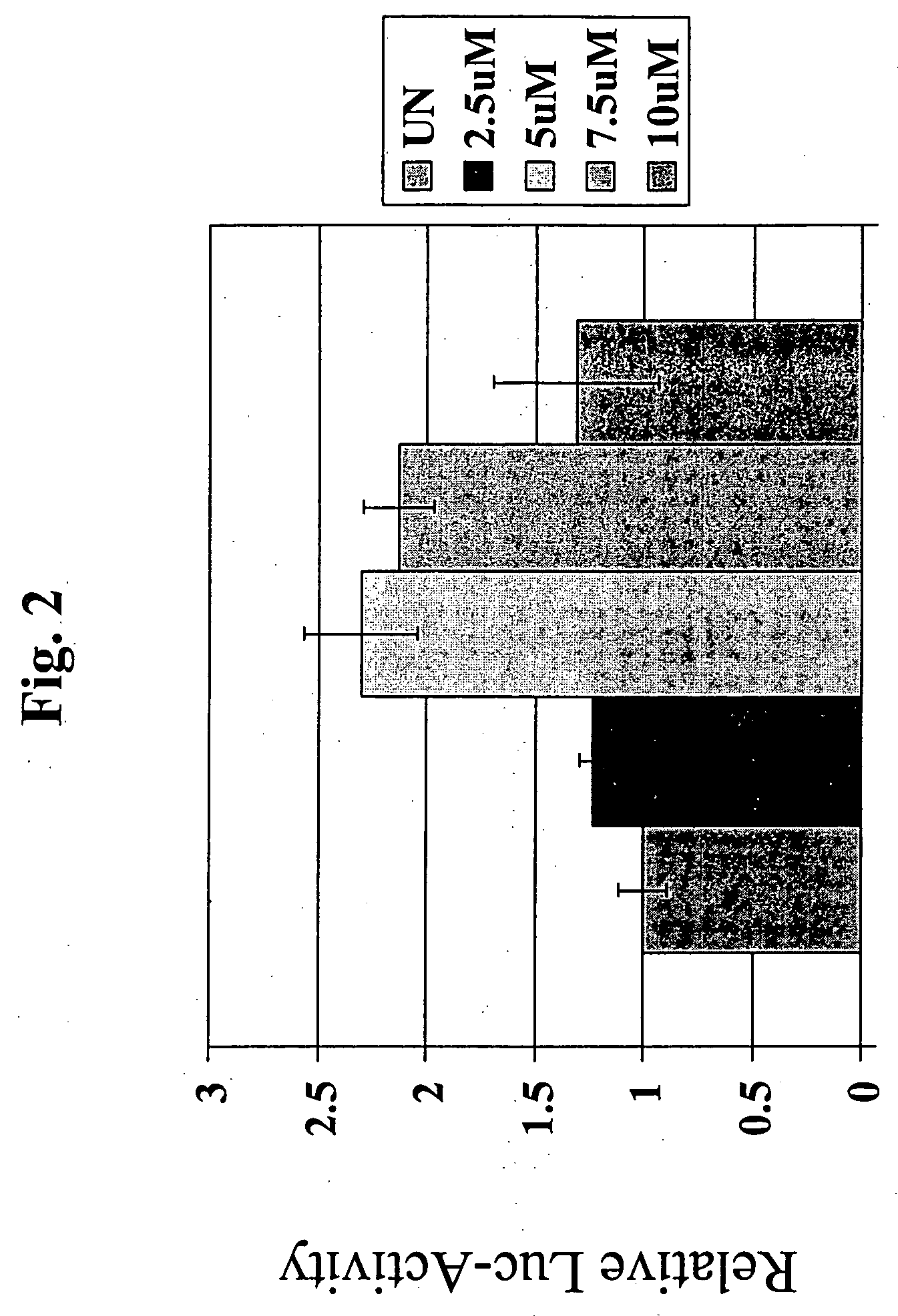
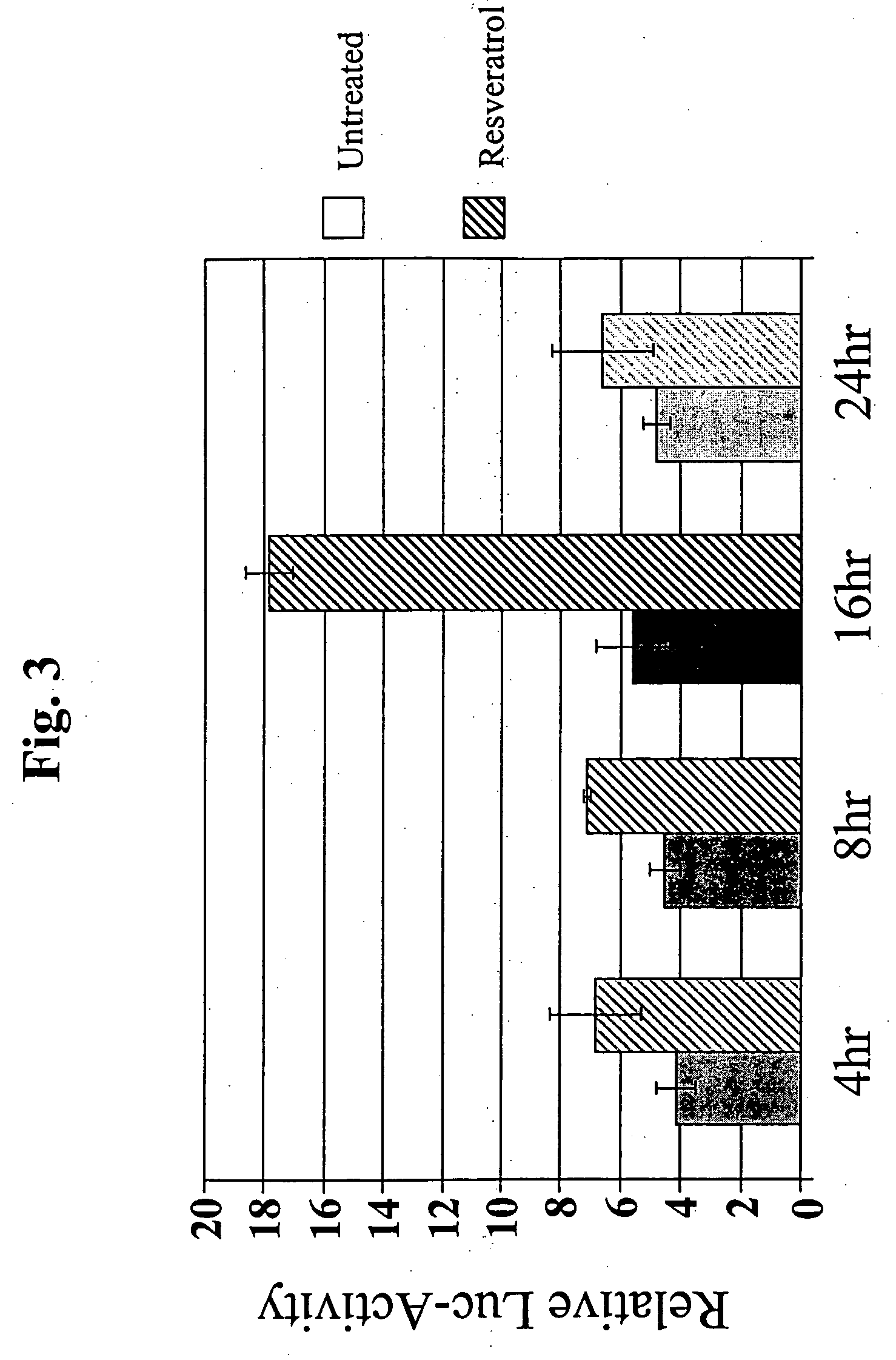
Use of resveratrol to regulate expression of apolipoprotein A1
InactiveUS20060147904A1Increase circulating APO A1/HDL levelIncreasing circulating HDL levelCompound screeningApoptosis detectionCell biologyResveratrol
Described are new methods for promoting the expression of apolipoprotein A1 (APO A1) for increasing levels of HDL, and assays for screening and identifying compounds for regulating expression of the APO A1 protein.
