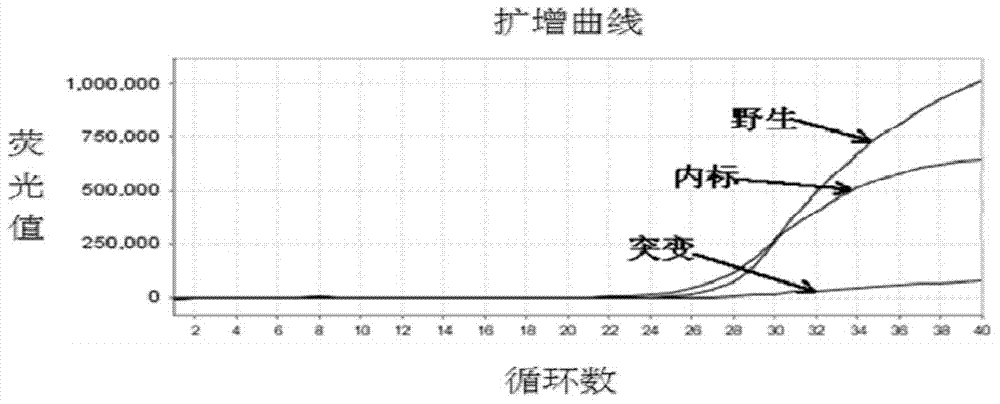
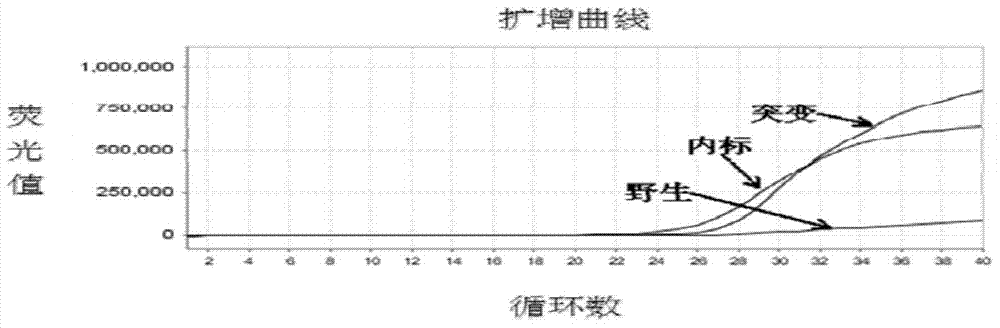
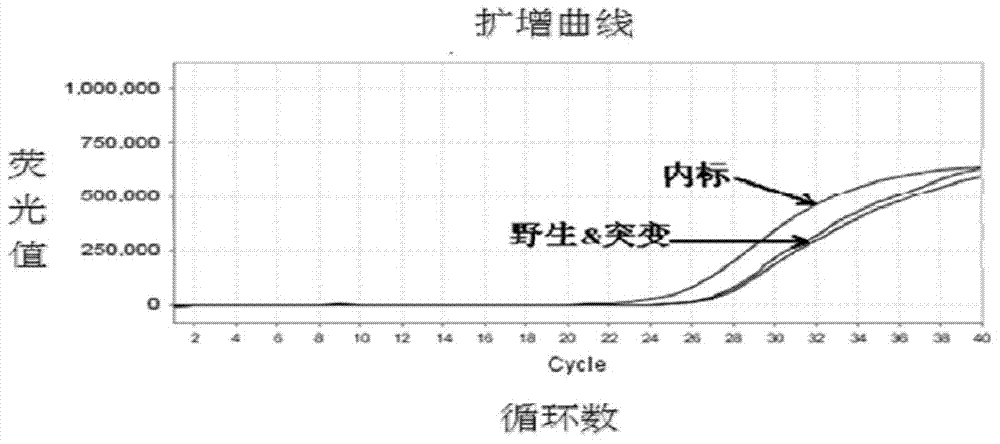
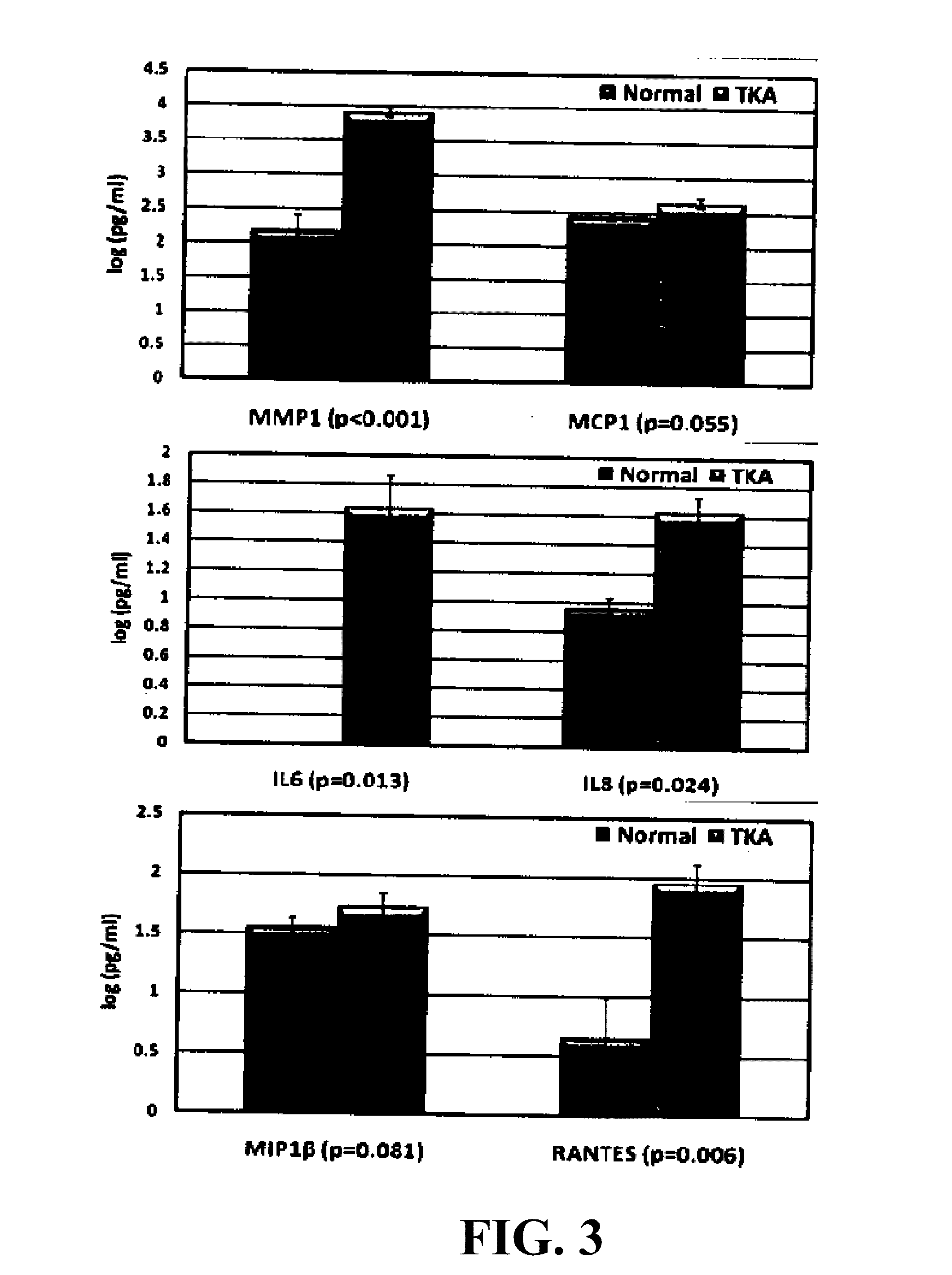
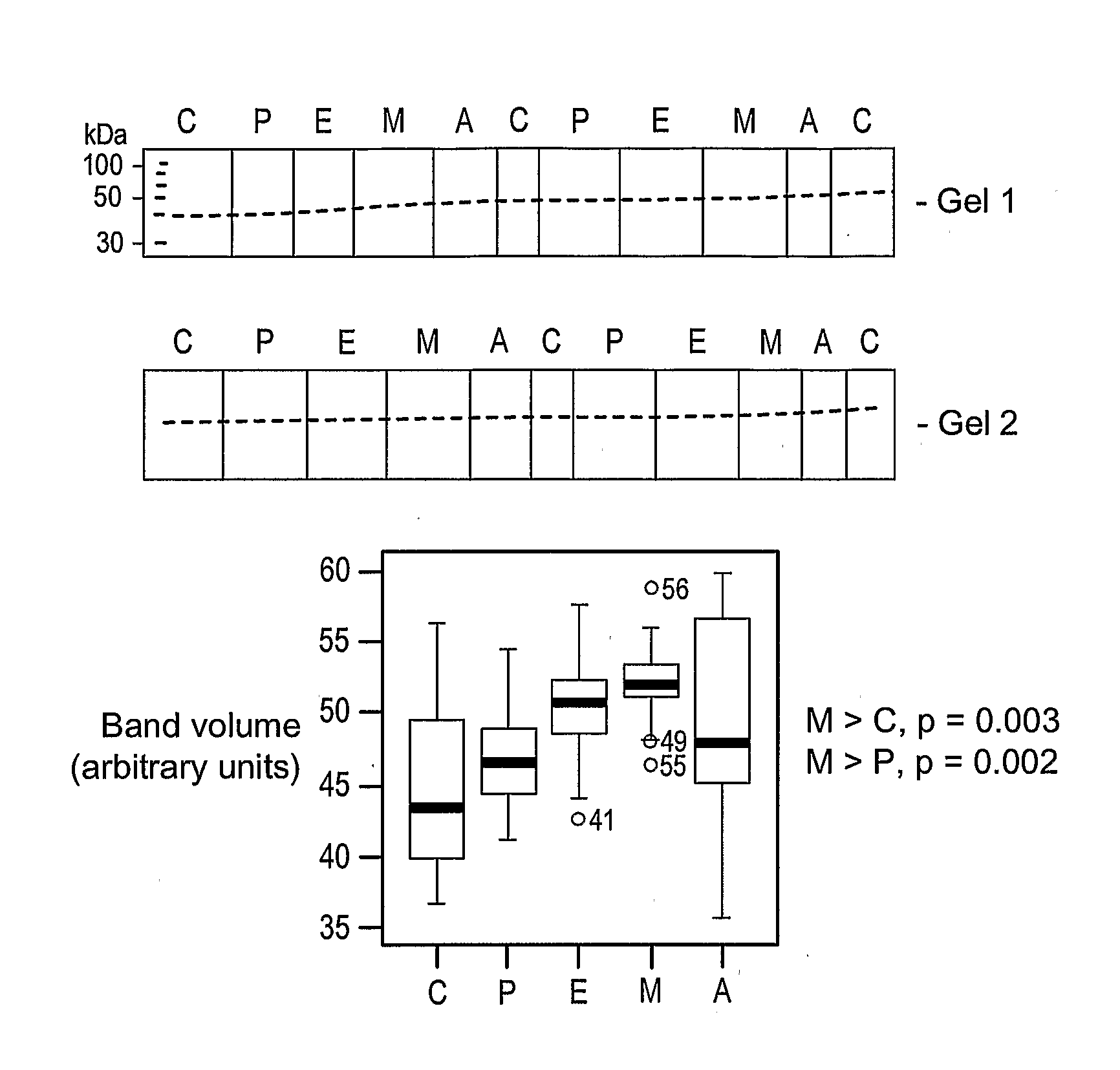
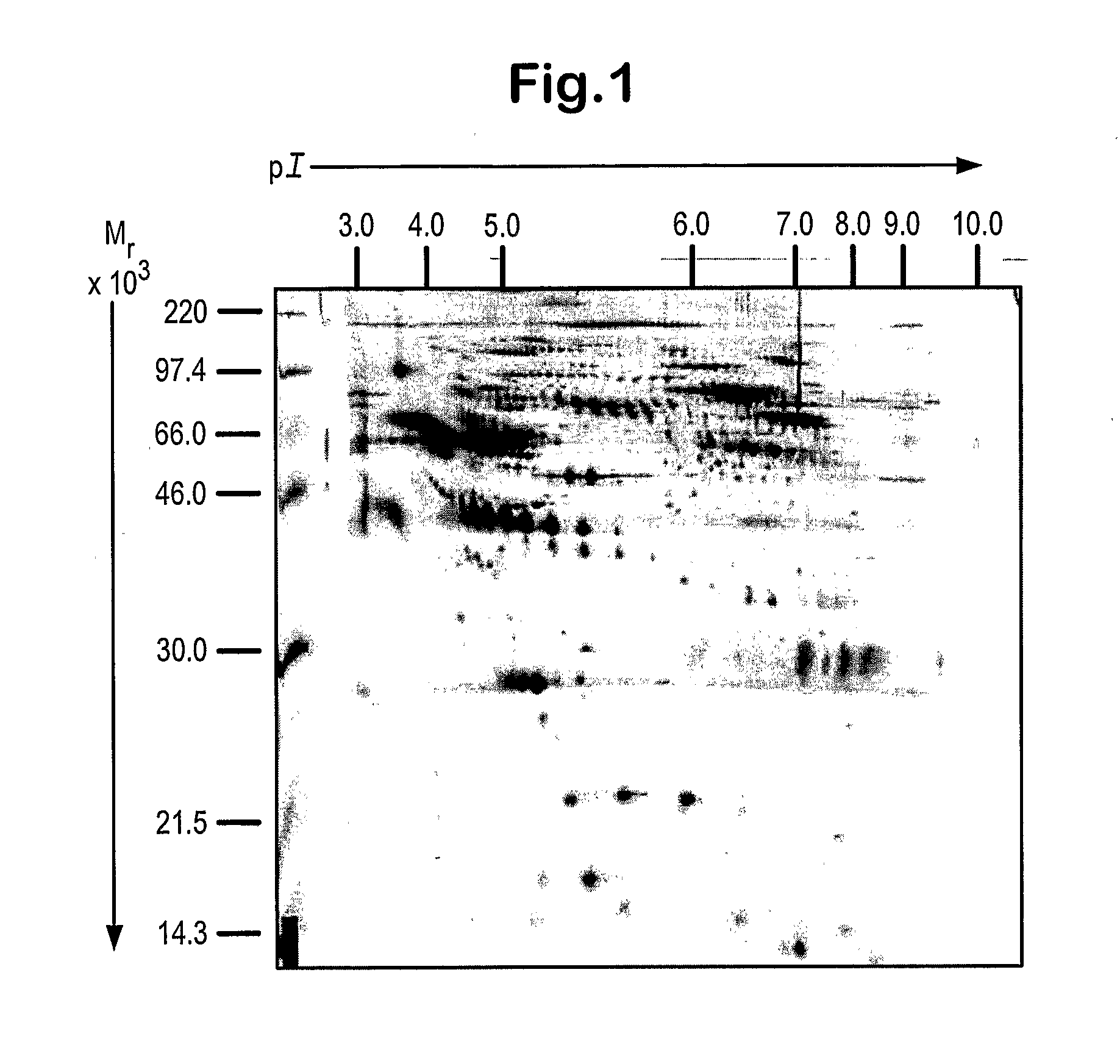
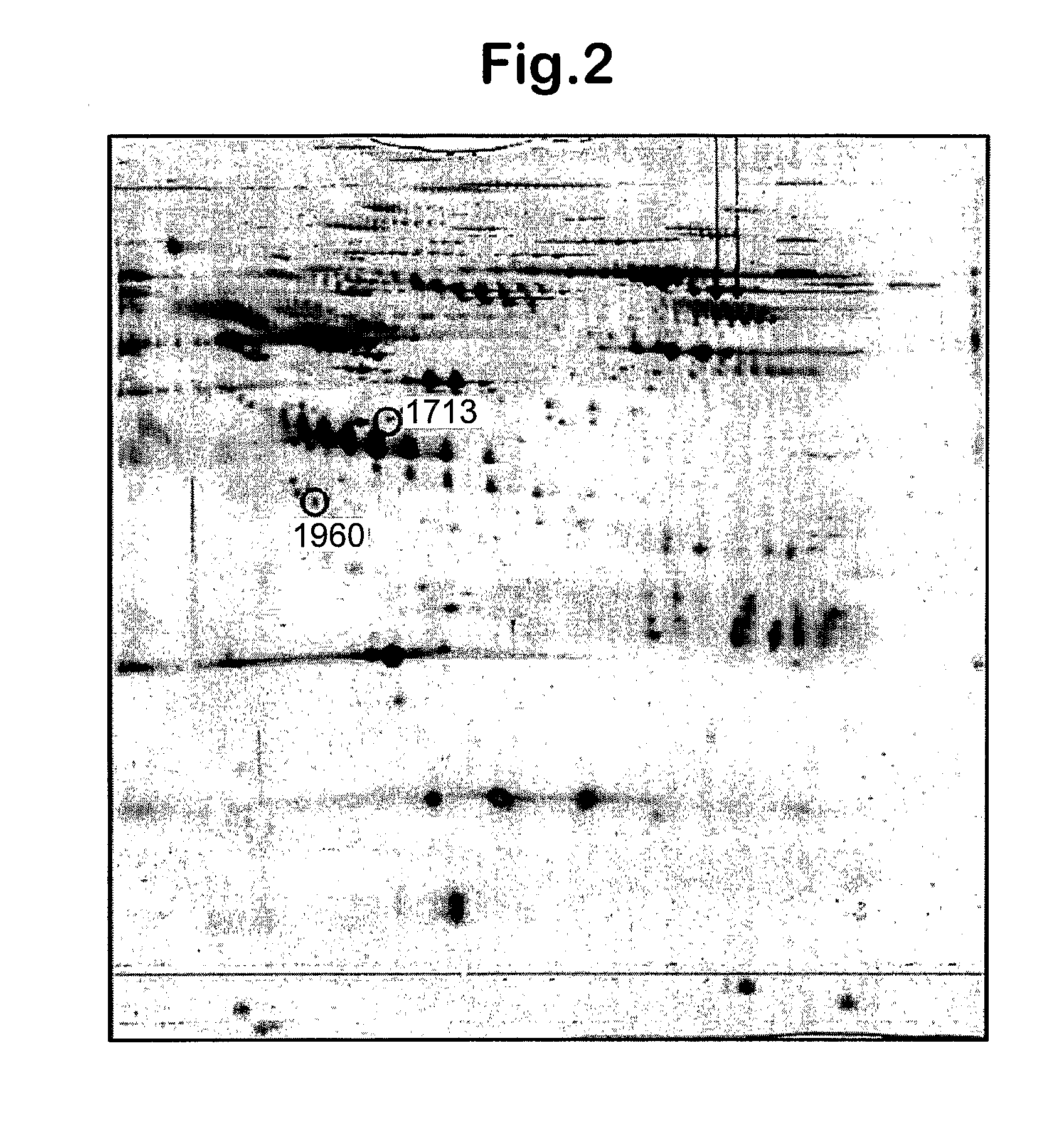
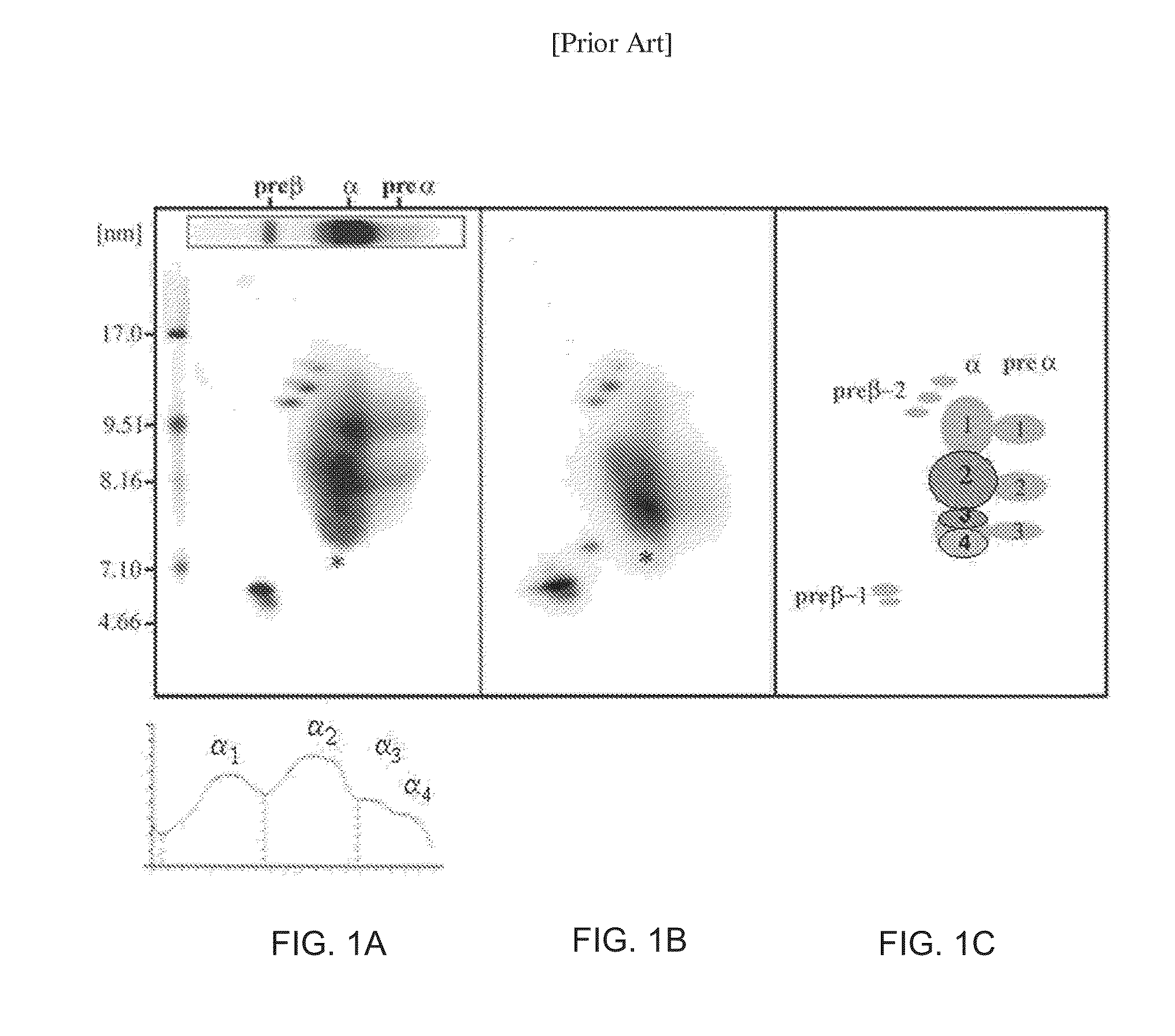
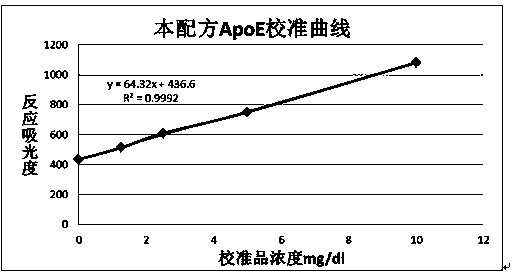
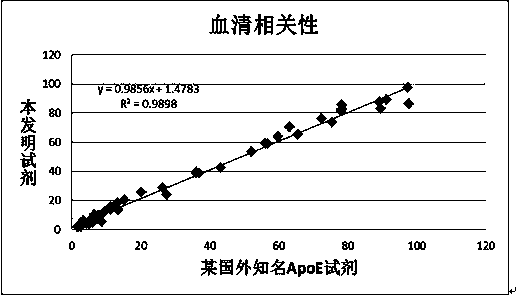
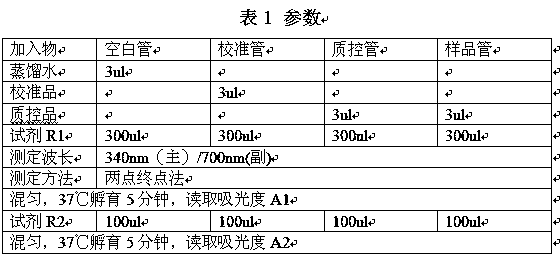
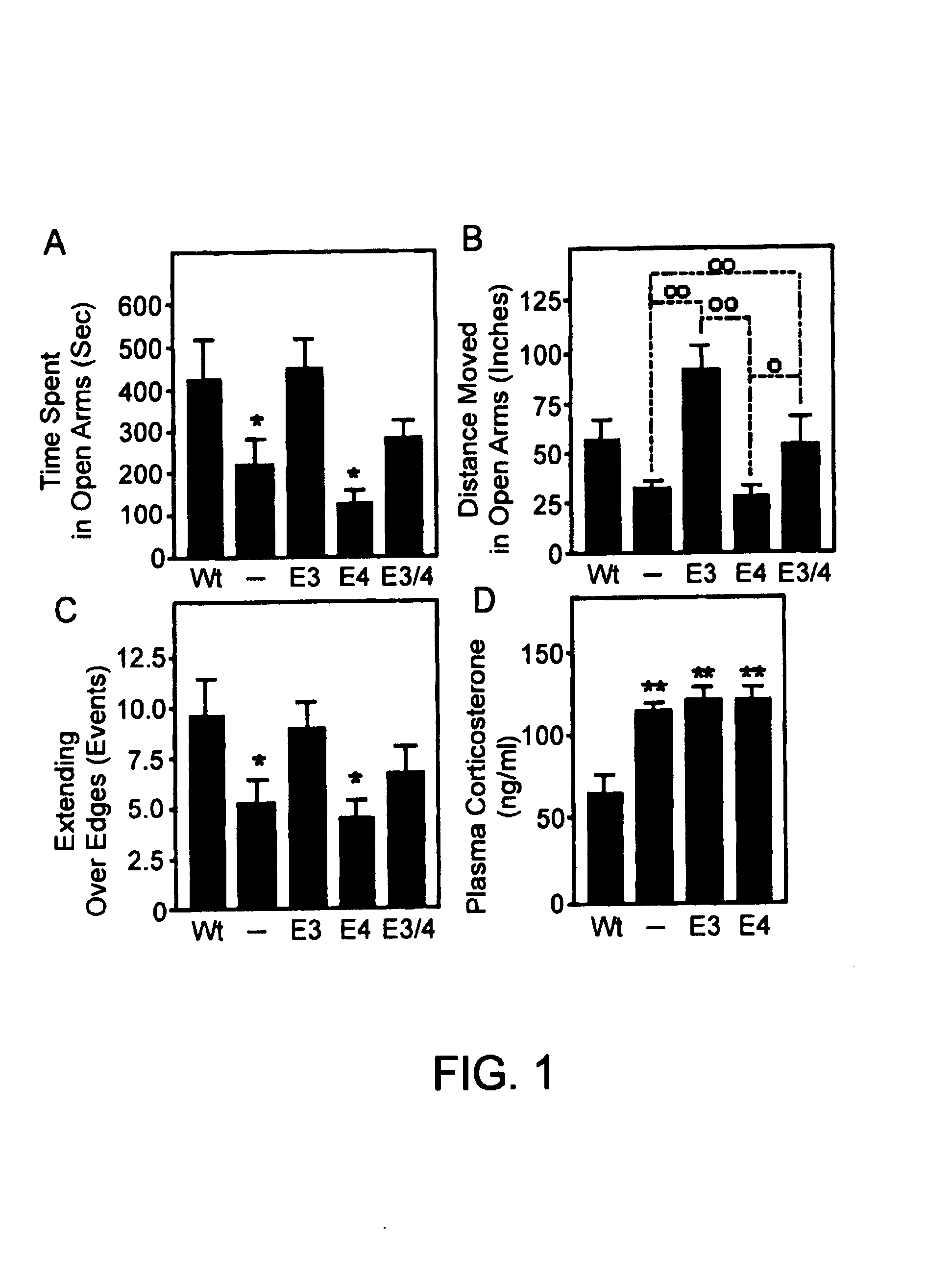
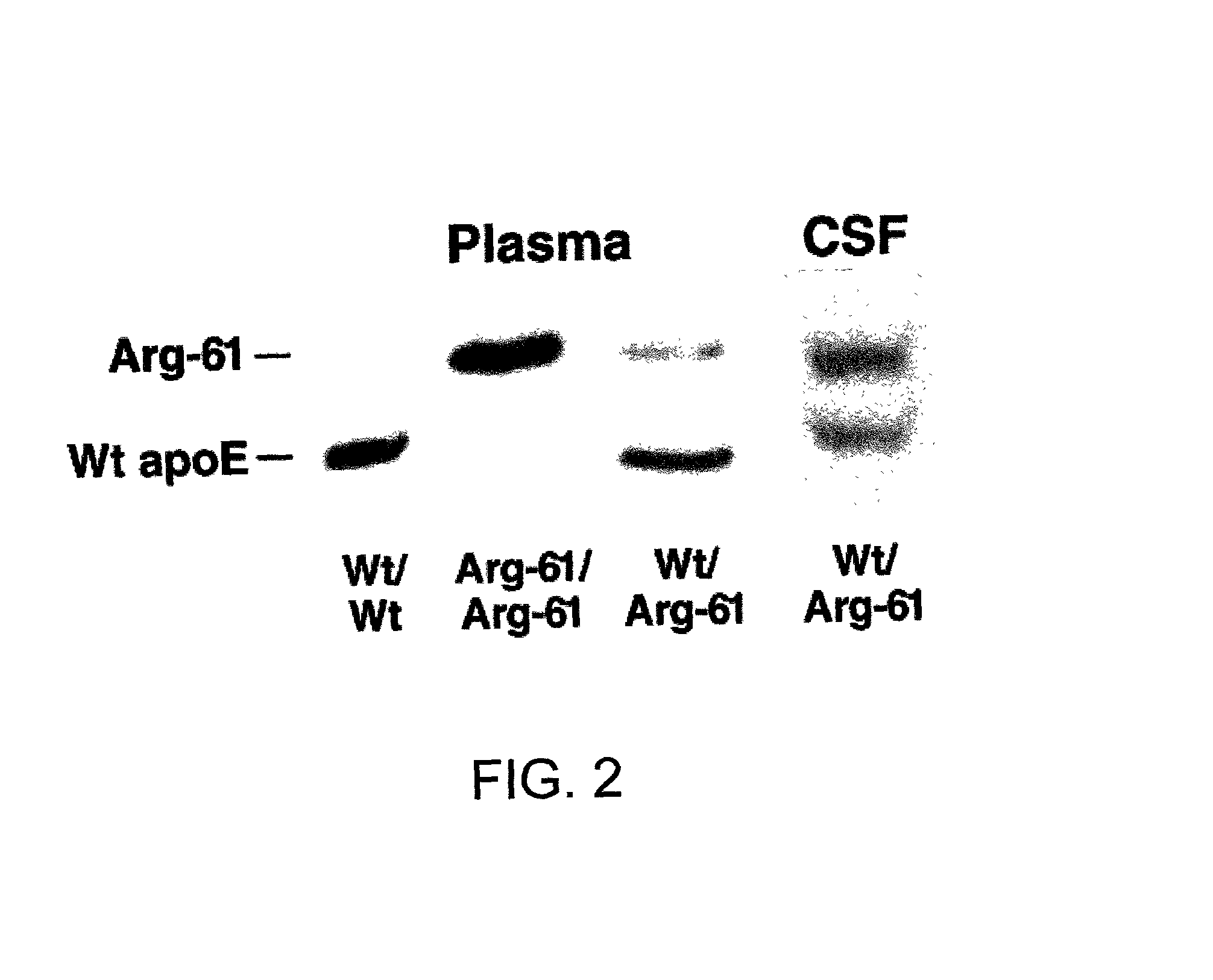
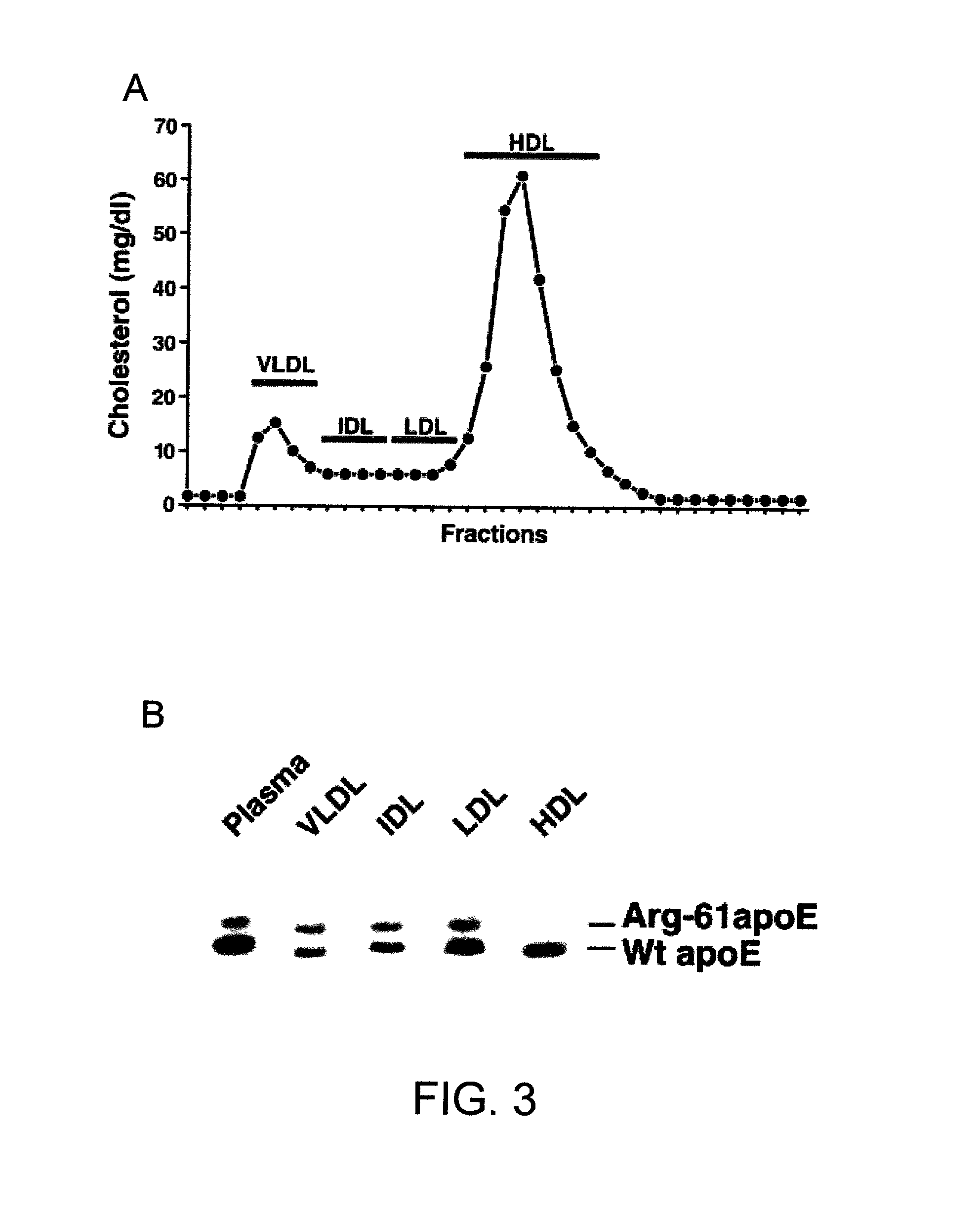
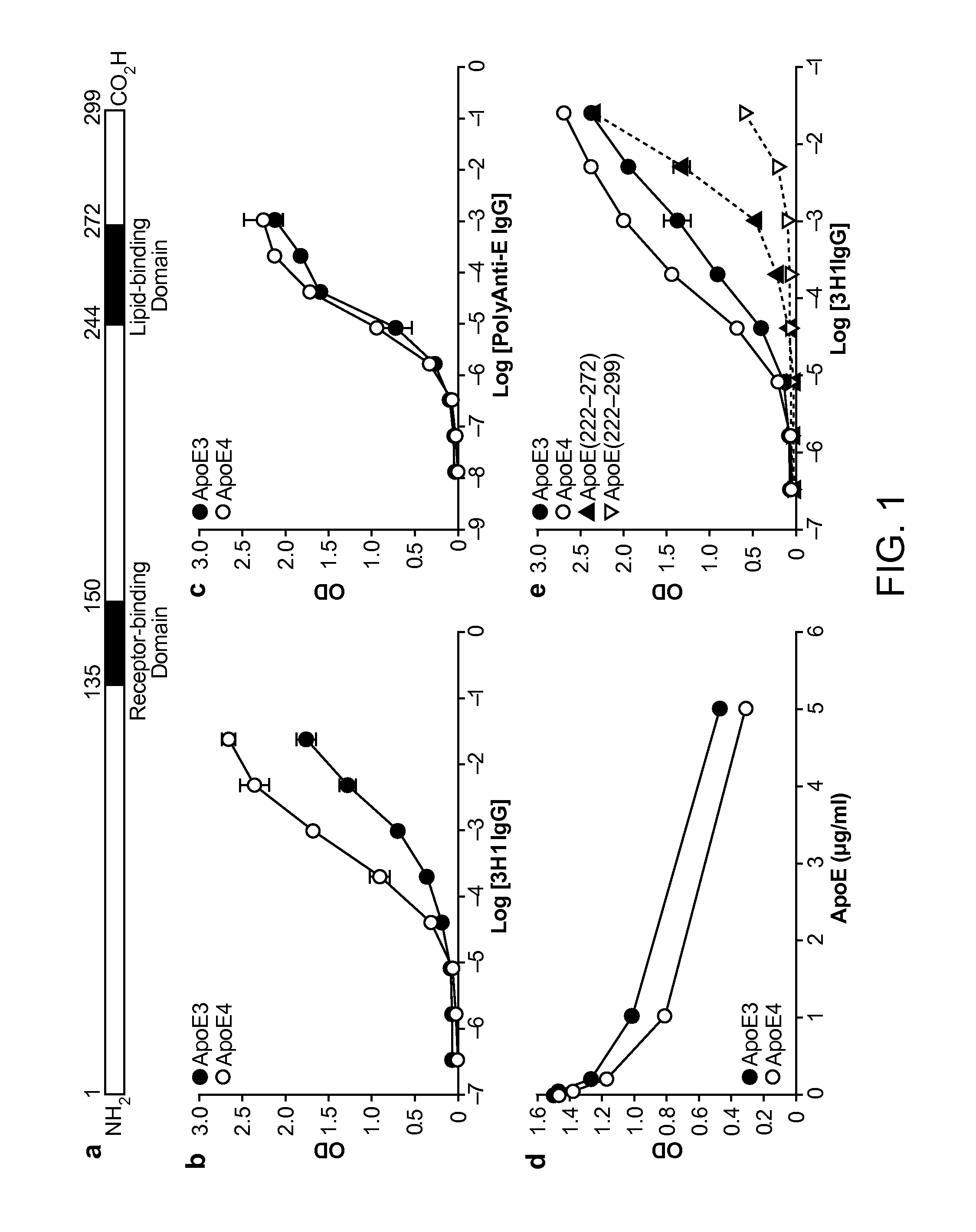
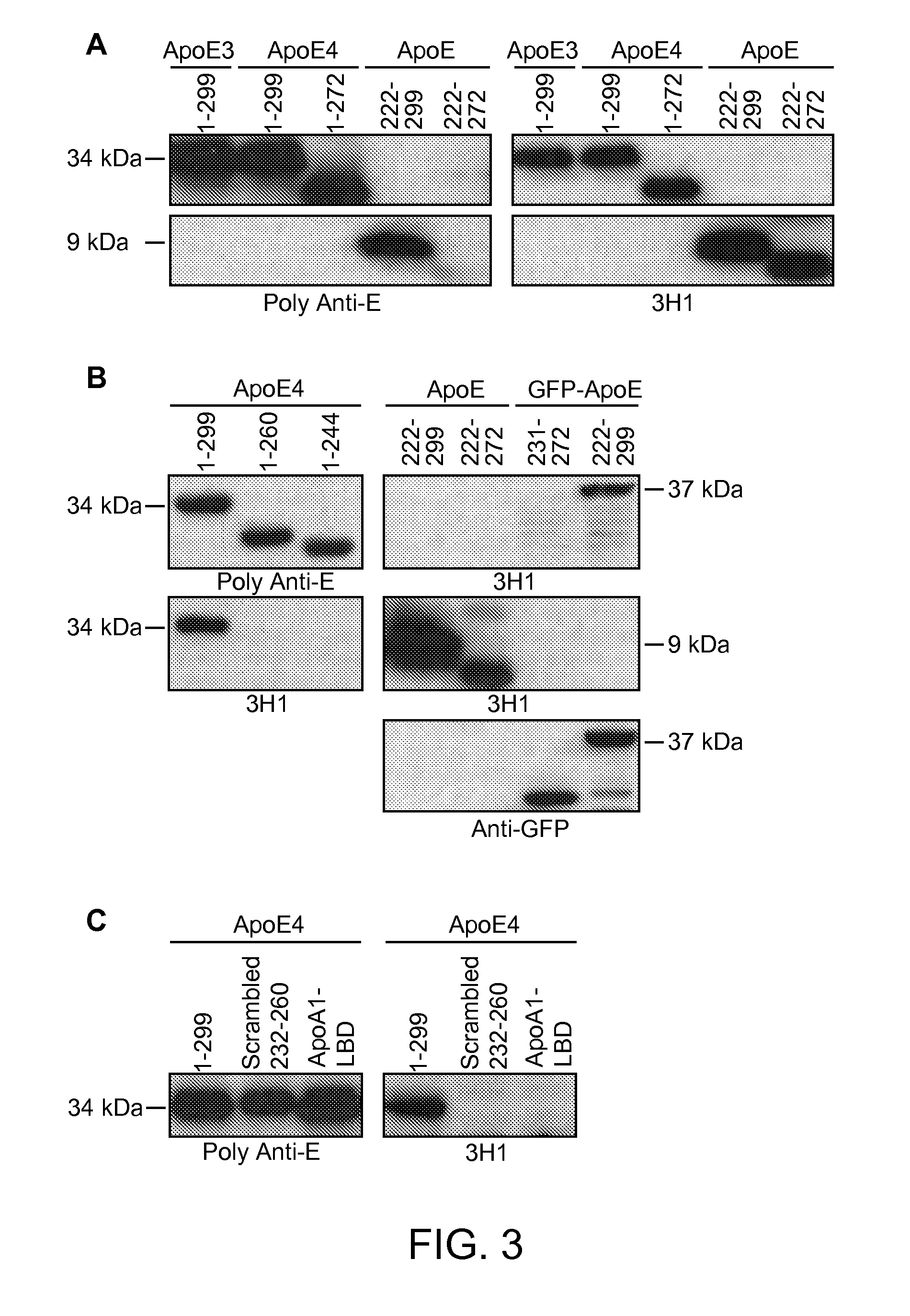
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
123 results about "Apolipoprotein e4" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
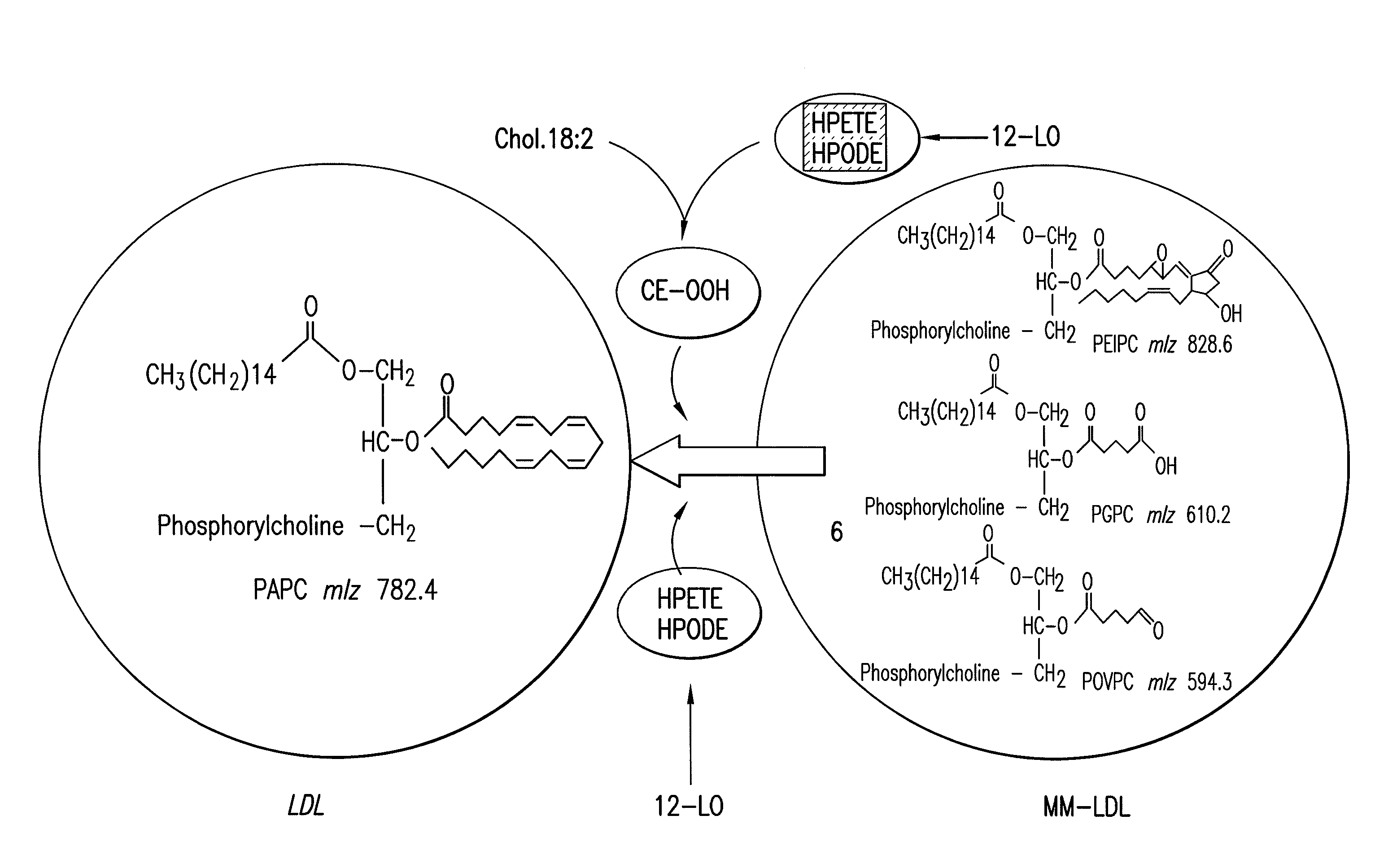
Apolipoprotein E (ApoE) is a class of proteins involved in the metabolism of fats in the body. It is important in Alzheimer's disease and cardiovascular disease.
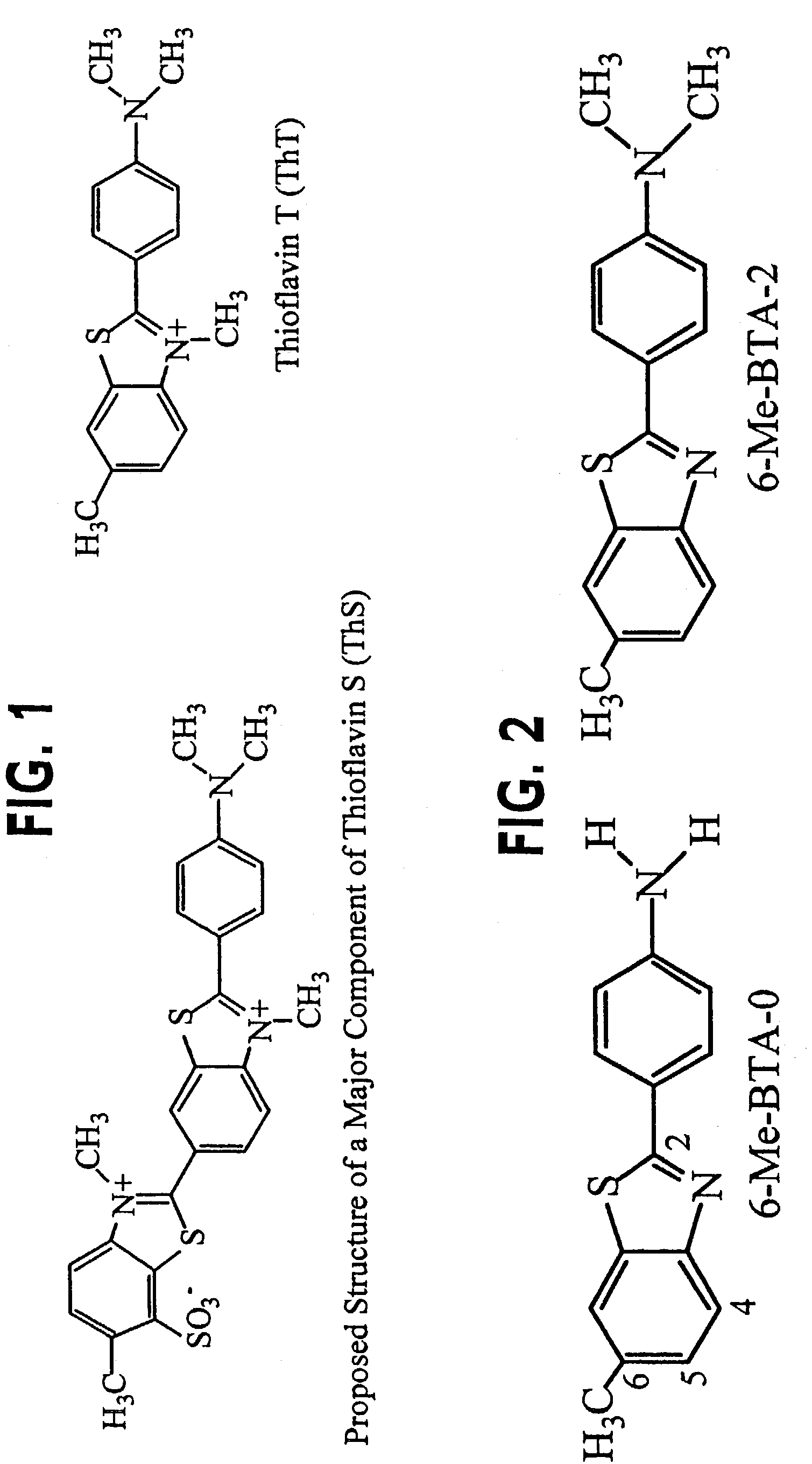
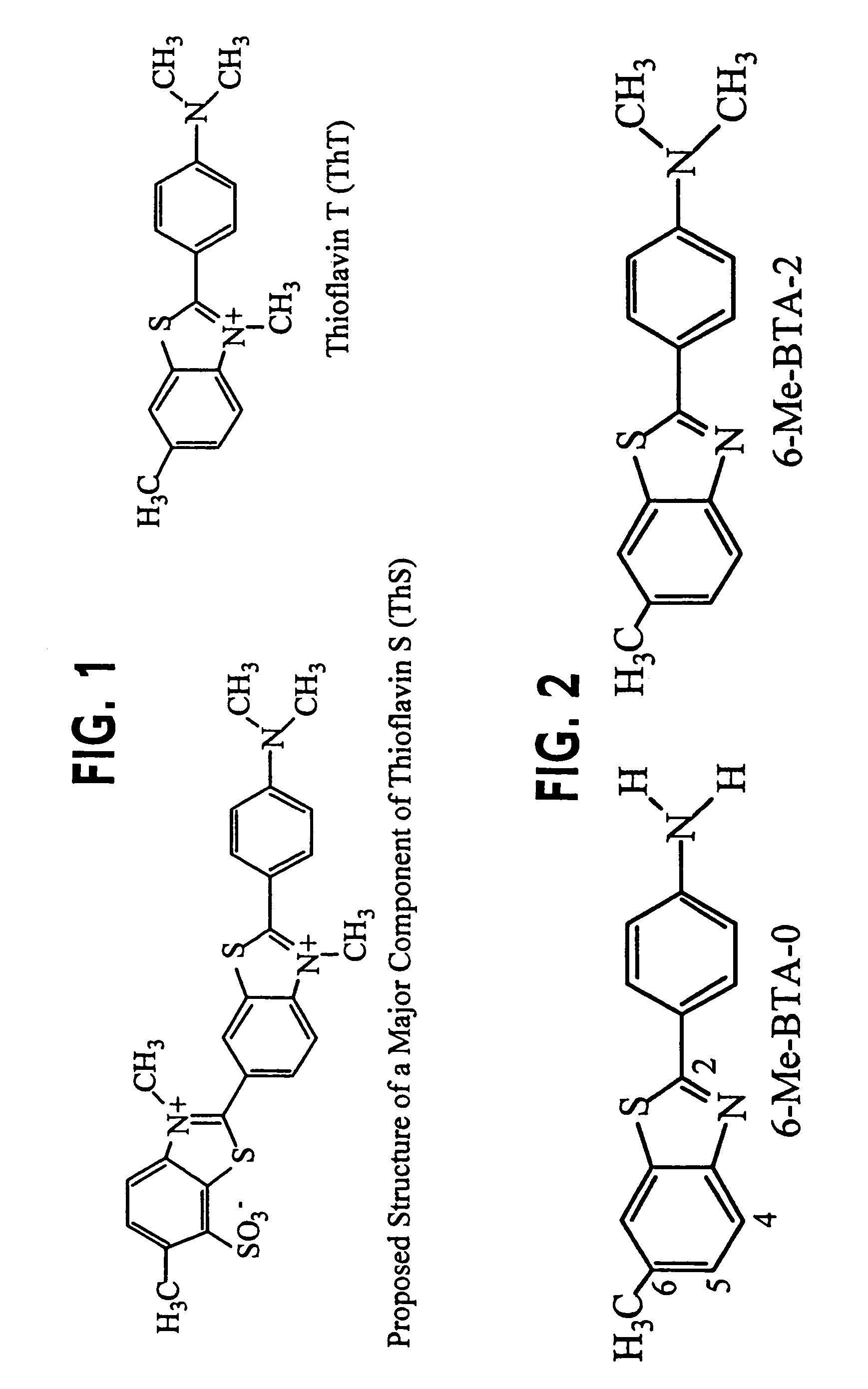
Thioflavin derivatives for use in antemortem diagnosis of Alzheimer's disease and in vivo imaging and prevention of amyloid deposition
This invention relates to novel thioflavin derivatives, methods of using the derivatives in, for example, in vivo imaging of patients having neuritic plaques, pharmaceutical compositions comprising the thioflavin derivatives and method of synthesizing the compounds. The compounds find particular use in the diagnosis and treatment of patients having diseases where accumulation of neuritic plaques are prevalent. The disease states or maladies include but are not limited to Alzheimer's disease, familial Alzheimer's disease, Down's Syndrome and homozygotes for the apolipoprotein E4 allele.
Owner:UNIVERSITY OF PITTSBURGH
Antibodies binding to a c-terminal fragment of apoliopoprotein e
InactiveUS20070104715A1Antibody mimetics/scaffoldsImmunoglobulins against animals/humansApolipoprotein e4Antibody fragments
A human antibody fragment, which antibody or fragment: (i) binds to a polypeptide having the amino acid sequence shown in SEQ ID NO: 1 of the C-terminal domain of Apolipoprotein E (ApoE-CTD) or the amino acid sequence of a part thereof; and (ii) binds to human plaques.