Owner:RESVERLOGIX
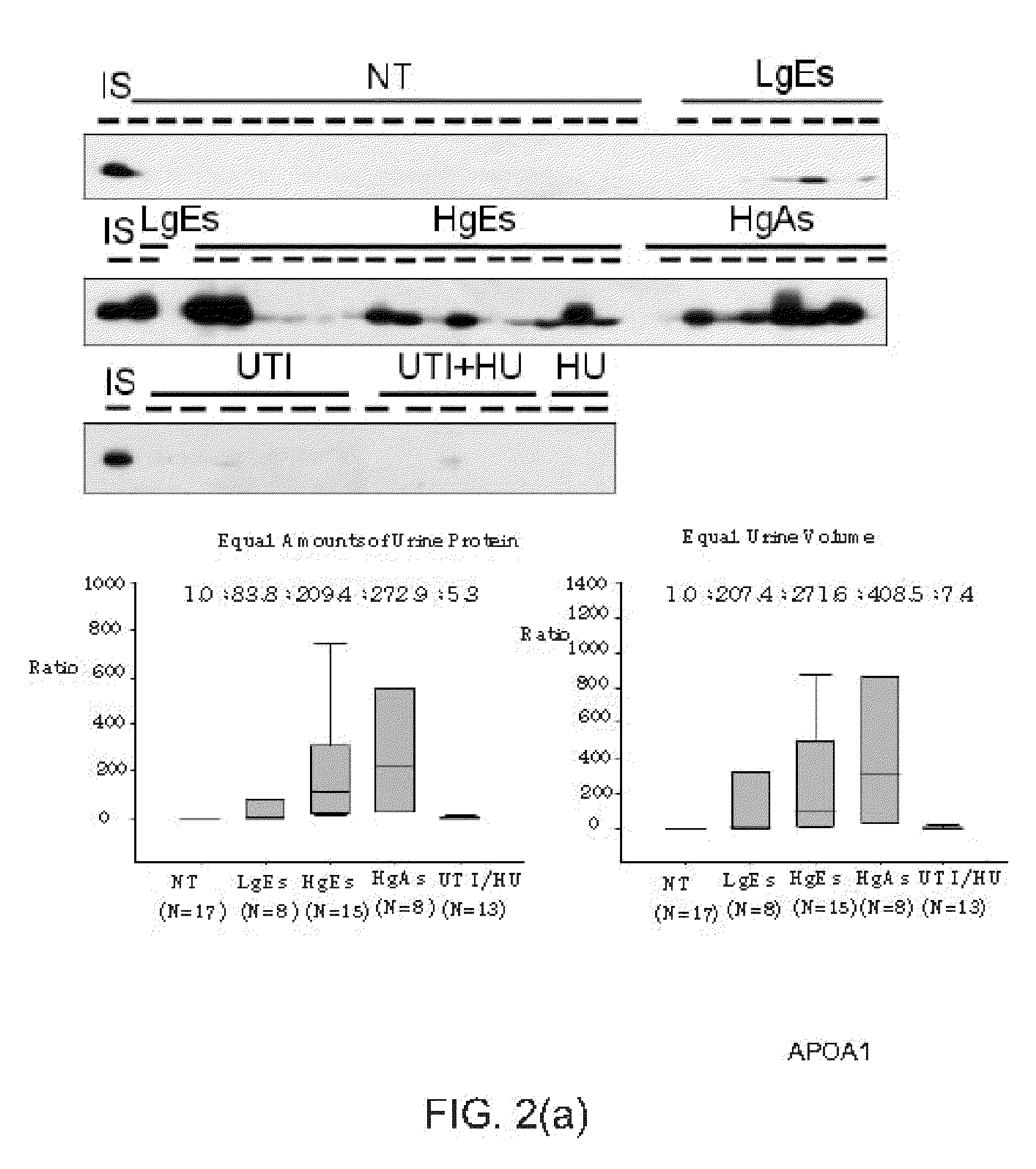
Bladder cancer biomarker and test method using the same
InactiveUS20110195478A1Diagnosing bladder cancerDetermining the aggressiveness of bladder cancerHydrolasesTransferasesHeparin cofactorTherapeutic effect
The present invention discloses a bladder cancer biomarker and a test method using the same. The biomarker contains at least one of the mentioned 69 compounds, such as apolipoprotein A1 (APOA1), apolipoprotein A2 (APOA2), peroxiredoxin 2 (PRDX2), heparin cofactor 2 precursor (HCII), and serum amyloid A-4 protein (SAA4), which exist in the urine specimen of a testee. The expression intensity of the biomarker can facilitate diagnosis of bladder cancer and evaluation of aggressiveness and malignancy of bladder cancer. Thereby, the physician can arrange an optimized treatment to achieve the best therapeutic effect.
Owner:CHANG GUNG UNIVERSITY
Method for producing anti-apolipoprotein A1 (ApoA1) multi-antibody serum by using Zhejiang sheep
InactiveCN103467600AImprove efficiencyConvenient medical diagnosisSerum immunoglobulinsImmunoglobulins against animals/humansSerum igeAnimal science
The invention discloses a method for producing anti-apolipoprotein A1 (ApoA1) multi-antibody serum by using a Zhejiang sheep. The method comprises the following steps: preparing immunogenic original emulsion, selecting the Zhejiang sheep to perform immunization for seven times, and the like, wherein the groin and the neck of the Zhejiang sheep are selected to inject during the immunization; finally, collecting the blood and separating the serum. The titer of the multi-antibody serum which is finally produced by the production method is 1:16; the multi-antibody serum has high benefit.
Owner:ZHENJIANG WANSHAN HONGBIAN AGRI PARK
Single-domain antibody aiming at apolipoprotein A1 and application thereof
InactiveCN103833851AEfficient expressionImmunoglobulins against animals/humansBiological testingEscherichia coliSingle-domain antibody
The invention discloses a VHH chain of a single-domain antibody for apolipoprotein A1. The VHH chain comprises a frame region FR and a complementary determining region CDR. The invention discloses amino acid sequences of the frame region FR selected from the following group of FR and the amino acid sequences of the complementary determining region CDR. The invention also discloses two types of single-domain antibodies for the apolipoprotein A1, also discloses two types of DNA molecules for coding the VHH chain of the single-domain antibody for the apolipoprotein A1 mentioned in the invention or the single-domain antibody for the apolipoprotein A1 mentioned in the invention, also discloses a host cell for expressing the single-domain antibody for the apolipoprotein A1, and also discloses the application of the single-domain antibody for the apolipoprotein A in detection of the apolipoprotein A1. Through the gene sequences and the host cell of the single-domain antibody disclosed by the invention, the single-domain antibody can be efficiently expressed in colon bacillus and applied in research and development of an apolipoprotein A1 detection reagent.
Owner:SOUTHEAST UNIV
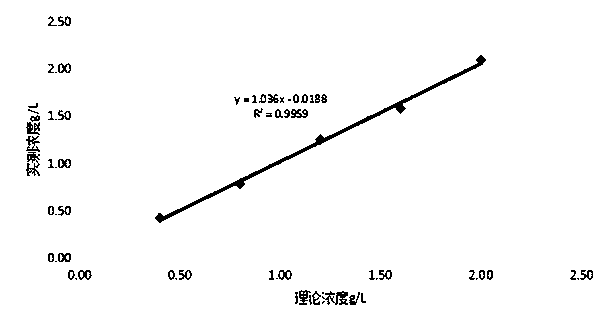
Preparation method for calibration matter for calibrating apolipoprotein A1 and apolipoprotein B
ActiveCN104020301ASimple methodWill not harmBiological testingVery low-density lipoproteinFreeze-drying
The invention discloses a preparation method for a calibration matter for calibrating apolipoprotein A1 and apolipoprotein B. The essentials of the technical scheme are as follows: the preparation method comprises the following steps: (1) adding a multi-anionic compound and divalent metal ions into blood serum to carry out pre-treatment; (2) extracting low density lipoprotein (LDL); (3) extracting high density lipoprotein (HDL); (4) mixing protein and base blood serum to prepare the calibration matter; (5) defining the value of the calibration matter. The preparation method has the advantages that the extraction of LDL and HDL components in the blood serum by a multi-anionic compound and divalent metal ion sub-step precipitation method is simplified; the content of the ApoB and the content of the ApoA1 are detected; the calibration matter can be mixed into the blood serum of healthy people according to the needed amount so that the ApoA1 and the ApoB reach the predicated content; the method is simple; a stabilizer and a turbidity-preventing agent are added into the mixed blood serum so that the prepared freeze-dried calibration matter is clarified after being re-dissolved; the stability is good and a base material effect does not exist; the calibration matter is diluted into five different concentrations and can be used for non-linear calibration of determining the ApoA1 and the ApoB on an automatic analyzer.