Owner:MEDIMMUNE LTD +1
Thioflavin derivatives for use in the antemortem diagnosis of Alzheimers disease and in vivo imaging and prevention of amyloid deposition
This invention relates to novel thioflavin derivatives, methods of using the derivatives in, for example, in vivo imaging of patients having neuritic plaques, pharmaceutical compositions comprising the thioflavin derivatives and method of synthesizing the compounds. The compounds find particular use in the diagnosis and treatment of patients having diseases where accumulation of neuritic plaques are prevalent. The disease states or maladies include but are not limited to Alzheimer's Disease, familial Alzheimer's Disease, Down's Syndrome and homozygotes for the apolipoprotein E4 allele.
Owner:UNIVERSITY OF PITTSBURGH
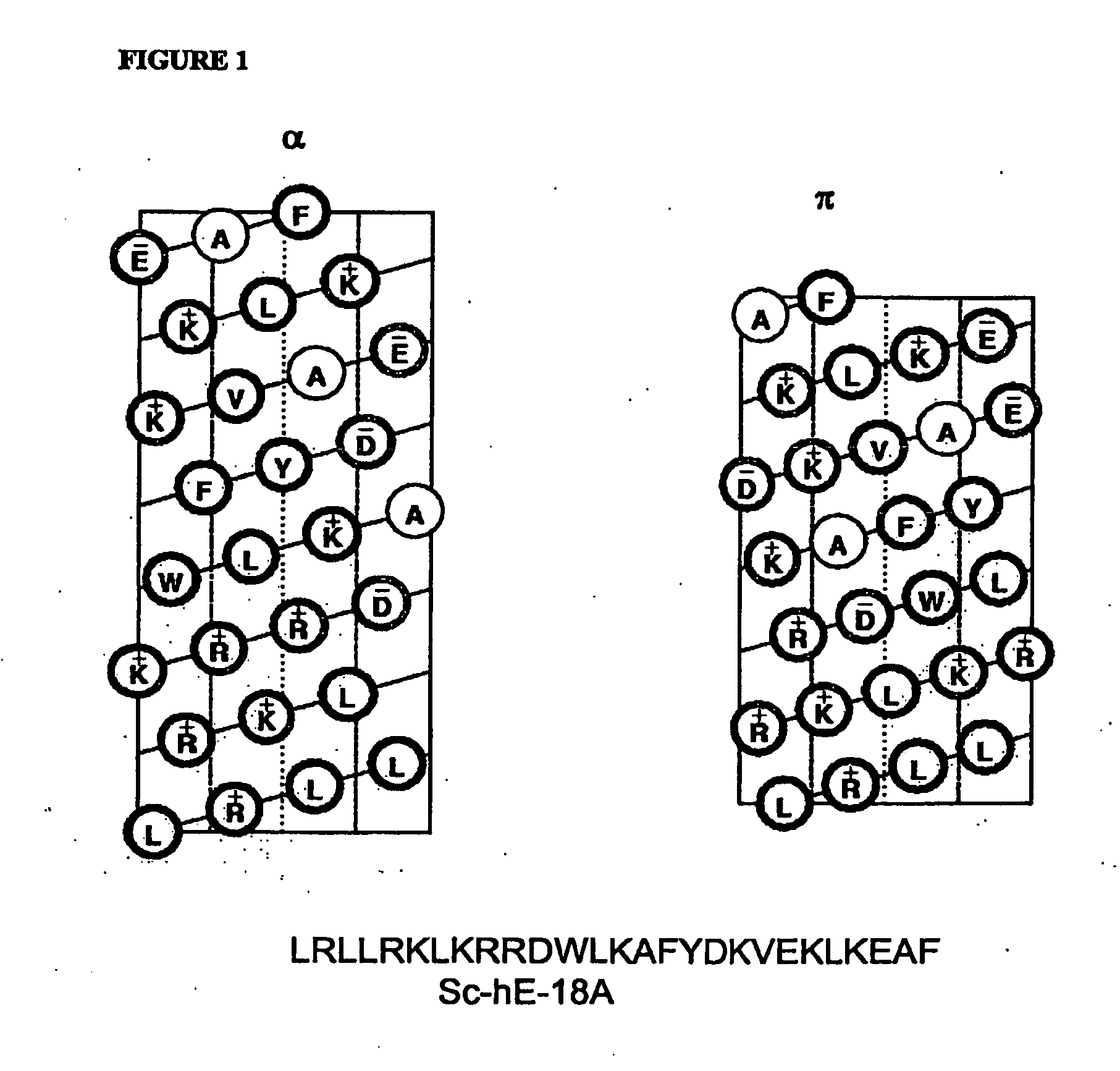
Synthetic apolipoprotein e mimicking polypeptides and methods of use
InactiveUS20100286025A1Antibacterial agentsSenses disorderAmphipathic helixVery low-density lipoprotein
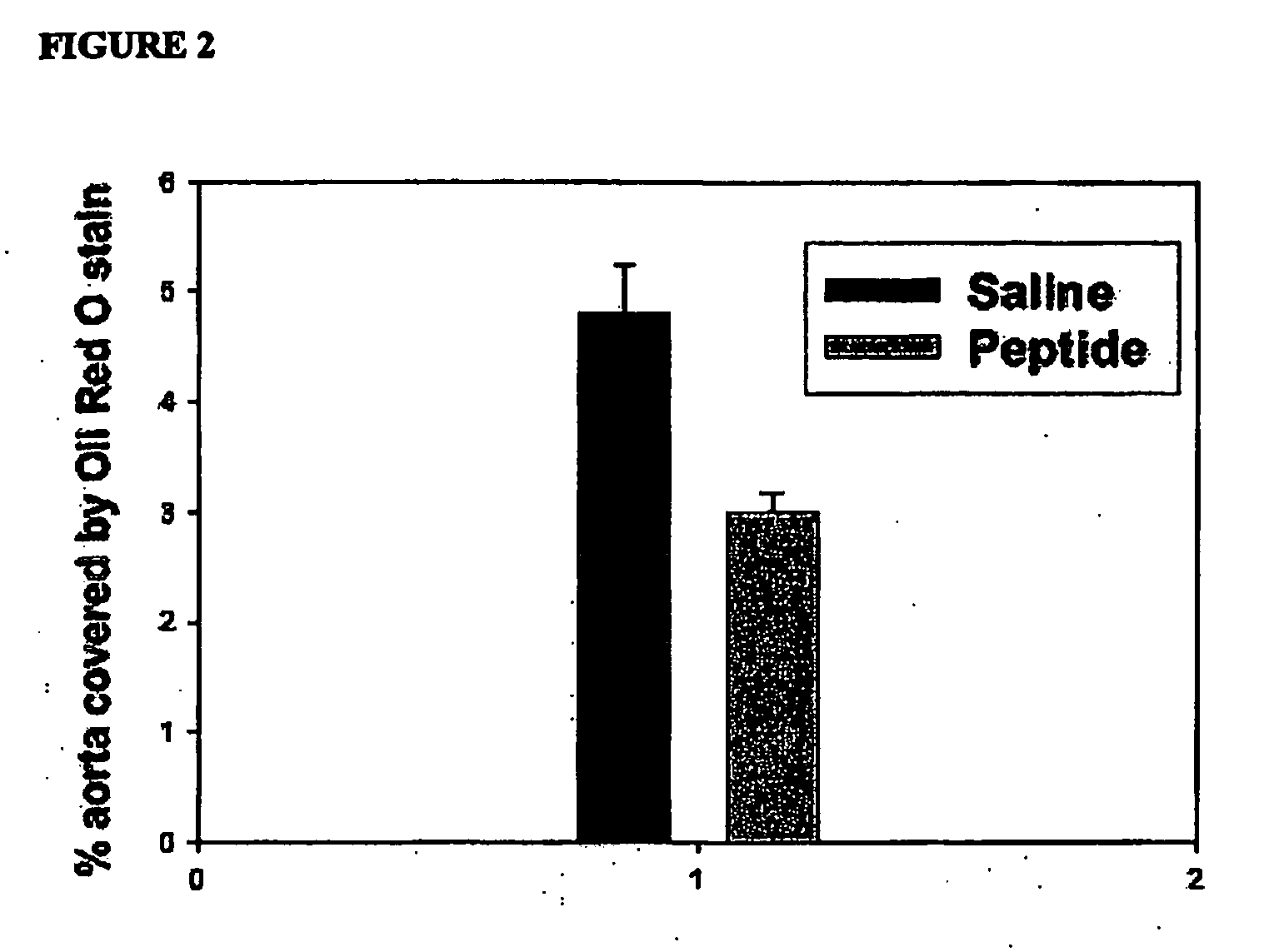
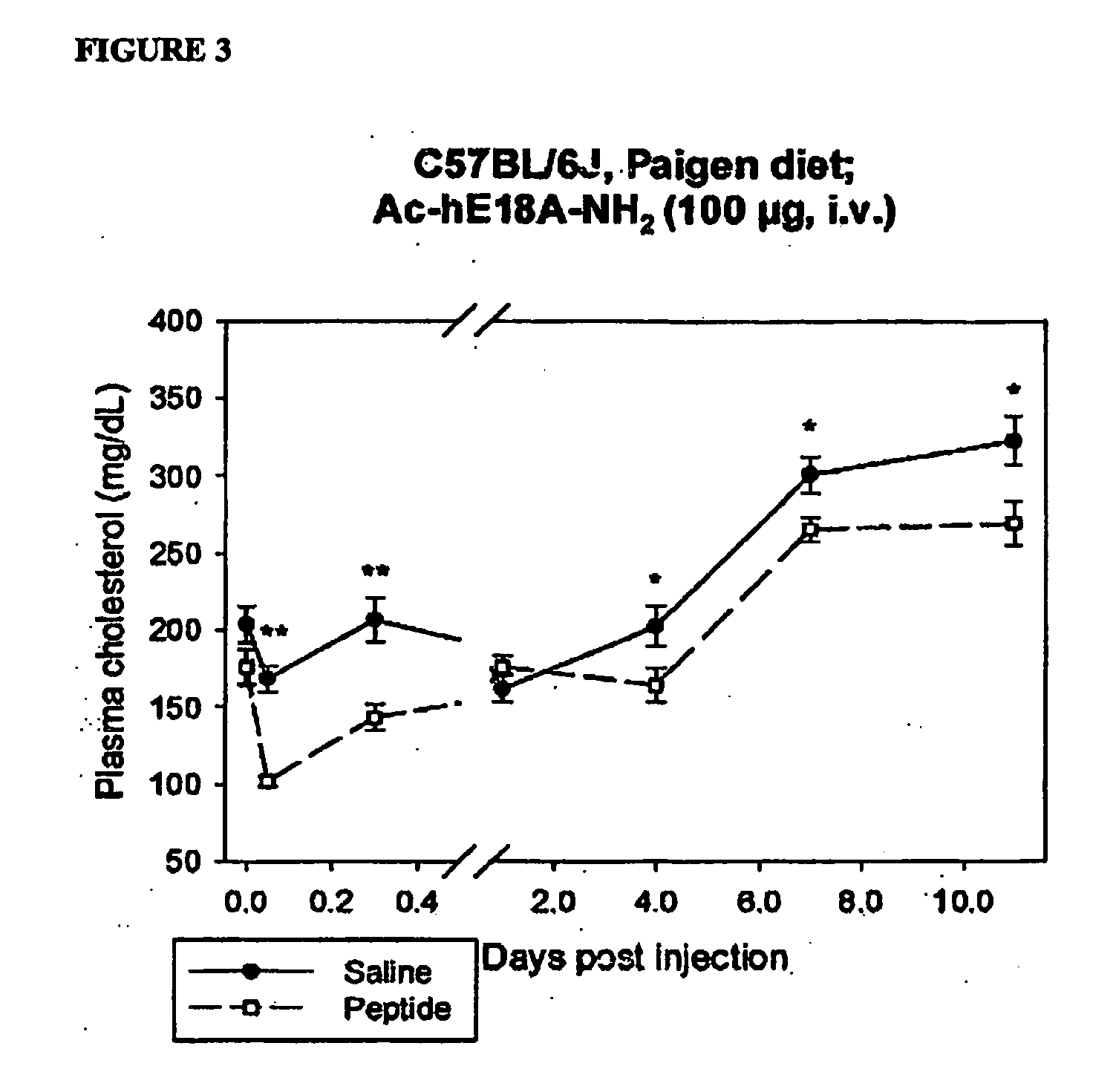
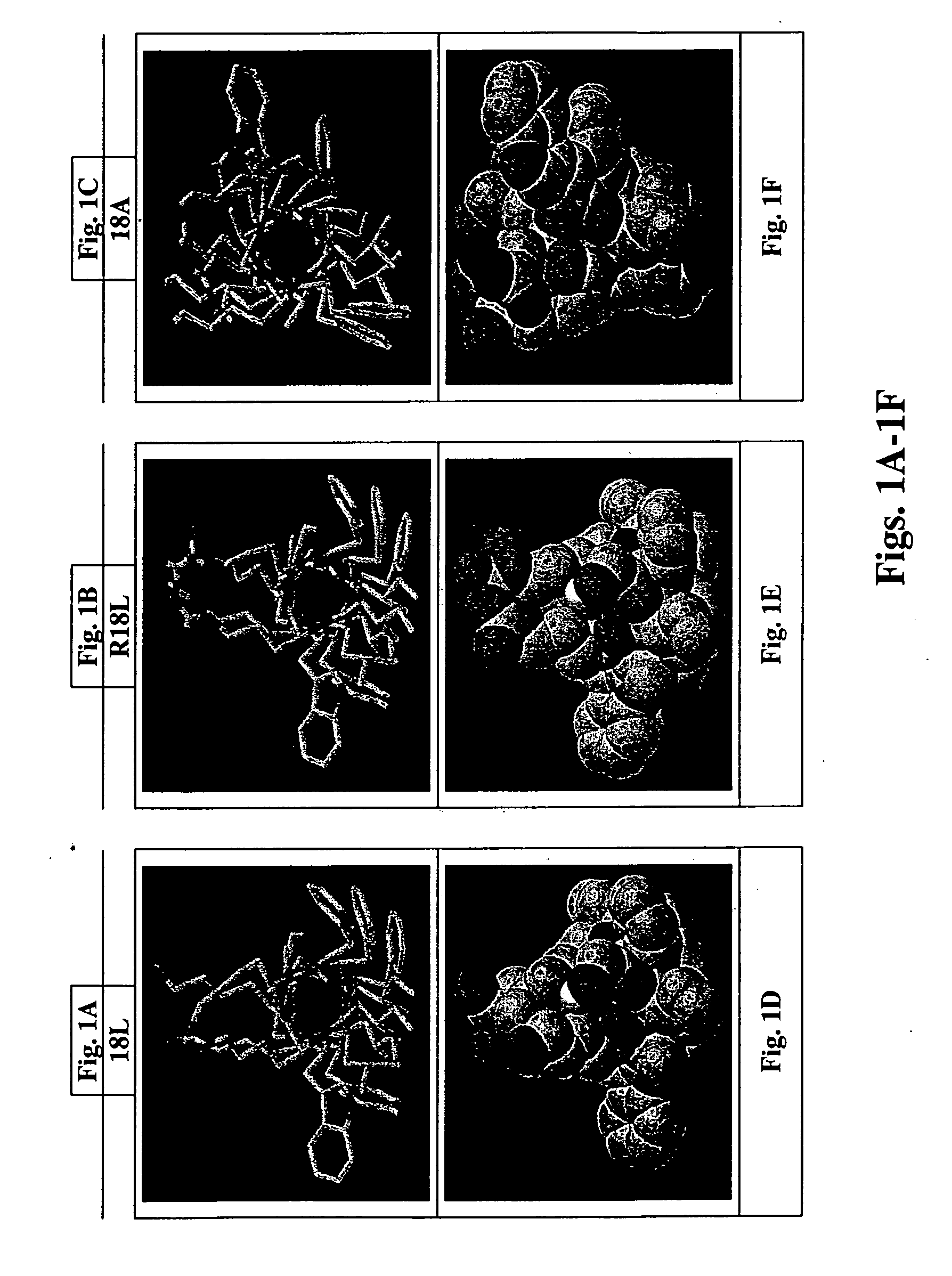
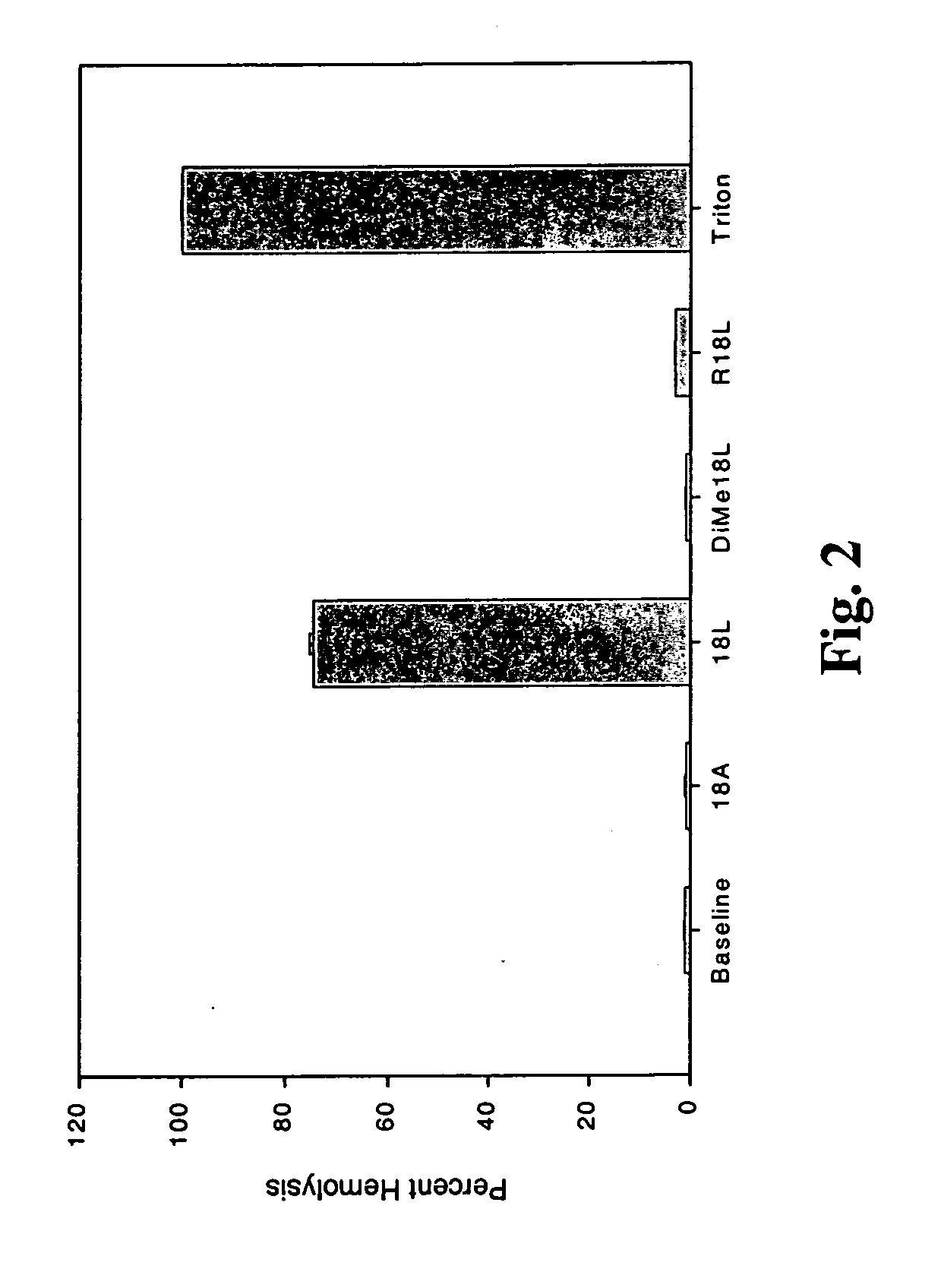
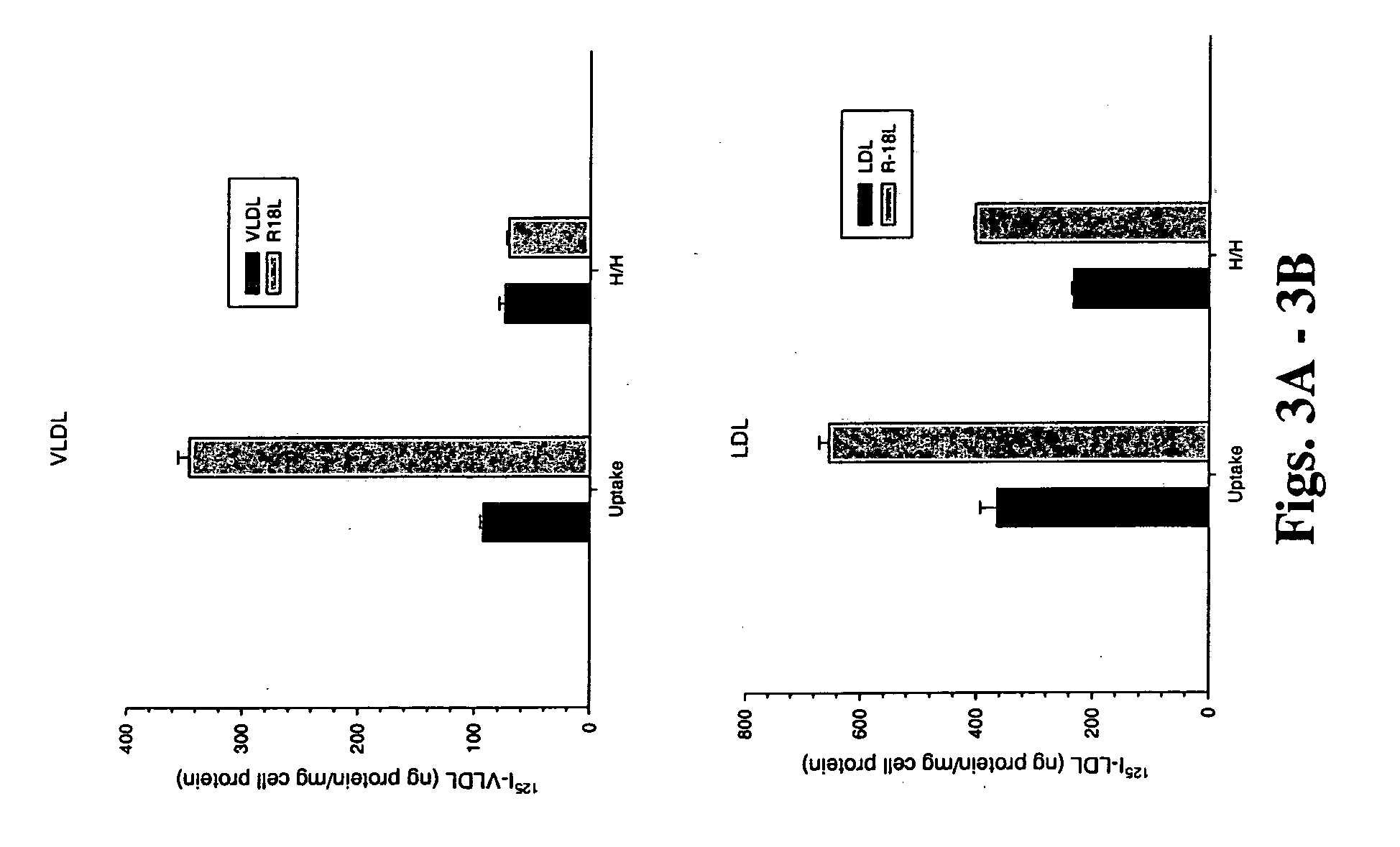
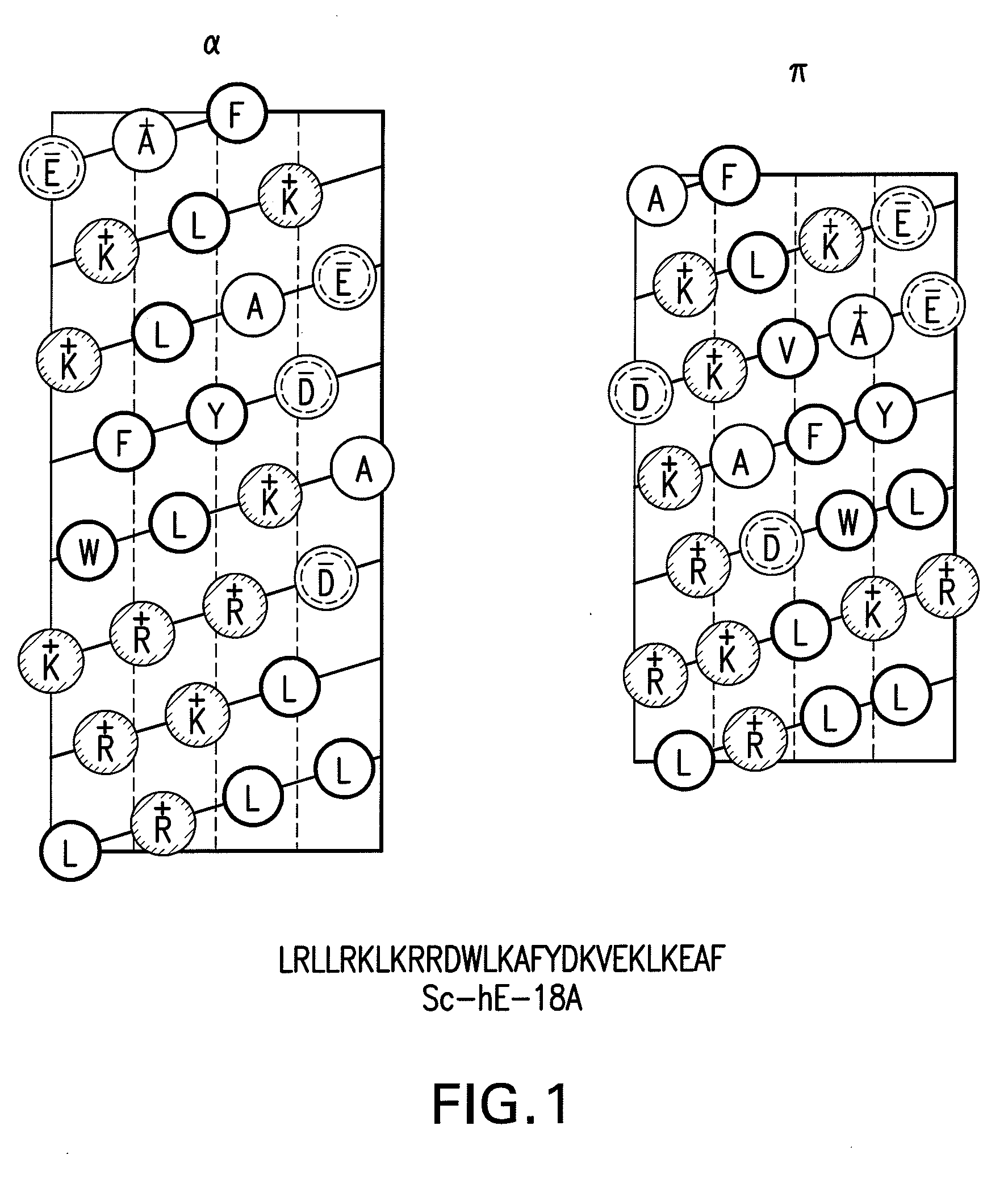
The present invention provides novel synthetic apolipoprotein E (ApoE)-mimicking peptides wherein the receptor binding domain of apolipoprotein E is covalently linked to 18A, the well characterized lipid-associating model class A amphipathic helical peptide, or a modified version thereof. Such peptides enhance low density lipoprotein (LDL) and very low density lipoprotein (VLDL) binding to and degradation by fibroblast or HepG2 cells. Also provided are possible applications of the synthetic peptides in lowering human plasma LDL / VLDL cholesterol levels, thus inhibiting atherosclerosis. The present invention also relates to synthetic peptides that can improve HDL function and / or exert anti-inflammatory properties.
Owner:UAB RES FOUND
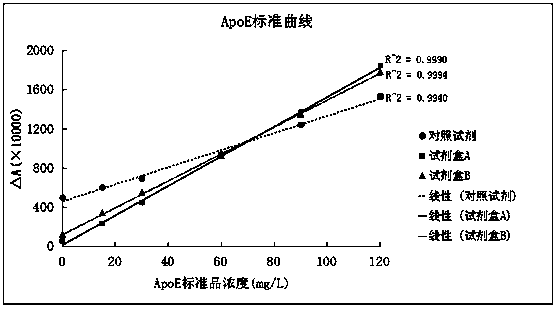
Apolipoprotein E testing reagent
InactiveCN101799475AImprove accuracyImprove anti-interference abilityColor/spectral properties measurementsBiological testingAnti jammingAntioxidant
The invention relates to a reagent for determining APOE in blood serum, aiming to provide a stable reagent which has simple operation, high accuracy, good repeatability and strong anti-jamming capability, uses excellent new-born calf serum to replace human blood serum as substrate preparation and uses liquid blood serum type constant value calibration solution to detect apolipoprotein E in combination, wherein the sample does not need thinning dilution. The reagent is characterized in that the apolipoprotein E testing reagent comprises: a. a reagent R1 comprising buffer solution, a surface active agent, an electrolyte, a polymer accelerant, a reaction promoter, a stabilizer, a proper amount of preservative, and the balance of purified water; b. a reagent R2, wherein a certain amount of the reagent R1 is added to anti-human-APOE antiserum and antioxidant with the vacuum ratio of 20-50%; and c. constant value calibration solution comprising buffer solution, APOE antigen, antioxidant, synergist, stabilizer, proper amount of preservative, and the balance of purified water.
Owner:浙江伊利康生物技术有限公司
Nucleic acid encoding apolipoprotein E-I3
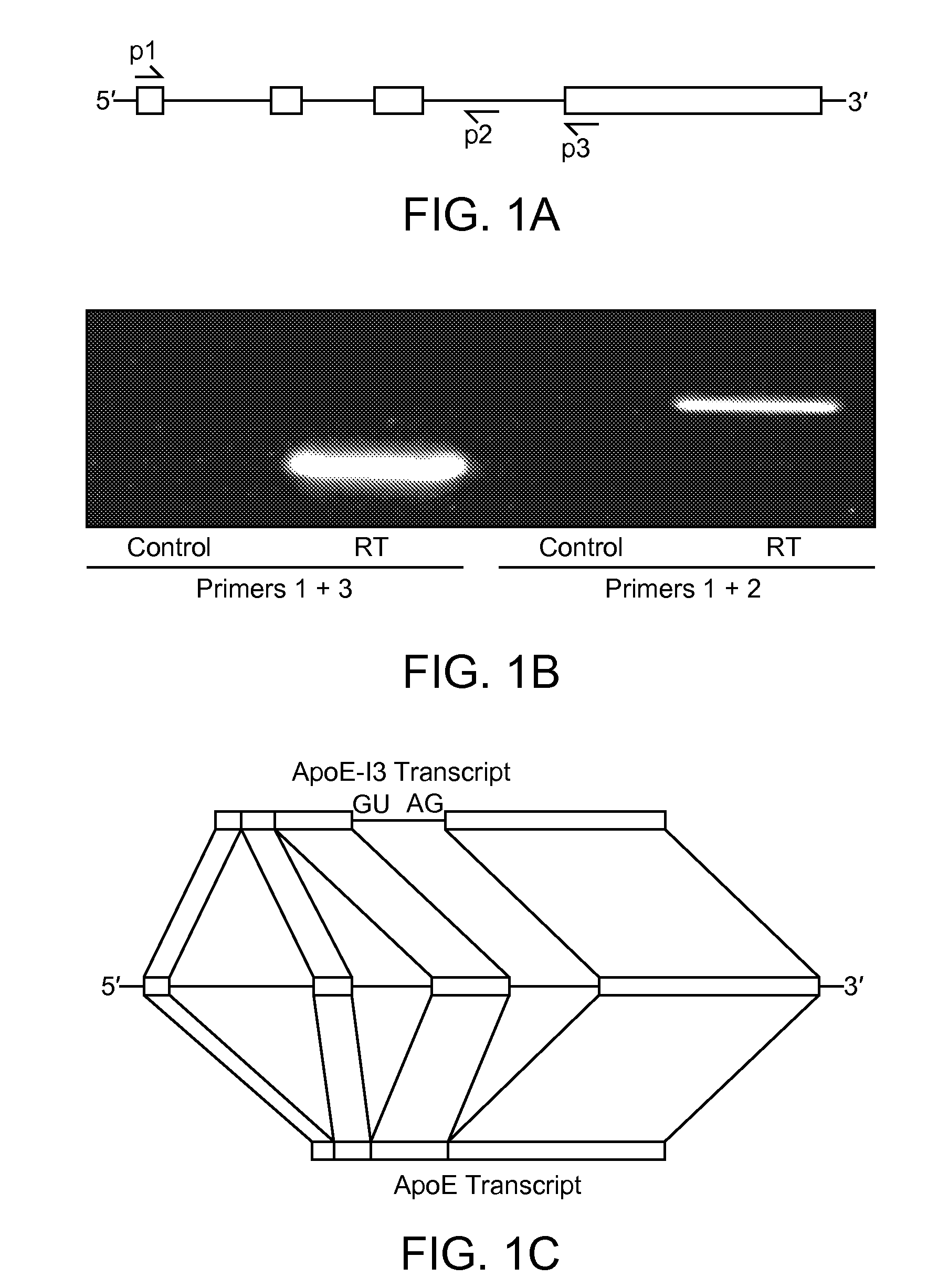
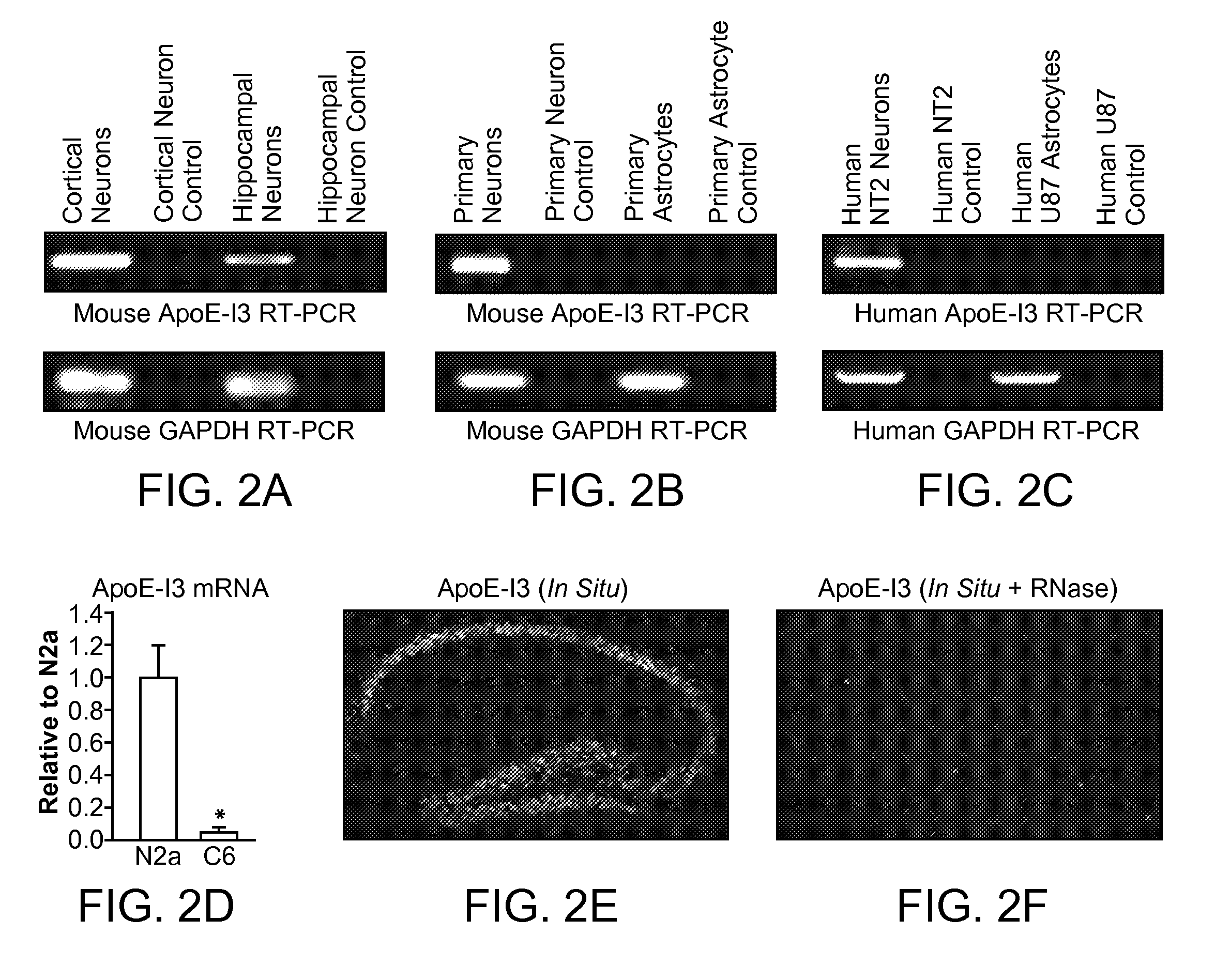
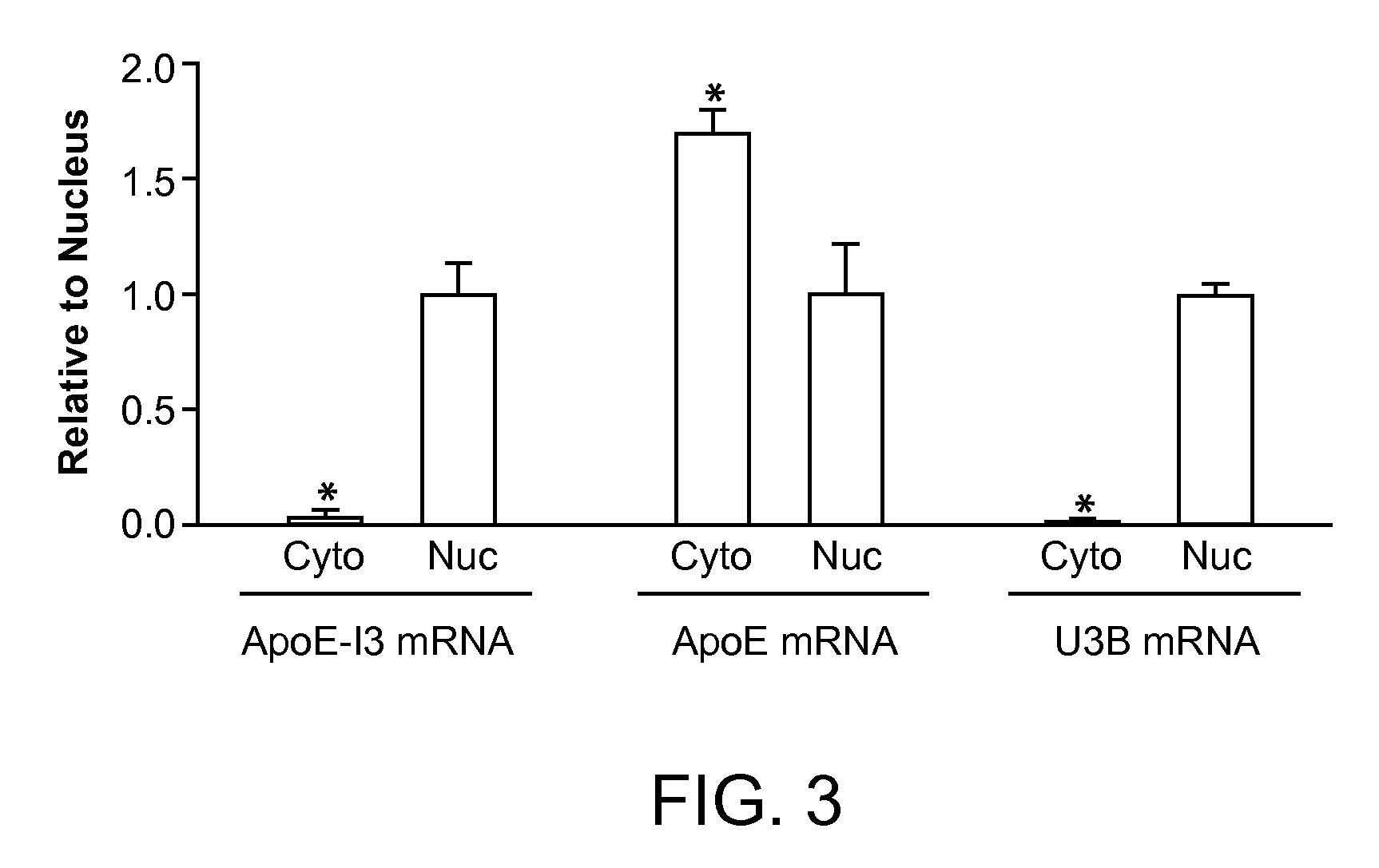
The present invention provides nucleic acids comprising a nucleotide sequence encoding an apolipoprotein E (apoE) splice variant, e.g., an unprocessed apoE, that retains intron 3; and vectors and host cells comprising same. The present invention further provides screening methods to identify agents that inhibit cleavage of intron-3 from the apoE splice variant. The present invention further provides methods of treating apoE-related neurological disorders, involving administering an agent that inhibits removal of intron-3 from a precursor apoE mRNA.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Human SLCO1B1 and ApoE (apolipoprotein E) gene polymorphism detection kit
InactiveCN104846085AEasy to detectAccurate Typing DetectionMicrobiological testing/measurementApolipoprotein e4SLCO1B1
The invention provides a human SLCO1B1 and ApoE (apolipoprotein E) gene polymorphism detection kit, comprising a PCR (polymerase chain reaction) buffer solution, dNTP (deoxy-ribonucleoside triphosphate), MgCl2, four groups of specific primers, four groups of specific probes, an internal standard system, HotStart Taq enzyme and UNG (uracil-N-glycosylase) enzyme. The detection kit has the advantages of high specificity, high sensitivity, ease and quickness of operation, high throughput, safety, objectiveness of result interpretation, and the like.
Owner:WUHAN YZY MEDICAL SCI & TECH
ApoE gene primer group, detection kit and detection method
ActiveCN105886608ARaise the ratioImprove detection accuracyMicrobiological testing/measurementDNA/RNA fragmentationApolipoprotein e4Agricultural science
The invention relates to the technical field of DNA (Deoxyribonucleic Acid) detection, and provides an ApoE (Apolipoprotein E) gene primer group, a detection kit and a detection method, wherein the ApoE gene primer group comprises a primer group for detecting an rs429358 site and a primer group for detecting an rs7412 site; an upstream primer in the primer group for detecting the rs429358 site has any one sequence in SEQ ID NO: 1-4; a downstream primer in the primer group for detecting the rs429358 site has any one sequence in SEQ ID NO: 5-8; an upstream primer in the primer group for detecting the rs7412 site has any one sequence in SEQ ID NO: 9-12; and a downstream primer in the primer group for detecting the rs7412 site has any one sequence in SEQ ID NO: 13-16. The primer group, the kit and the method provided by the invention have the advantage that the correct signal proportion in the ApoE gene detection process can be improved.
Owner:WUHAN CONSIDERIN GENE & HEALTH TECH CO LTD
Biomarkers of osteoarthritis
Owner:UNIVERSITY OF MISSOURI
Apolipoprotein E ELISA reagent box and method of producing the same
The invention discloses an apolipoprotein E4 ELISA kit and a preparation method thereof, the invention applies the purified human apolipoprotein E4 to prepare an anti-human ApoE4 antibody, and an ELISA plate is coated after the compatible purification. The kit composition includes the ELISA plate coated by the anti-human ApoE4 monoclonal antibody, an enzyme-labeled antibody, ApoE4 standard frozen powder, sample dilution solution, TMB substrate color developing solution, concentration washing liquid and reaction termination liquid. The ApoE4 in the antibody capture standard solution or the sample solution which is fixed on the microporous surface of the ELISA plate is identified by and combined with the enzyme-labeled antibody, so as to form the antibody-ApoE4-antibody-enzyme compound, the absorbance is measured after the reaction and color development of the enzyme and the substrate and the concentration of the ApoE4 in the sample can be calculated by the standard curve. The kit can detect the consistency of the apolipoprotein E4 in human serum, plasma or cerebrospinal fluid, the invention has the advantages of sensitivity, rapidness, simpleness and accuracy, so the invention provides the effective means for in vitro diagnosis and determination of ApoE4 gene type and the gene dosage.
Owner:杭州浙大生科生物技术有限公司
Diagnosis Of Neurodegenerative Diseases
InactiveUS20080286263A1Improve trustNervous disorderElectrolysis componentsHemoglobin Beta ChainApolipoprotein C-II
The invention relates to a method of diagnosis of Huntington's Disease in a diagnostic sample of a valid body tissue taken from a human subject, which comprises detecting an altered concentration of a protein in the diagnostic sample, compared with a sample of a control human subject, the protein being selected from: Swiss Prot accession number: Protein name; P10909: Clusterin precursor; P00738: Haptoglobin precursor; P01009: Alpha-1-antitrypsin precursor; P01024: Complement C3 precursor; P01620: 1 g kappa chain V-III region; P01834: 1 g kappa chain C region P01842: 1 g lambda chain C regions; P01857: 1 g gamma-1 chain C region; P01859: Ig gamma-2 chain C region; P01876: 1 g alpha-1 chain C region P02647: Apolipoprotein A-I precursor; P02649: Apolipoprotein E precursor; P02652: Apolipoprotein A-II precursor; P02655: Apolipoprotein C-II precursor; P02656: Apolipoprotein C-II precursor P02671: Fibrinogen alpha / alpha-E chain precursor; P02763: Alpha-1-acid glycoprotein 1 precursor; P02766: Transthyretin precursor; P02768: Serum albumin precursor; P02787: Serotransferrin precursor; P04196: Histidine-rich glycoprotein precursor; P06727: Apolipoprotein A-IV precursor; P19652: Alpha-1-acid glycoprotein 2 precursor; P68871 / P02042: Hemoglobin beta chain / Hemoglobin delta chain; P60709: Beta actin.
Owner:ELECTROPHORETICS LTD
Reagent for detecting risk factor of alzheimer's disease, detection kit therefor and method of detecting risk factor of alzheimer's disease using the same
InactiveUS20050266501A1Low costImprove accuracyNervous disorderImmunoglobulins against animals/humansBULK ACTIVE INGREDIENTBiology