Owner:玉环市南方试剂有限公司
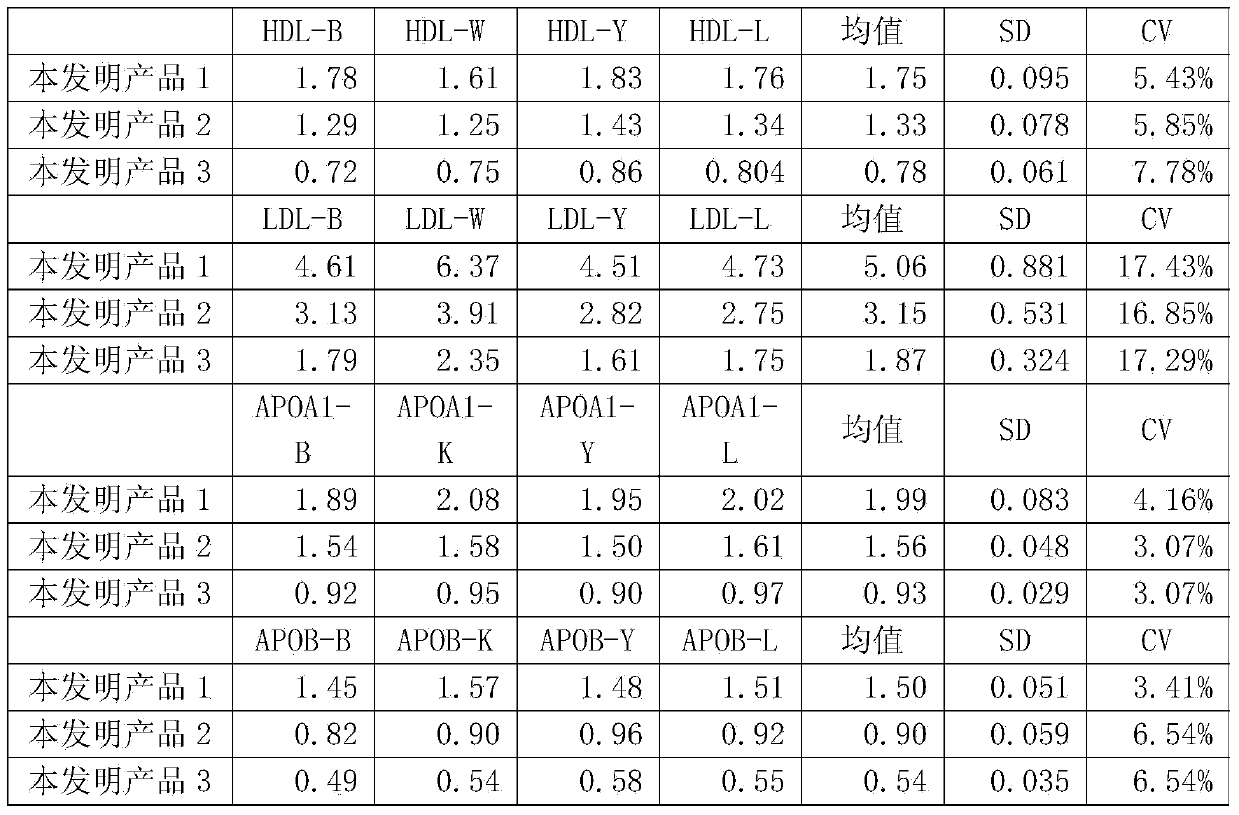
Quality Control Substance for Blood Lipid Test
This application discloses a quality control substance for a blood lipid test. Specifically, a disclosed composition comprises serum matrix, protective agent, preservative, total cholesterol, triglyceride, high density lipoprotein cholesterol, low density lipoprotein cholesterol, apolipoprotein A1, apolipoprotein B, lipoprotein (a), optional apolipoprotein E, optional phospholipid, optional homocysteine, optional C-reactive protein, and optional small and low density lipoprotein cholesterol. The application also relates to the use of this combination in the preparation of quality control substances. The quality control substance according to this application has excellent uniformity in and between bottles, while maintaining good stability.
Owner:BEIJING STRONG BIOTECH INC
Method of diagnosing non-alcoholic steatohepatitis (nash) using molecular markers
InactiveUS7314720B2Ease of evaluationBioreactor/fermenter combinationsBiological substance pretreatmentsAntioxidant proteinMitochondrial ATPase