A reagent for detecting a risk factor of Alzheimer's disease which contain, as the active ingredients, an antibody specific to apolipoprotein E4 and an antibody against all of apolipoprotein E2, apolipoprotein E3 and apolipoprotein E4; a detection method; and a detection kit. Thus, apolipoprotein E4 genotype can be economically and sensitively detected compared with the PCR method which is employed for gene examination today.
Owner:IMMUNO BIOLOGICAL LABIRATORIES CO LTD
Extended risk assessment panel for individualized treatment of cardiovascular disease, and methods related thereto
ActiveUS20140088072A1More treatmentDetailed informationBiocideOrganic active ingredientsSterolIndividualized treatment
Disclosed is a personalized diagnostic and treatment solution for cardiovascular disease. The invention comprises an extended CVD risk assessment panel, combining tests for traditional and new important risk markers, and methods for devising a personalized treatment plan for a patient via the use of a CVD diagnosis and treatment protocol algorithm. The new important risk markers include HDL subpopulation profile by two-dimensional gel-electrophoresis, plasma sterols, direct measurement of sdLDL-C, determination of CRP molecular forms, glycated albumin as a percentile of total albumin, and other specialized testing pertaining to apolipoprotein E and Factor V Leiden genotyping, NT-proBNP and adiponectin. This solution provides a more complete risk assessment of an individual than merely measuring traditional CVD risk markers, and enables the healthcare practitioner to optimize therapy for patients with or without established CVD. This solution presents the advantages of greater accuracy, savings in time and cost over existing testing and treatment methods.
Owner:BOSTON HEART DIAGNOSTICS
Synthetic single domain polypeptides mimicking apolipoprotein E and methods of use
ActiveUS20070101448A1Improve efficiencyReduce riskPeptide/protein ingredientsAerosol deliveryApolipoprotein e4Antibody
The present invention is directed to a synthetic apolipoprotein-E mimicking polypeptide consisting of a single domain. The invention is also directed to nucleic acid encoding the polypeptide, vectors including the nucleic acid, antibodies specific for the polypeptide, and compositions comprising the same and methods of using the same.
Owner:UAB RES FOUND
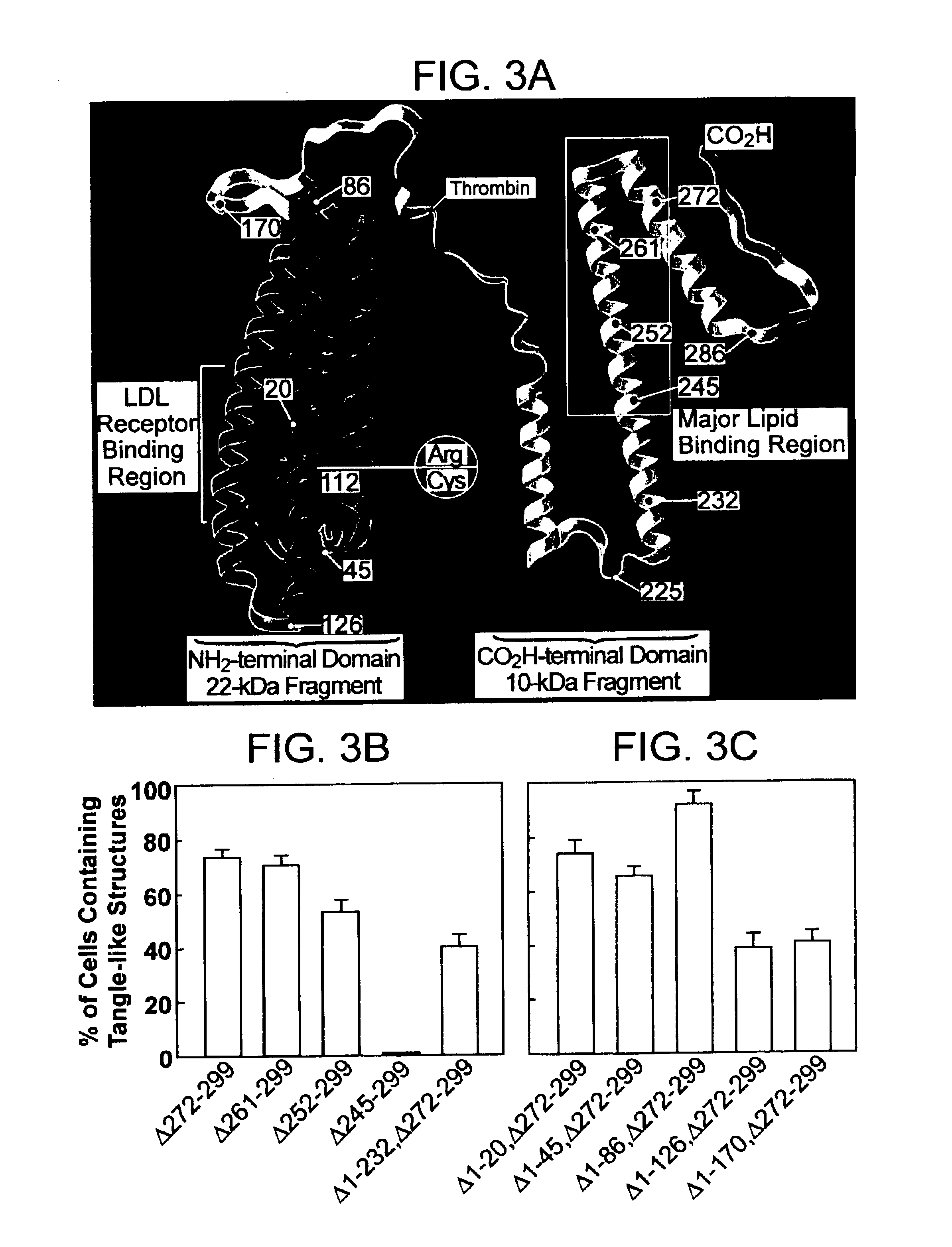
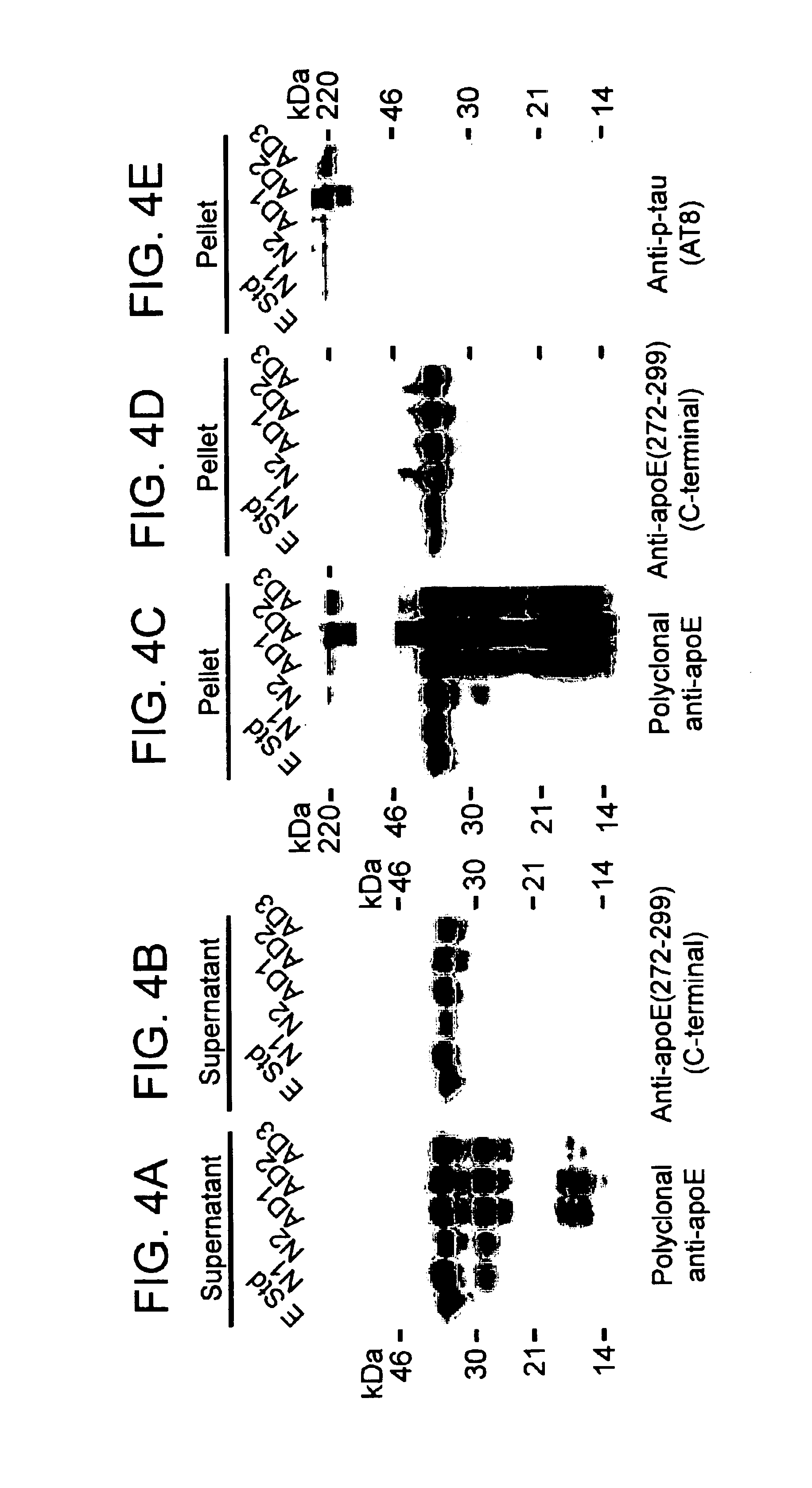
Methods of treating disorders related to apoE
InactiveUS6787519B2Inhibition formationLower Level RequirementsNervous disorderPeptide/protein ingredientsFiberHuman animal
The present invention provides methods inhibiting formation of neurofibrillary tangles; and methods for treating disorders relating to apolipoprotein E (apoE) in a subject. The methods generally involve reducing the level of a carboxyl-terminal truncated form of apoE in a neuronal cell of a subject. The invention further provides isolated cells comprising a nucleic acid molecule encoding a carboxyl-terminal truncated form of apoE; and methods of screening compounds using the cells. The invention further provides compounds that inhibit an apoE cleavage enzyme, and that reduce the formation of neurofibrillary tangles in a neuronal cell. The invention further provides transgenic non-human animals that include as a transgene a nucleic acid that encodes a carboxyl-terminal truncated form of apoE; as well as methods of screening compounds using transgenic animals.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
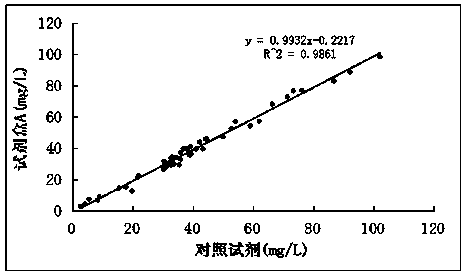
Apolipoprotein E detection kit
The invention discloses an apolipoprotein E detection kit which comprises a reagent R1, a reagent R2 and a calibration product. The reagent R1 mainly comprises the following components: 10-50mmol / L of phosphate buffer, 10-40g / L of polyethylene glycol, 50-200mmol / L of sodium chloride, 0.02-0.2% of tween 20, 0.1-1% of BSA (Bovine Serum Albumin) and 0.1% of NaN3, wherein polyethylene glycol is low-polymerization polyethylene glycol. The reagent R2 mainly comprises the following components: 10-50mmol / L of phosphate buffer, 50-200mmol / L of sodium chloride, 0.02-0.2% of tween 20, 10-50% (v / v) of ApoE antibody and 0.1% of NaN3; the calibration product mainly comprises the following components: 10-50mmol / L of phosphate buffer, 50-200mmol / L of sodium chloride, 0.1-1% of BSA, 0.2-5mmol / L of EDTA (Ethylene Diamine Tetraacetic Acid), 0.02-0.2% of tween 20, 0.1% of NaN3 and 0-120mg / L of ApoE antigen. The apolipoprotein E detection kit is convenient to use, good in reagent stability, good in detection result accuracy, good in repeatability, convenient to use, low in cost and convenient to popularize and apply.