The invention relates to a method of diagnosing non-alcoholic steatohepatitis (NASH) using molecular markers. The inventive method consists in detecting and quantifying, in vitro in a hepatic tissue sample, the levels of a protein which can be used as a NASH molecular marker and which is selected from apolipoprotein A1, sub-unit β of the mitochondrial ATPase, leukotriene A4 hydrolase, keratin 18, guanidine acetate N-methyltransferase, superoxide dismutase, albumin, antioxidant protein 2 (isoform 1), prohibitin 1, methionine adenosyl transferase, long-chain acyl CoA dehydrogenase, selenium binding protein, antioxidant protein 2 (isoform 2), and combinations of same. The invention further consists in comparing the results obtained with the normal values of said proteins in healthy hepatic tissue. Said method can be used to diagnose NASH and / or to assess a patient's potential risk of developing NASH.
Owner:ONE WAY LIVER GENOMICS
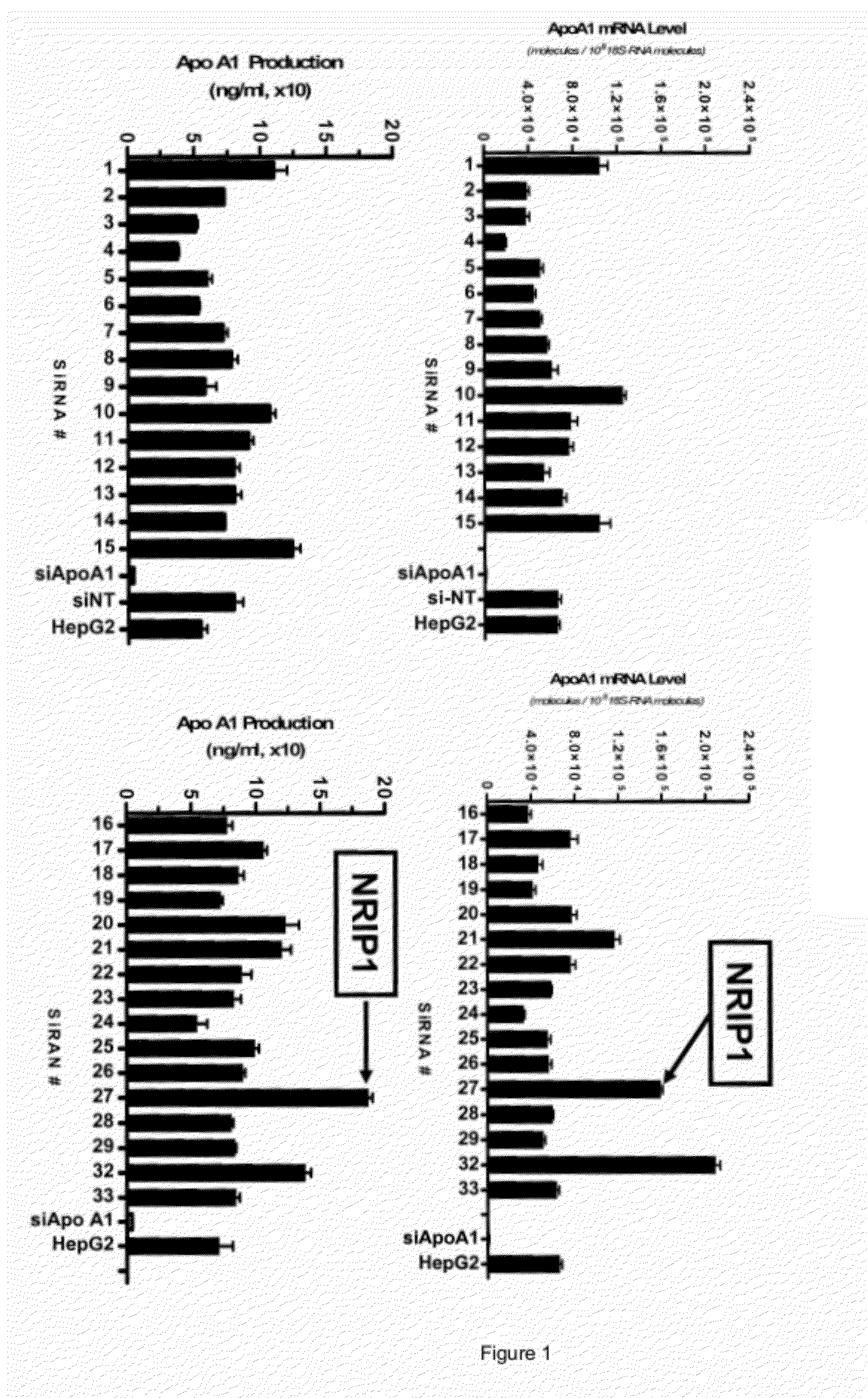