Owner:NINGBO RUI BIO TECH
Immunoturbidimetric assay apolipoprotein E detection kit
InactiveCN103399160ASimple and fast operationHigh sensitivityColor/spectral properties measurementsBiological testingHuman apolipoproteinAntiserum
The invention relates to an immunoturbidimetric assay apolipoprotein E detection kit, and relates to the technical field of kit. The kit is composed of three parts which are an R1 reagent, an R2 reagent, and a calibrator. The R1 reagent is composed of 40-50mmol / L of a buffer solution with a pH value of 7, 70-150mmol / L of a surfactant, 15-30mmol / L of disodium ethylenediamine tetraacetate, and 0.1-1% of a preservative. The R2 reagent is prepared by adding anti-human-apolipoprotein-E antiserum with a volume ratio of 30-50% into a certain amount of the R1 reagent. The calibrator is 80-200g / L ApoE antigen, and also comprises 0.2-15% of a preservative, 1-4% of a stabilizing agent, and 1-10% of a surfactant, wherein the percentages are human serum matrix volume amount percentages. The kit has the advantages of simple operation, high sensitivity, good specificity, fast determination, and accurate and reliable result.
Owner:上海睿康生物科技有限公司
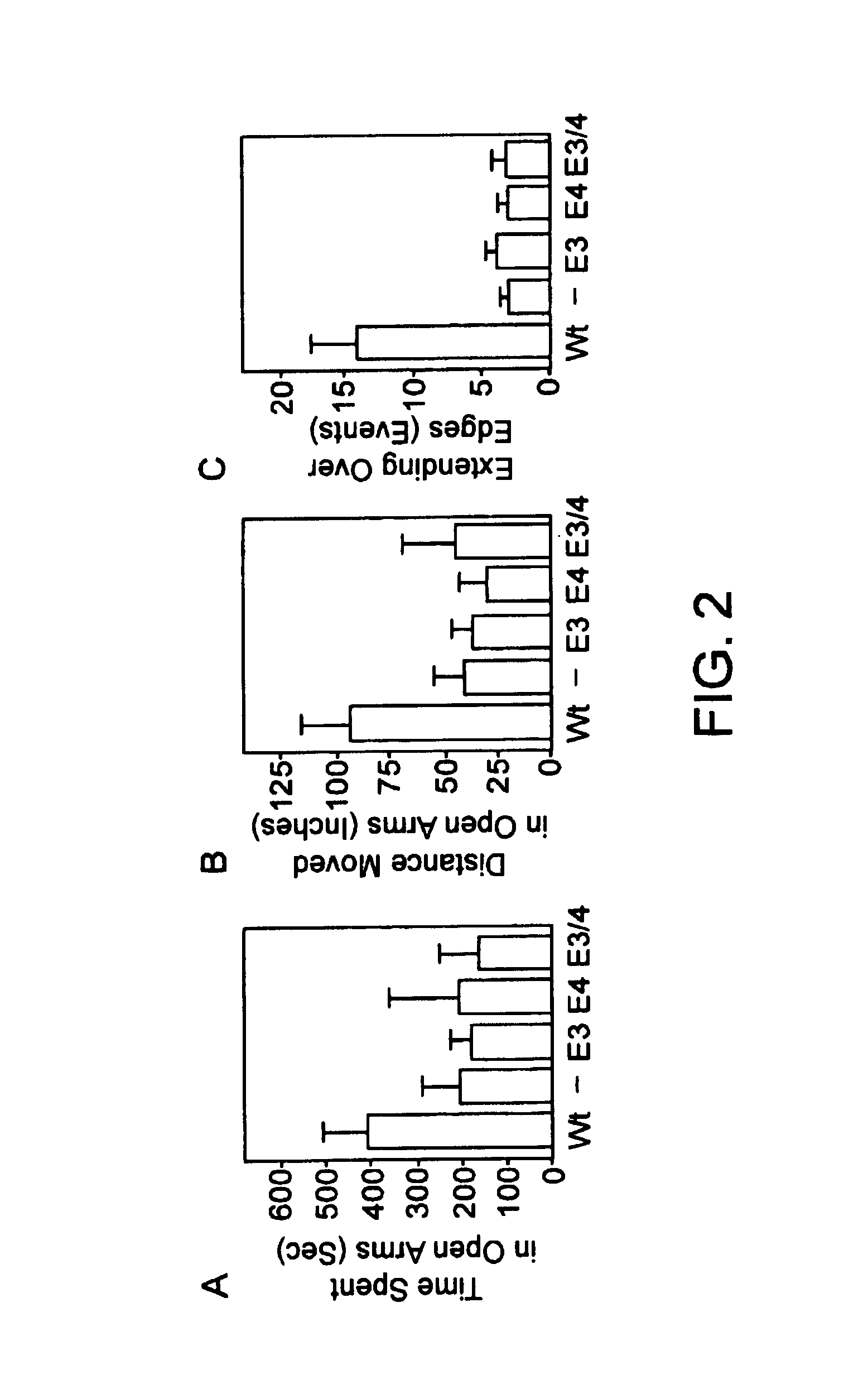
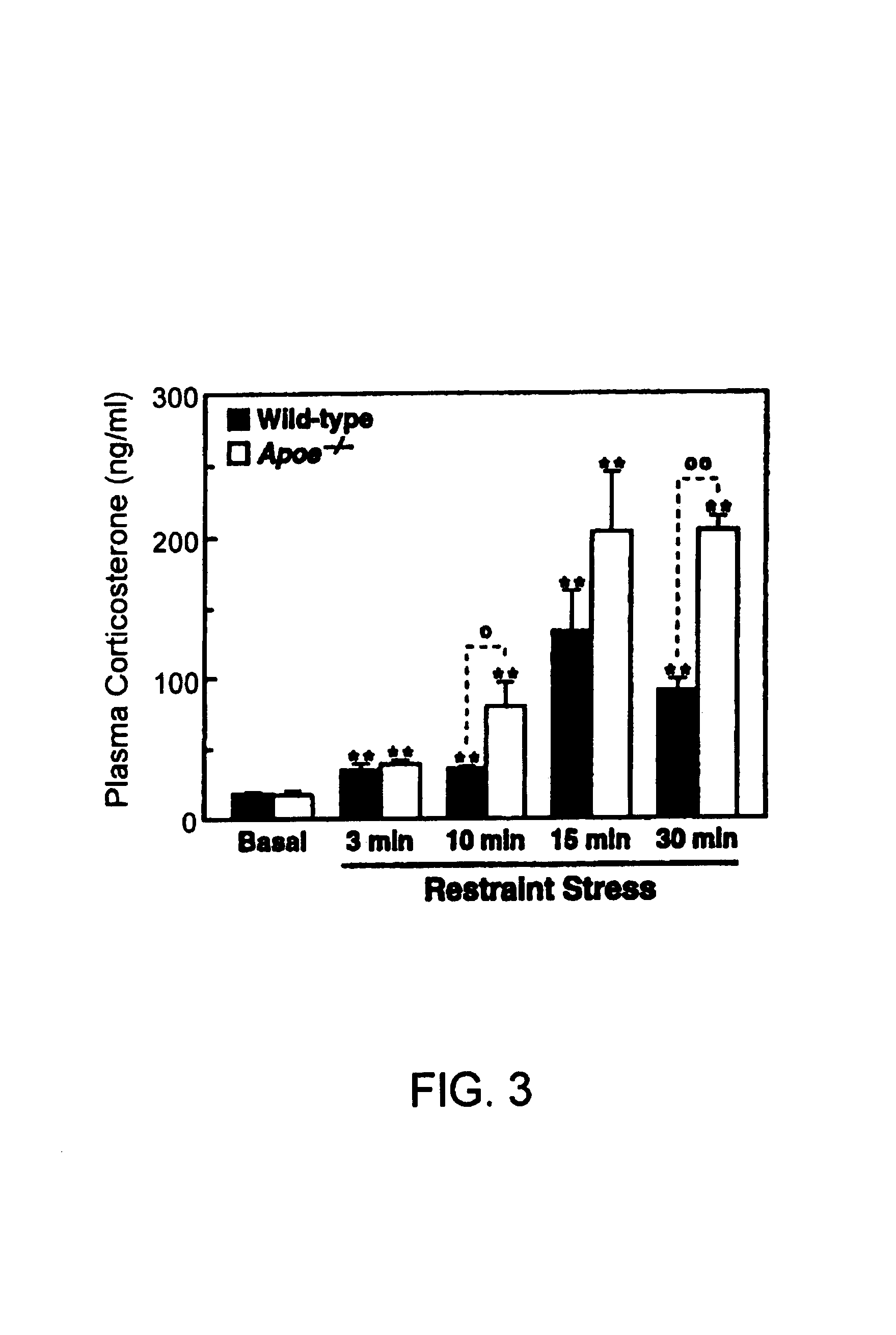
Method of screening a compound for anxiolytic activity in an Apolipoprotein e knockout animal
InactiveUS6982361B1Peptide/protein ingredientsGenetic material ingredientsStress inducedApolipoprotein e4
Methods and compositions for use in treating anxiety are provided. In the subject methods, an effective amount of an agent having ApoE3 activity is administered to a host suffering from an anxiety, e.g. excessive anxiety, unwanted anxiety, an anxiety disorder, etc. Also provided are methods and compositions for modulating adrenal steroidogenesis and / or release, particularly stress induced adrenal steroidogenesis and / or release, and the hippocampal-pituitary-adrenal (HPA) axis. In these methods, an effective amount of an ApoE activity modulating agent, e.g. an ApoE agonist or antagonist, is administered to the host.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
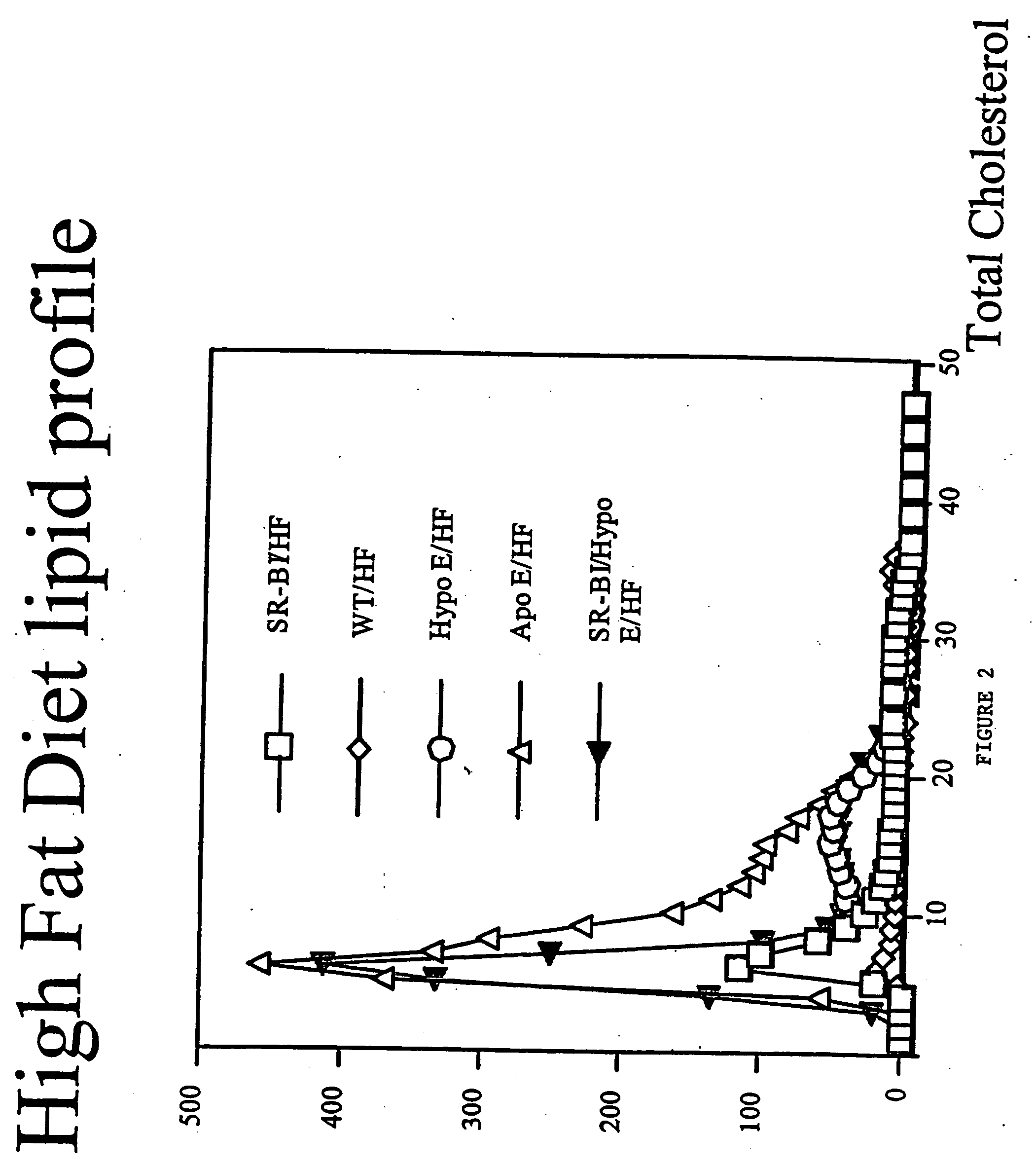
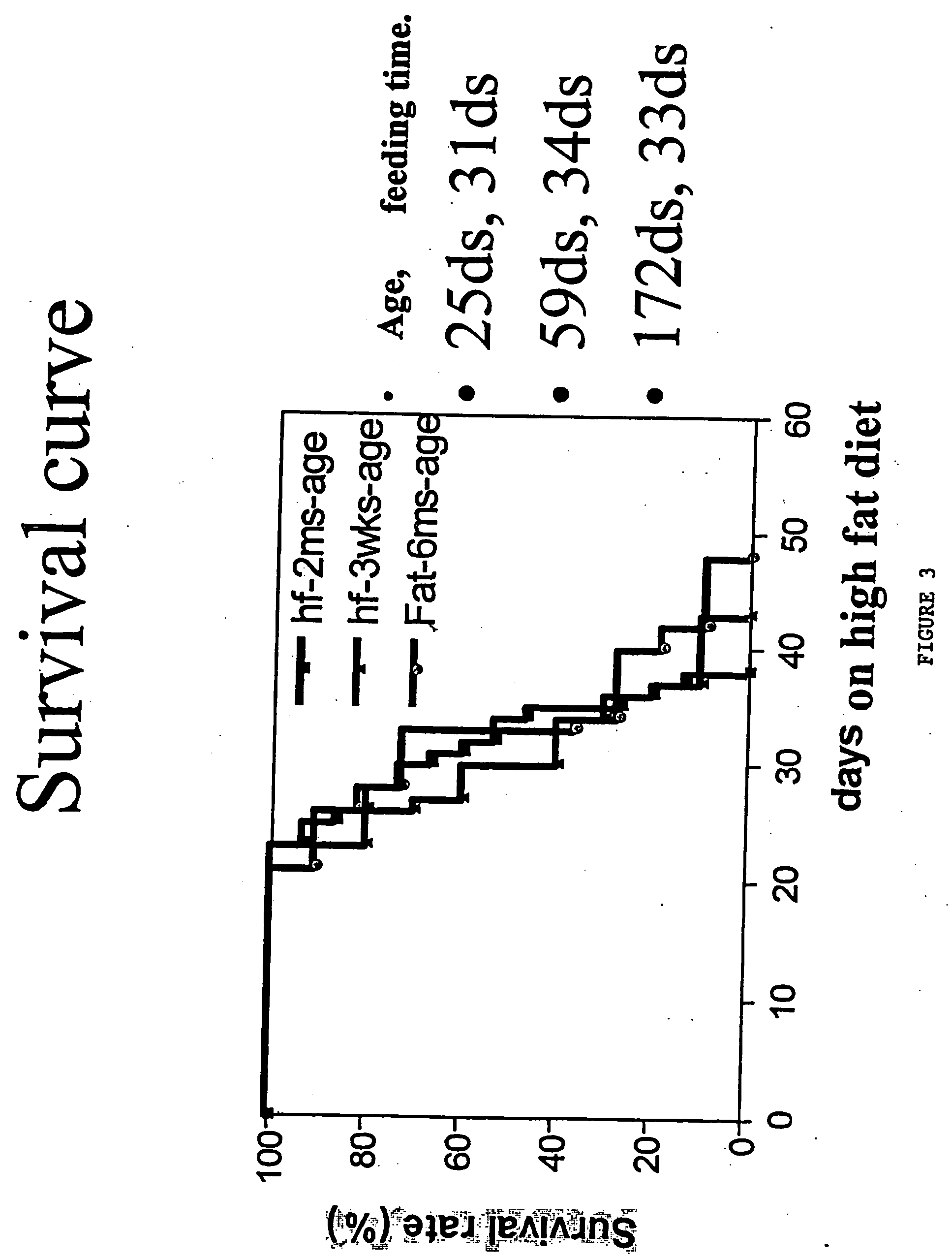
Inducible heart attack animal model
An animal model of coronary heart disease has been developed where myocardial infarct can be induced by altering the animal's diet. In all embodiments, this animal model is a result of reduced activity of scavenger receptor class BI (SR-BI) and apolipoprotein E (ApoE). In a preferred embodiment, the model is a result of crossbreeding two transgenic mouse lines: a knockout of SR-BI (SR-BI− / −) and an impaired ApoE expressor (hypoE). The impaired ApoE gene results in only 2-5% expression of ApoE and a reduction in cholesterol homeostasis. Resulting animals are predisposed to hypercholesterolemia but can live longer than a year on a normal low fat diet. Serum plasma levels can be significantly elevated by changing the animal's diet to one containing high levels of fat and cholesterol. Within a month on a high fat, high cholesterol diet, animals develop atherosclerosis and myocardial infarction occurs. Survival depends on the nature of the diet and the conditions of animal husbandry and can typically be around 20-30 days after administration of the modified diet depending on the specific conditions. Housing the animals alone or in groups significantly affects survival of these animals on a high fat diet. Analysis of B- and T-cell deficient SR-BI / ApoE / RAG2 triple knockout mice established that B- and T-lymphocytes do not play a key role in the pathophysiology of the SR-BI ApoE dKO model of human disease. These animal models can be used to study mechanisms and progression of CHD as a function of diet, treatment with drugs to be screened for efficacy or undesirable side effects, and social environmental effects.