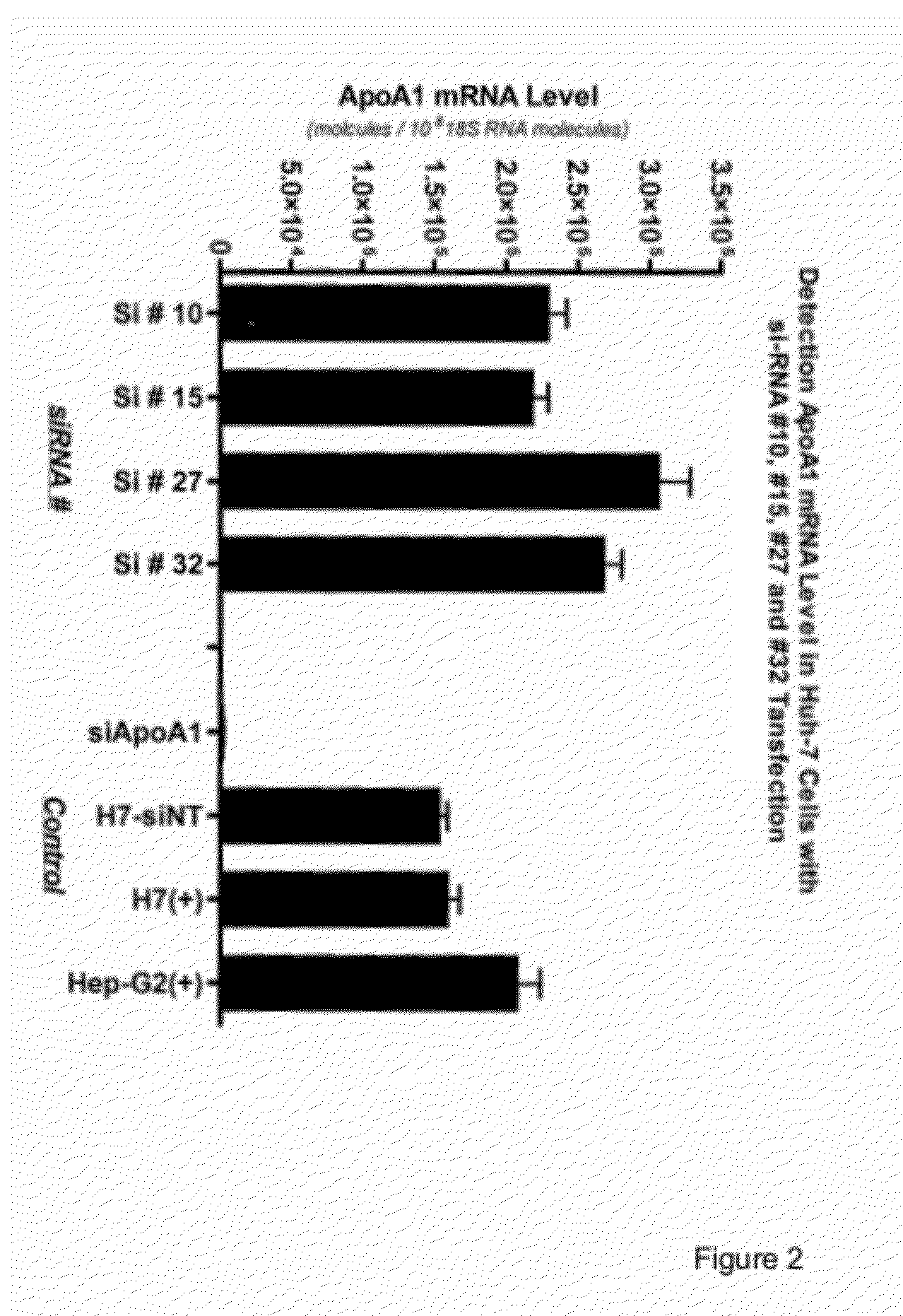
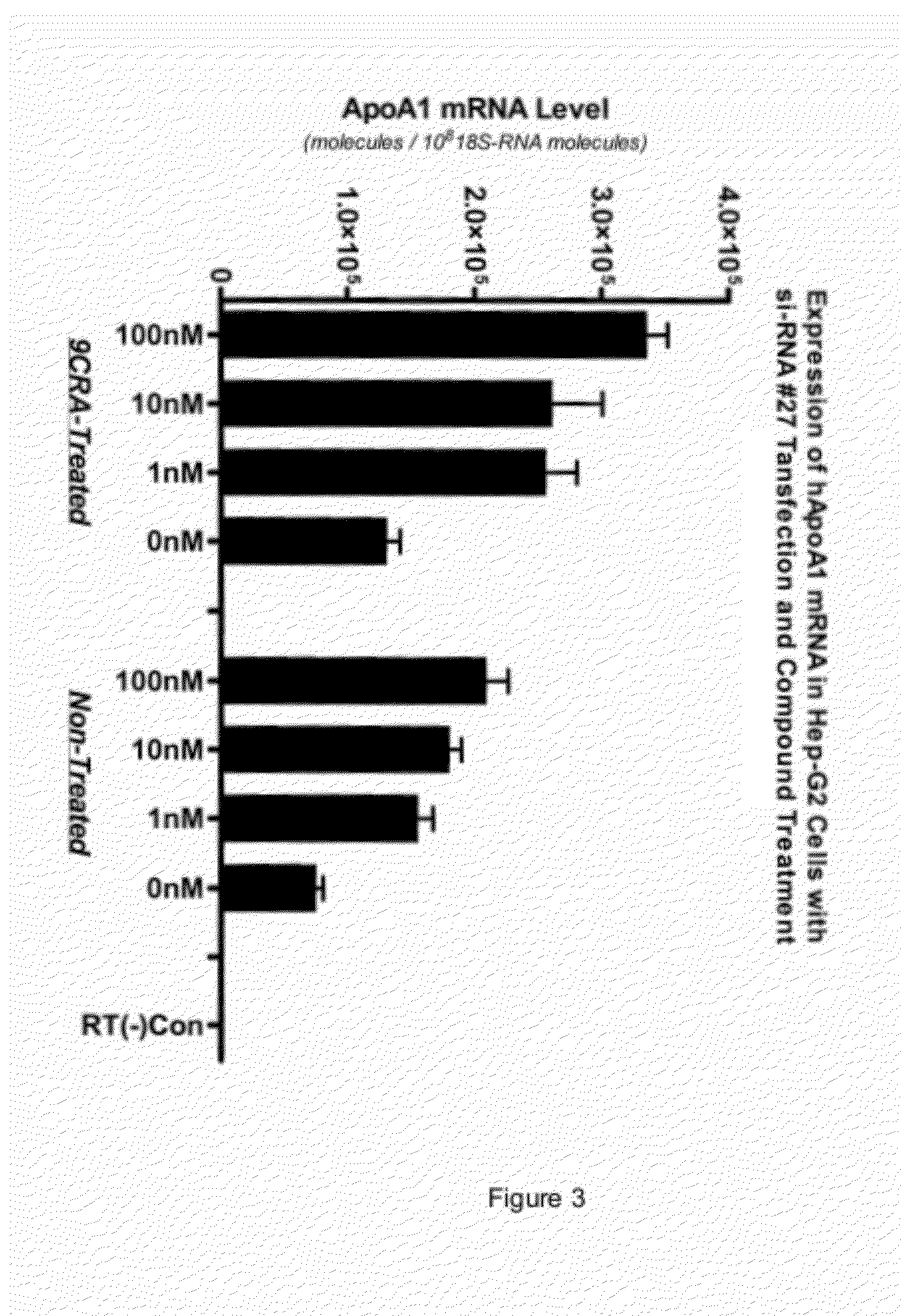
NRIP1 regulation of apolipoprotein A1
InactiveUS8257921B1Reduced activityReduce expressionMicrobiological testing/measurementBiological material analysisApolipoproteins ECancer research
Owner:MERCK SHARP & DOHME CORP
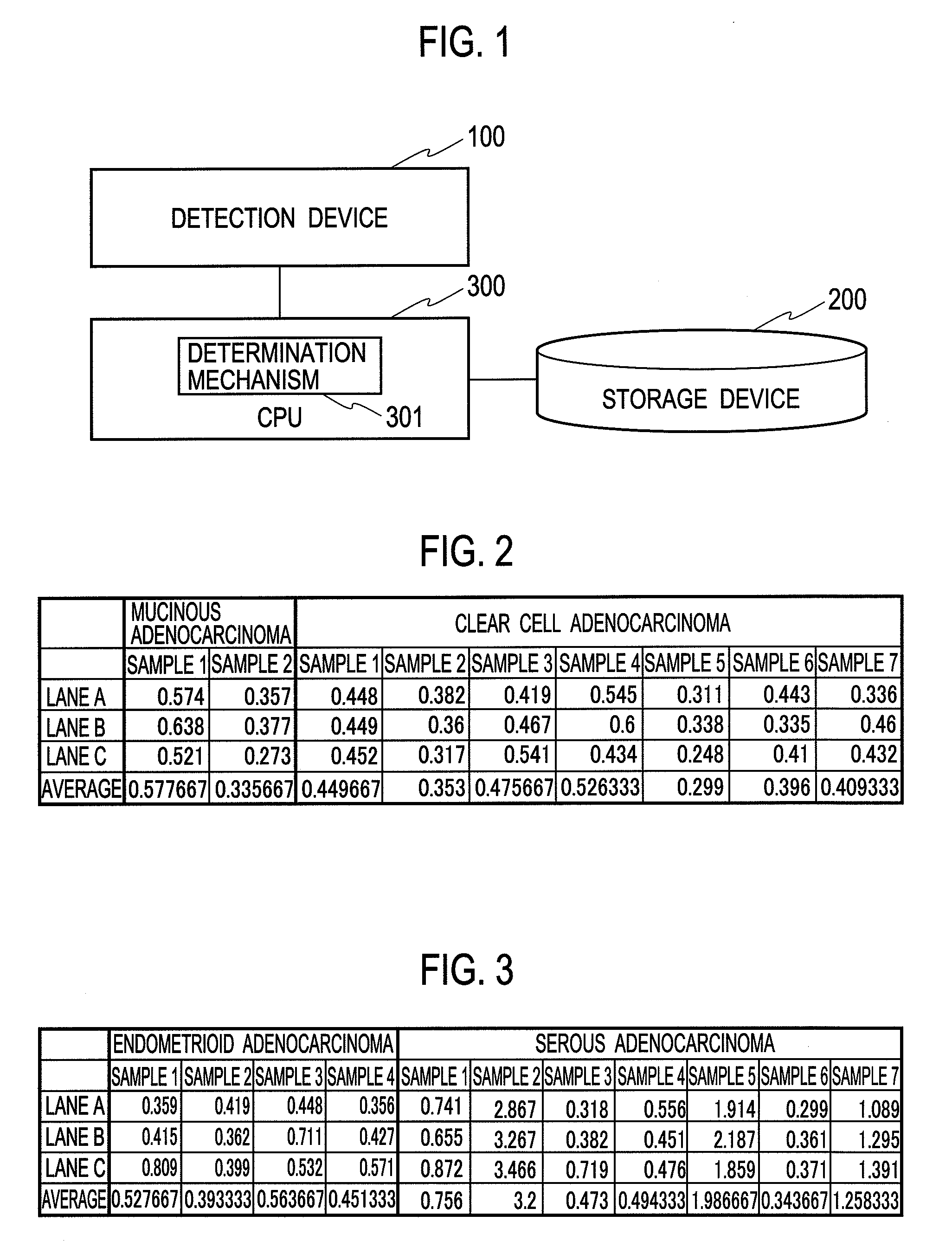
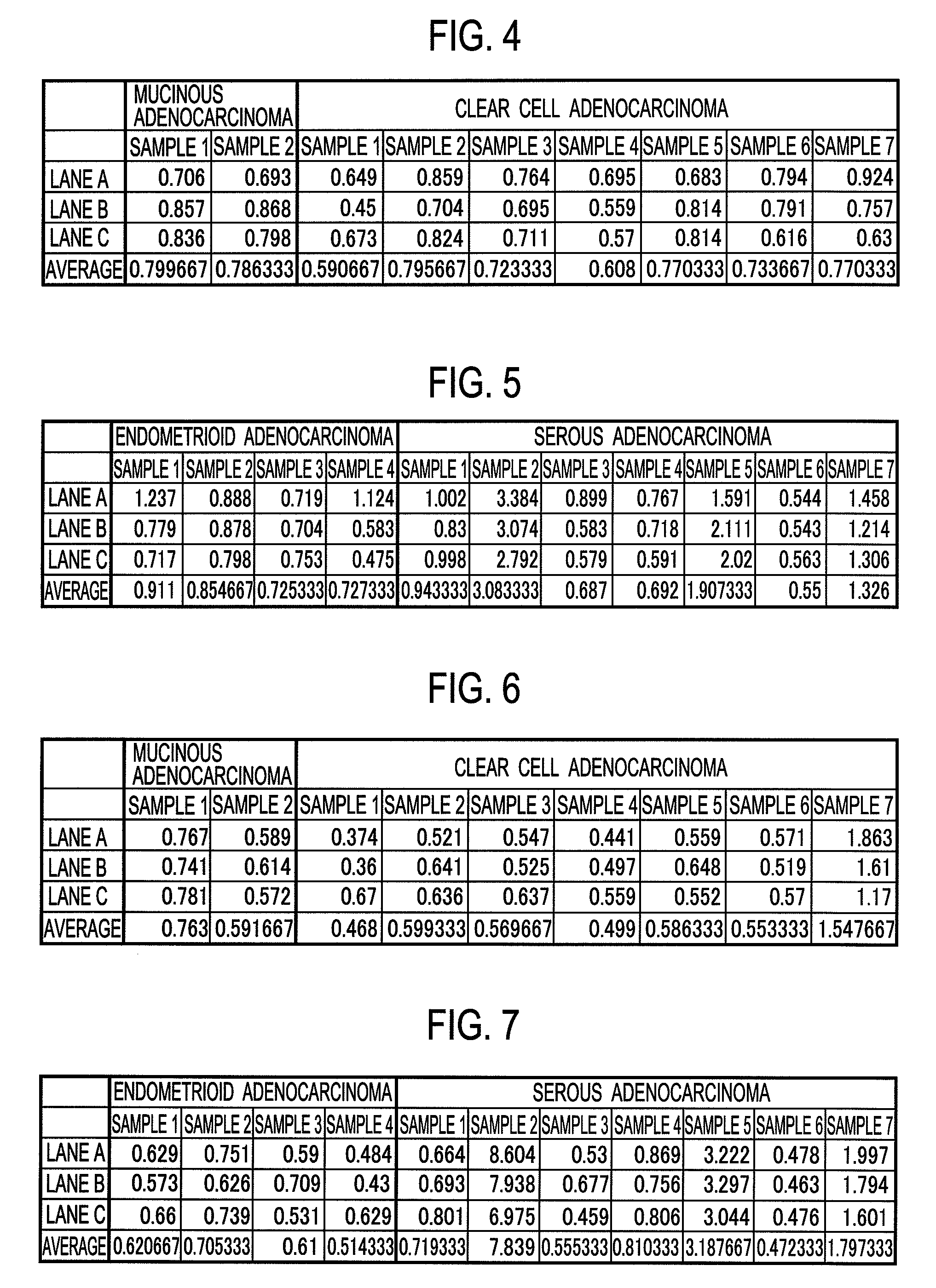
Molecular diagnosis of ovarian cancers
InactiveUS20090246769A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMedicineOvarian tissue
A molecular diagnosis system of ovarian cancers encompasses a detection device configured to obtain a detected value of an expression amount of an apolipoprotein A1 gene in ovarian tissue as a diagnosis subject, a storage device configured to store a normal value of the expression amount of the apolipoprotein A1 gene in normal ovarian tissue, and a determination mechanism configured to determine that the ovarian tissue as the diagnosis subject is clear cell adenocarcinoma when the detected value is lower than the normal value.
Owner:SHIMADZU CORP +1
Entecavir high-density lipoprotein enveloping preparation, and preparation method and use thereof
ActiveCN103655477AImprove securityReduce dosePowder deliveryDigestive systemPhospholipidHigh-density lipoprotein
The invention discloses an entecavir high-density lipoprotein enveloping preparation, and a preparation method and a use thereof. The entecavir high-density lipoprotein enveloping preparation contains entecavir, phospholipid, cholesterol and ApoAl in the weight ratio of 5: 7: 3: (5-20).
Owner:唐为钢
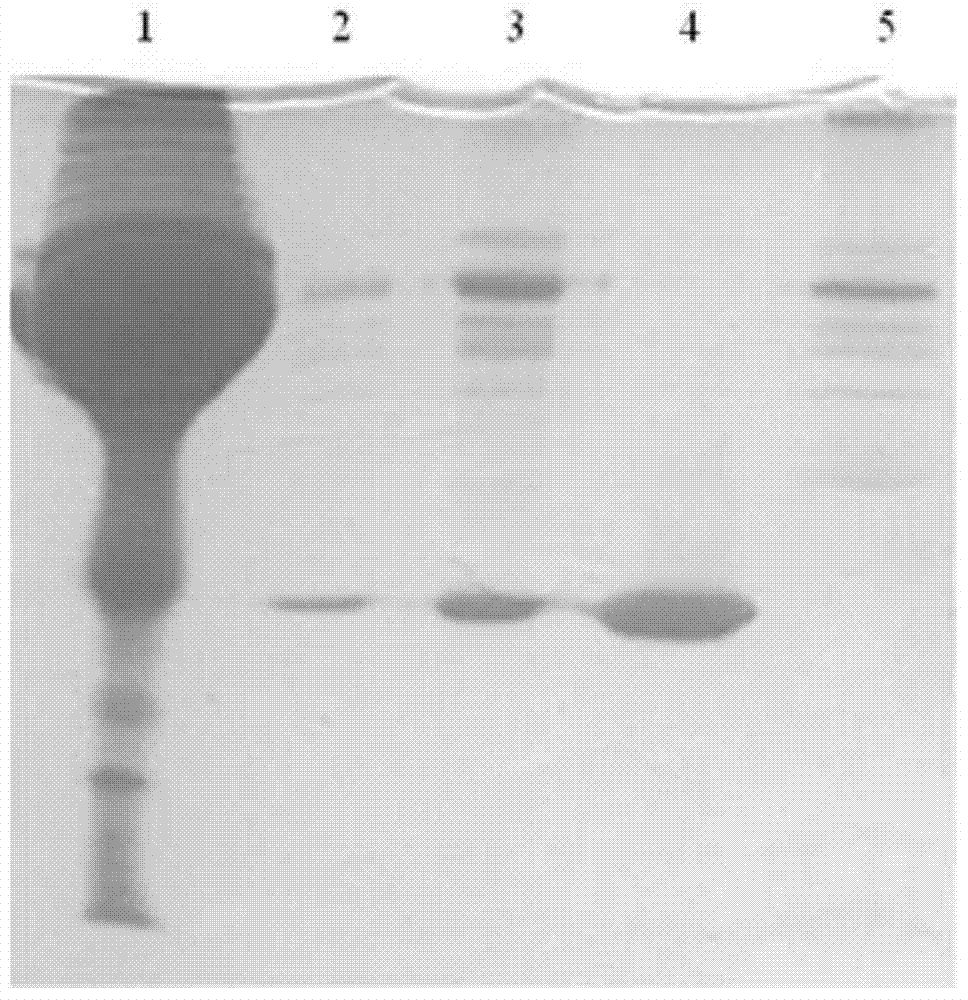
Apolipoprotein A1 purification method and ApoAI protein injection antigen
The invention provides an apolipoprotein A1 purification method. The method is as below: obtaining a plasma sample, and clarifying the sample; conducting a first hydrophobic chromatography on the plasma sample after clarification, so as to obtain a first purification product; degreasing the first purification product; and conducting a second hydrophobic chromatography on the first purification product after degreasing treatment. The first purification elution includes twice elution, the first elution uses 5-10% organic alcohol, and the second elution uses 25-30% alcohol; and the second hydrophobic chromatography uses alcohol eluate with elution strength lower than 25-30%. The method of the invention uses strong hydrophobic mechanism of the lipid in an HDL structure for the first purification, and then the lipoprotein structure with destroyed HDL makes the apolipoprotein A1 separate from the lipid, so that hydrophobicity difference between the apolipoprotein A1 and complex protein with the same high hydrophobicity increases, hydrophobic separation occurs again, and apolipoprotein A1 with high purity can be obtained. The method is simple and novel, and has large sample treatment amount and high efficiency.
Owner:深圳瑞亚力集团有限公司
Apolipoprotein A1 mimetics and uses thereof
The present invention provides peptidomimetics derived from Apolipoprotein A-I, which is useful for beneficially influencing lipid parameters and / or plasma cholesterol levels. The invention also provides pharmaceutical compositions and methods of treatment for elevated levels of plasma cholesterol.
Owner:TRUSTEES OF TUFTS COLLEGE
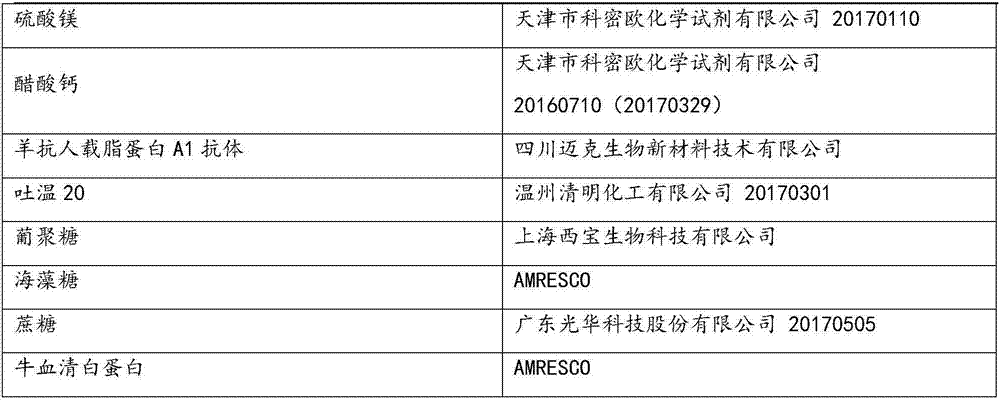
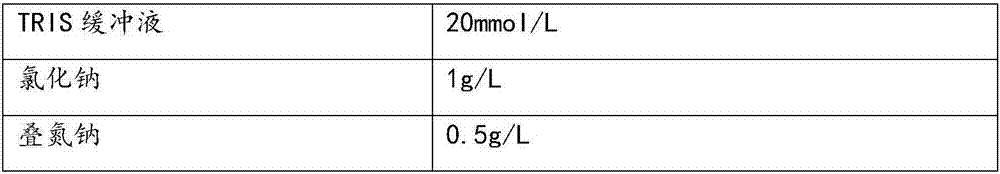
Serum apolipoprotein A1 determination kit, preparation method and application thereof
InactiveCN111537754ANo reconstitution requiredImprove stabilityBiological material analysisBiological testingA lipoproteinActive agent
The invention provides an apolipoprotein A1 (ApoA1) determination kit, which contains a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from the following components: a 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) buffer solution, NaCl, guanidine hydrochloride, sodium decyl sulfate, tetramethylurea, polyethylene glycol 6000, a surfactant and a preservative, and the reagent R2 is prepared from a 4-hydroxyethylpiperazine ethanesulfonic acid (HEPES) buffer solution, goat anti-human ApoA1 antibody coated latex particles, a surfactant, a stabilizer and a preservative. The invention also provides a preparation method and application of the kit, wherein the kit is beneficial to exposure of antigen sites in lipoprotein and promotion of antigen-antibody reaction due to mutualsynergy of multiple components, and is a liquid kit with strong stability, high sensitivity, good repeatability and low cost.
Owner:中拓生物有限公司 +2
Composite calibration product containing six indexes and having high anti-interference ability, and preparation method thereof
InactiveCN110057641ANo interferenceImprove stabilityPreparing sample for investigationMANNITOL/SORBITOLApolipoproteins b
The invention discloses a composite calibration product containing six indexes and having high anti-interference ability, and a preparation method thereof, and relates to the field of biotechnology. The matrix of the composite calibration product is serum, the six index components are apolipoprotein A1, apolipoprotein B, high density lipoprotein cholesterol, low density lipoprotein cholesterin, total cholesterol and triglyceride, and the matrix contains anti-interference substances: mannitol, Genapol X 80, ascorbic acid, Emulgen A90, FAD, ADP, polyoxyethylene 8000 and glutathione. According tothe composite calibration product disclosed by the invention, the anti-interference substances are fully and evenly mixed at first, and then the 6 indexes are added in sequence, so that full play isgiven to the anti-interference substances added to the serum matrix, and the prepared composite calibration product can not only reduce the interference between a plurality of indexes in the product,but also can improve the stability.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
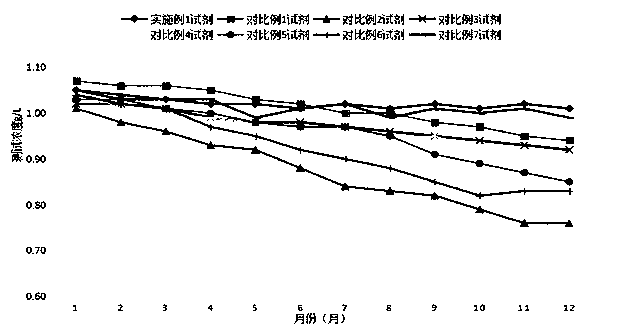
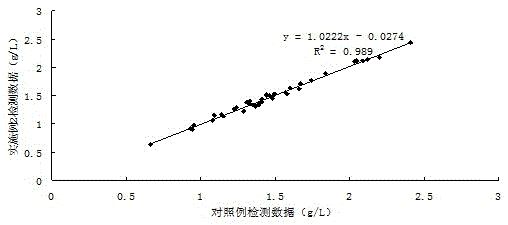
Apolipoprotein A1 detection kit and detection method
ActiveCN107462729AImprove stabilityImprove uniformityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsMagnesium saltLime acetate
The invention provides an apolipoprotein A1 detection kit. The apolipoprotein A1 detection kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises 0.8 to 51g / L of octylphenoxypolyethoxyethanol; the reagent R2 comprises 0.8 to 51g / L of octylphenoxypolyethoxyethanol, 0.01 to 15.5g / L magnesium salt, 0.01 to 20g / L of lime acetate, and an antibody. The kit is good in antibody performance and repeatability, and accurate in reagent detection result, and can meet the use requirement.
Owner:SICHUAN MACCURA BIOTECH CO LTD
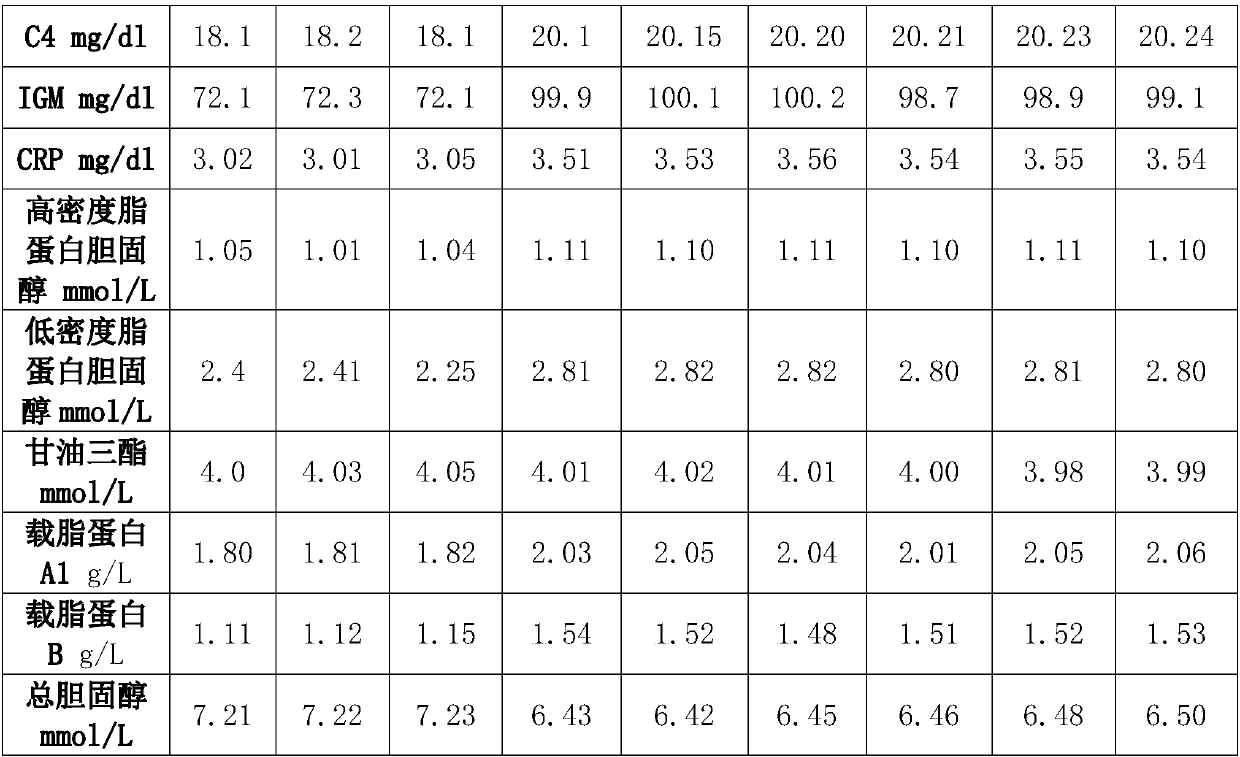
Blood-lipoid and special protein composite quality control product
InactiveCN109613263AGuaranteed softGuarantee later traceabilityBiological testingFreeze-dryingPolyethylene glycol
The invention provides a blood-lipoid and special protein composite quality control product. The blood-lipoid and special protein composite quality control product is prepared from twelve components of C3, C4, apolipoprotein A1, apolipoprotein B, high-density lipoprotein, low-density lipoprotein, C-reactive protein, IGA, IGM, IGG, total cholesterol and glycerinum, and addition is carried out according to the order. A used matrix is calf serum, and inductive anti-interference matter is 2.0-10% of BSA, 5ml / L of polyethylene glycol, 1-20g / L of PEG20000, 2g / L of sodium fluoride and 0.5-1.5% of ATP. Composite quality control freeze dried aquatic products are prepared with the help of a freeze-drying technology.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Apolipoprotein A1 immunoturbidimetry detection kit
InactiveCN105527449AHigh analytical sensitivityDoes not affect accuracyBiological material analysisBiological testingMagnetite NanoparticlesBovine serum albumin
The invention discloses an apolipoprotein A1 immunoturbidimetry detection kit, and belongs to the technical field of clinical in-vitro detection reagent. The kit comprises a reagent R1, a reagent R2, and a calibration material. By adding a certain amount of silica coated magnetic nanoparticles into the reagent R1 and adding a certain amount of bovine serum albumin and Kathon-CG in the reagent R2, the kit stability and analysis sensitivity are effectively improved, the linear range is also relatively good, the reagent accuracy is high, and the kit is conducive to further promotion and use in the market.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Assays and methods for the diagnosis of ovarian cancer
Provided are methods for diagnosing ovarian cancer or assessing the risk of developing ovarian cancer in a subject by measuring, in a biological sample from the subject, the amount of IL-6 and comparing the amount of IL-6 measured to a predetermined IL-6 cutoff value. Also provided are methods that further include measuring, in the biological sample, the amount of two or more biomarkers selected from the group consisting of transthyretin, apolipoprotein A1, transferrin, β-2 microglobulin, and CA 125 II. The amount of IL-6 and biomarkers are useful in the diagnosis of ovarian cancer, and individuals can be identified as having ovarian cancer when the amount of IL-6 is greater than the IL-6 cutoff value and / or the biomarker score is greater than the biomarker score cutoff value.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
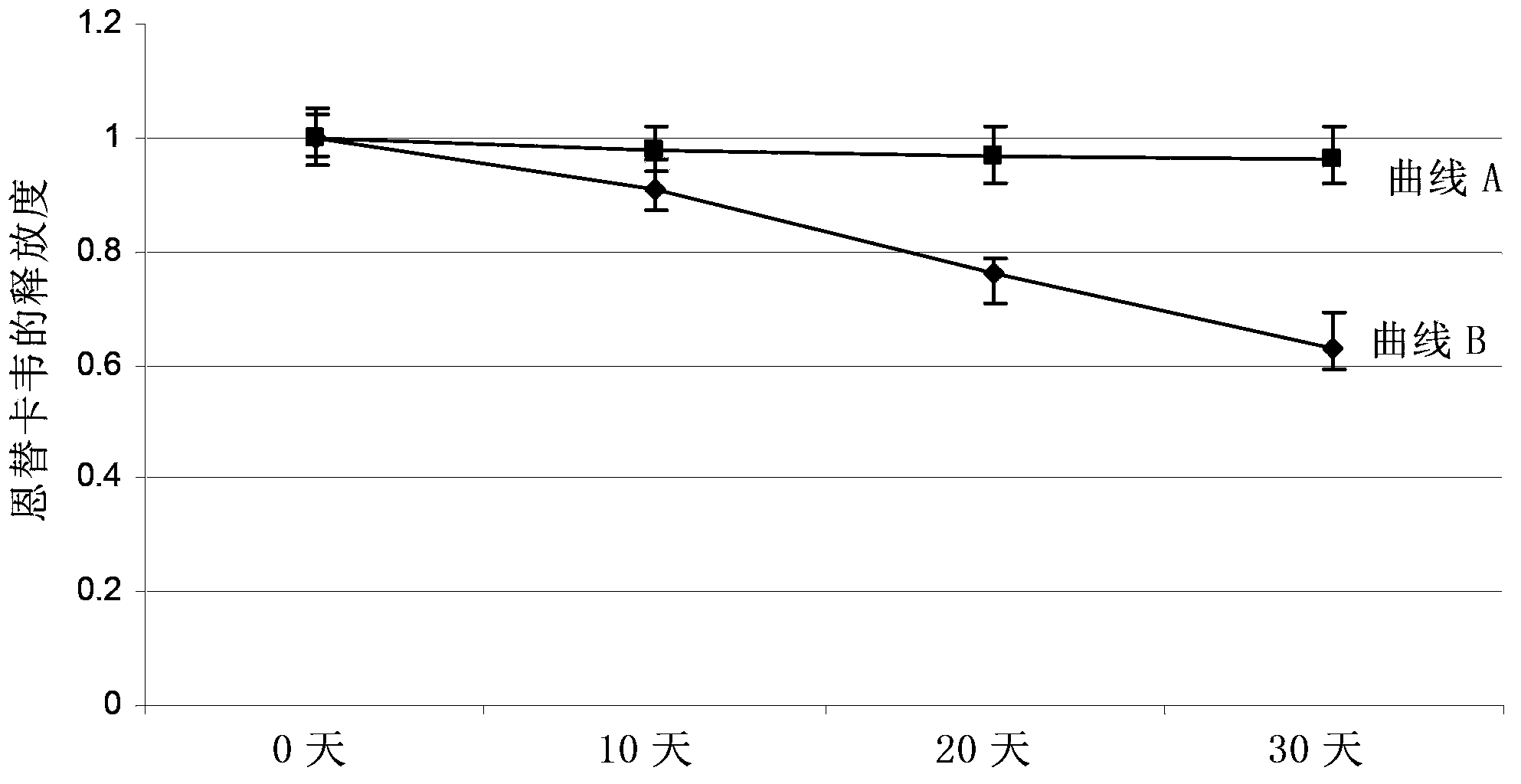
Methods to increase reverse cholesterol transport in the retinal pigment epithelium (RPE) and Bruch's membrane (BM)
InactiveUS7470659B2Increasing reverse cholesterol transportReduce accumulationBiocideSenses disorderReverse cholesterol transportCholesterol
The present invention addresses the treatment of age-related macular degeneration using regulation of pathogenic mechanisms similar to atherosclerosis. In further specific embodiments, compositions that increase reverse cholesterol transport are utilized as therapeutic targets for age-related macular degeneration. In a specific embodiment, the lipid content of the retinal pigment epithelium, and / or Bruch's membrane is reduced by delivering Apolipoprotein A1, particularly a mimetic peptide.
Owner:RGT UNIV OF CALIFORNIA
Protease-sensitive site in apolipoprotein A1, therapeutic and diagnostic implications
InactiveUS8343932B2Ameliorates cholesterol balanceEasy to transportApolipeptidesPeptide/protein ingredientsCholesterolReverse cholesterol transport
The invention relates to the identification of a naturally occurring internal proteolytic cleavage site in the ApoA1 protein, which leads to inactivation of the mature protein. Specific modification of this cleavage site leads to a stabilised ApoA1 protein, which is beneficial for the reverse cholesterol transport. The invention therefore encompasses pharmaceutical compositions comprising a recombinant stabilised variant ApoA1 protein or rHDL particles comprising such a protein, for use in the treatment of patients having reduced HDL or hampered reverse cholesterol transport.
Owner:PRONOTA NV
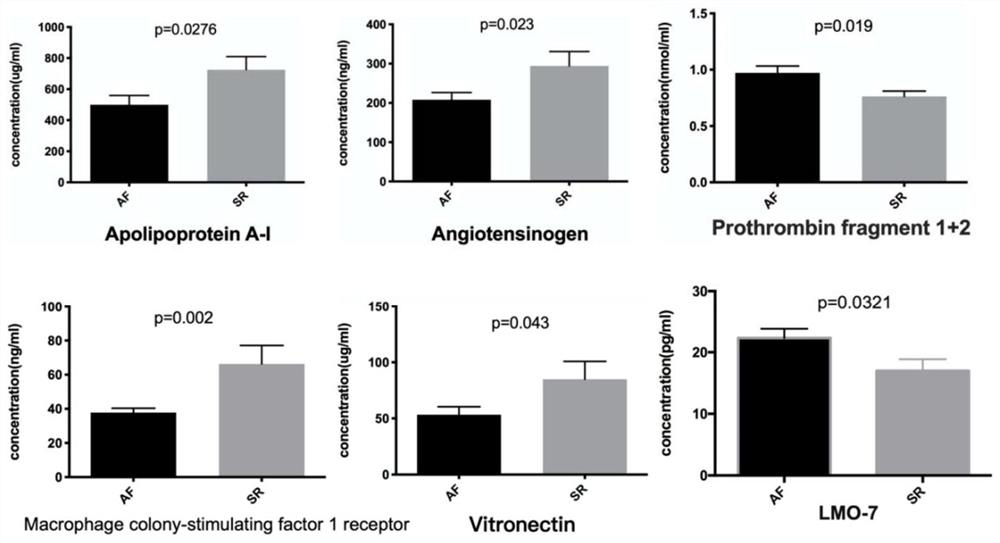
Multi-protein combined biomarker, application and heart valvular disease complicated with atrial fibrillation diagnostic kit
The invention provides a multi-protein combined biomarker, application and a heart valvular disease complicated with atrial fibrillation diagnostic kit. The multi-protein combined biomarker is composed of an apolipoprotein A1 protein antibody, an angiotensin antigen antibody, an LIM structural domain protein 7, a macrophage colony stimulating factor receptor 1, a vitronectin antibody and a prothrombin fragment F1 + 2 antibody. Modern biological technology and bioinformatics analysis find that combined application of the six proteins has the application of predicting valvular heart disease complicated with atrial fibrillation, can be applied to preparation of targeted drugs for early intervention of related diseases, and can also be applied to preparation of a heart valvular disease complicated with atrial fibrillation screening kit. According to the kit, modeling is carried out after the concentrations of the six proteins are measured, and good evaluation efficiency and potential hugeapplication value are achieved on whether atrial fibrillation happens to a patient suffering from the heart valvular disease or not.
Owner:TEDA INT CARDIOVASCULAR HOSPITAL
Methods for diagnosis of acute coronary syndrome
InactiveUS20090215077A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
Owner:RULES BASED MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com