Owner:MASSACHUSETTS INST OF TECH
Detection kit for apolipoprotein E and preparation method thereof
InactiveCN102818900AImprove stabilityImprove accuracyBiological testingApolipoprotein e4Latex particle
A detection kit for apolipoprotein E. The kit body comprises: a reagent R1 containing a buffer solution, polyethylene glycol, sodium azide, sodium EDTA and bovine serum albumin; and a reagent R2 containing a buffer solution, latex particles with apolipoprotein E monoclonal antibodies, Twain-20, sodium azide, sodium EDTA and bovine serum albumin. A sample and the reagents are mixed in a certain volume ratio to perform a series of reactions; and then the reactants are placed in a half / full automatic biochemical analyzer for detection of absorbency change speed at a dominant wavelength of 340 nm, so as to calculate the concentration of apolipoprotein E. The kit has the advantages of accuracy, stability and convenience.
Owner:乐普(北京)诊断技术股份有限公司
Quality Control Substance for Blood Lipid Test
This application discloses a quality control substance for a blood lipid test. Specifically, a disclosed composition comprises serum matrix, protective agent, preservative, total cholesterol, triglyceride, high density lipoprotein cholesterol, low density lipoprotein cholesterol, apolipoprotein A1, apolipoprotein B, lipoprotein (a), optional apolipoprotein E, optional phospholipid, optional homocysteine, optional C-reactive protein, and optional small and low density lipoprotein cholesterol. The application also relates to the use of this combination in the preparation of quality control substances. The quality control substance according to this application has excellent uniformity in and between bottles, while maintaining good stability.
Owner:BEIJING STRONG BIOTECH INC
Antibody Specific for Apolipoprotein and Methods of Use Thereof
The present disclosure provides synthetic antibodies specific for an epitope present on an apolipoprotein E polypeptide. The antibodies are useful in various treatment, diagnostic, and monitoring applications, which are also provided.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Methods to treat alzheimer's disease using apoe inhibitors
InactiveUS20150337030A1Avoid defectsTreating and preventing and delaying onsetNervous disorderPolymorphism usesAntibody inhibitorLate onset
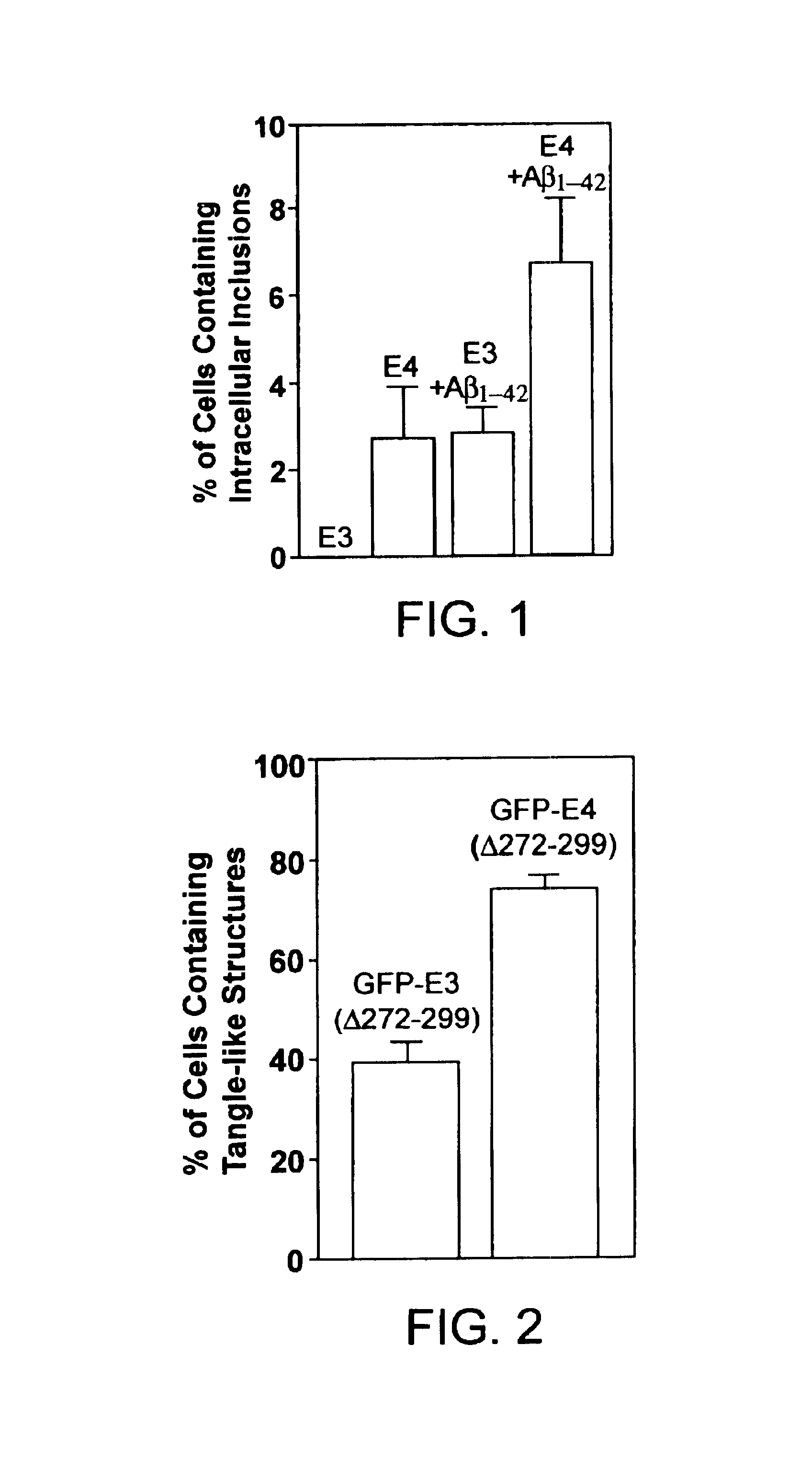
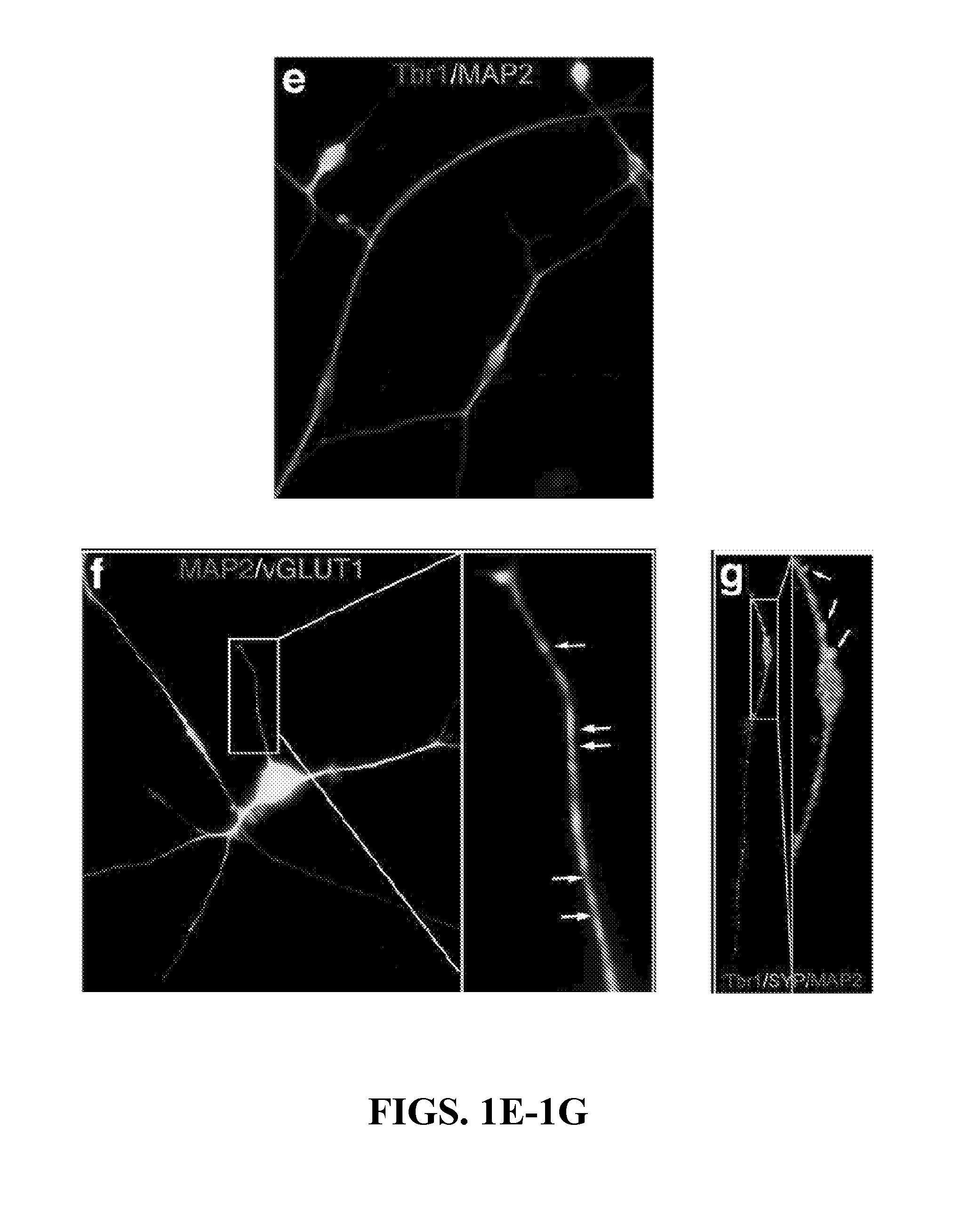
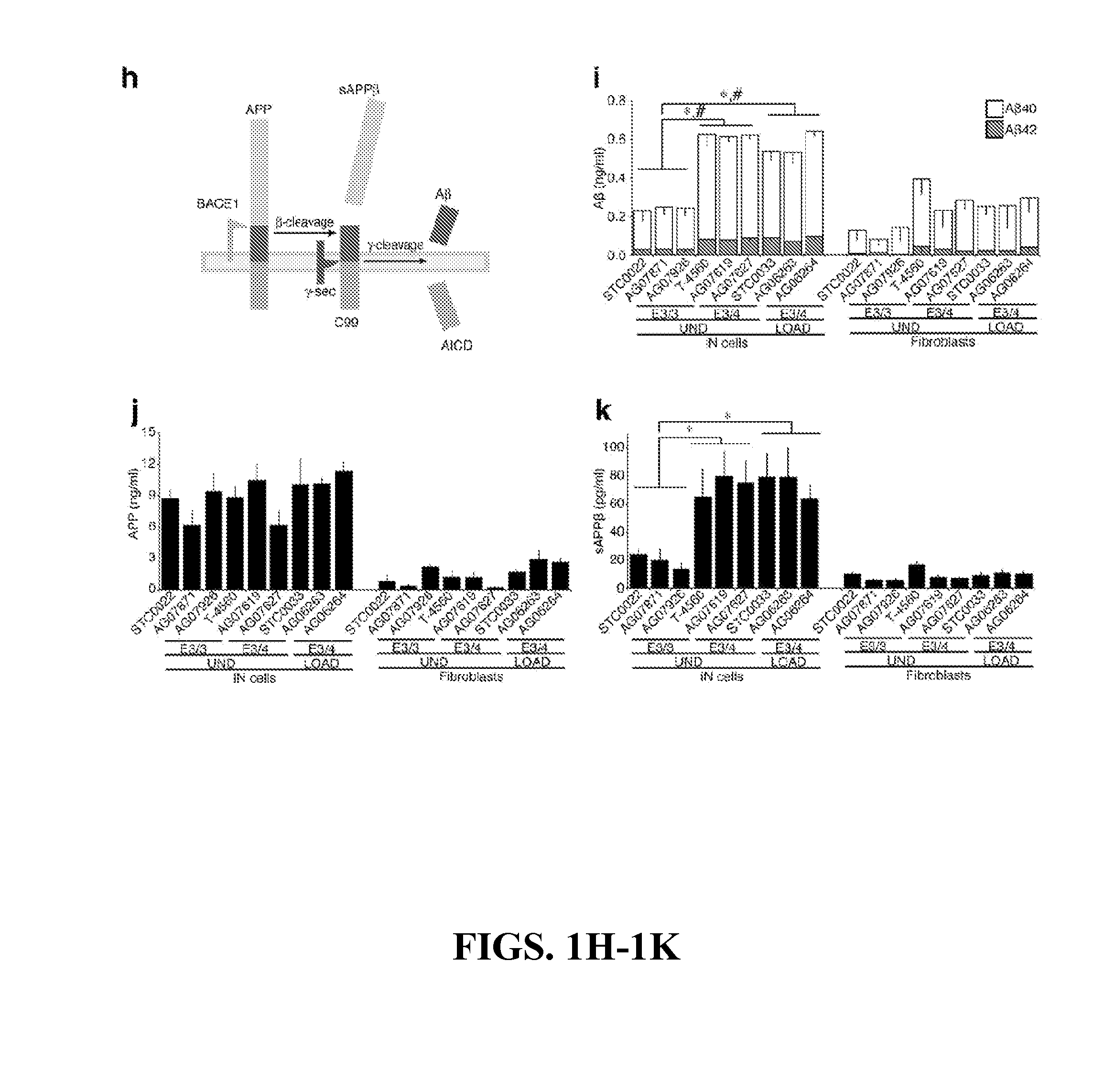
Non-familial late-onset Alzheimer's disease (LOAD), a condition associated with the accumulation of the amyloid precursor protein-derived (APP) Abeta fragment in the brain, can be the consequence of combined genetic and environmental risk factors. One of these risk factors is the presence of the apolipoprotein E4 (APOE4) allele. This invention provides for a neuron model that can be used to screen and identify compounds that can prevent the APOE4-induced pre-LOAD state. This invention provides for methods for the treatment and / or prevention of a neurodegenerative disorder by using an inhibitor of APOE4, such as an antibody inhibitor, or by using an excess of APOE3 protein.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Synthetic apolipoprotein e mimicking polypeptides and methods of use
InactiveUS20120245101A1Antibacterial agentsSenses disorderVery low-density lipoproteinAmphipathic helix
The present invention provides novel synthetic apolipoprotein E (ApoE)-mimicking peptides wherein the receptor binding domain of apolipoprotein E is covalently linked to 18A, the well characterized lipid-associating model class A amphipathic helical peptide, or a modified version thereof. Such peptides enhance low density lipoprotein (LDL) and very low density lipoprotein (VLDL) binding to and degradation by fibroblast or HepG2 cells. Also provided are possible applications of the synthetic peptides in lowering human plasma LDL / VLDL cholesterol levels, thus inhibiting atherosclerosis. The present invention also relates to synthetic peptides that can improve HDL function and / or exert anti-inflammatory properties.
Owner:UAB RES FOUND
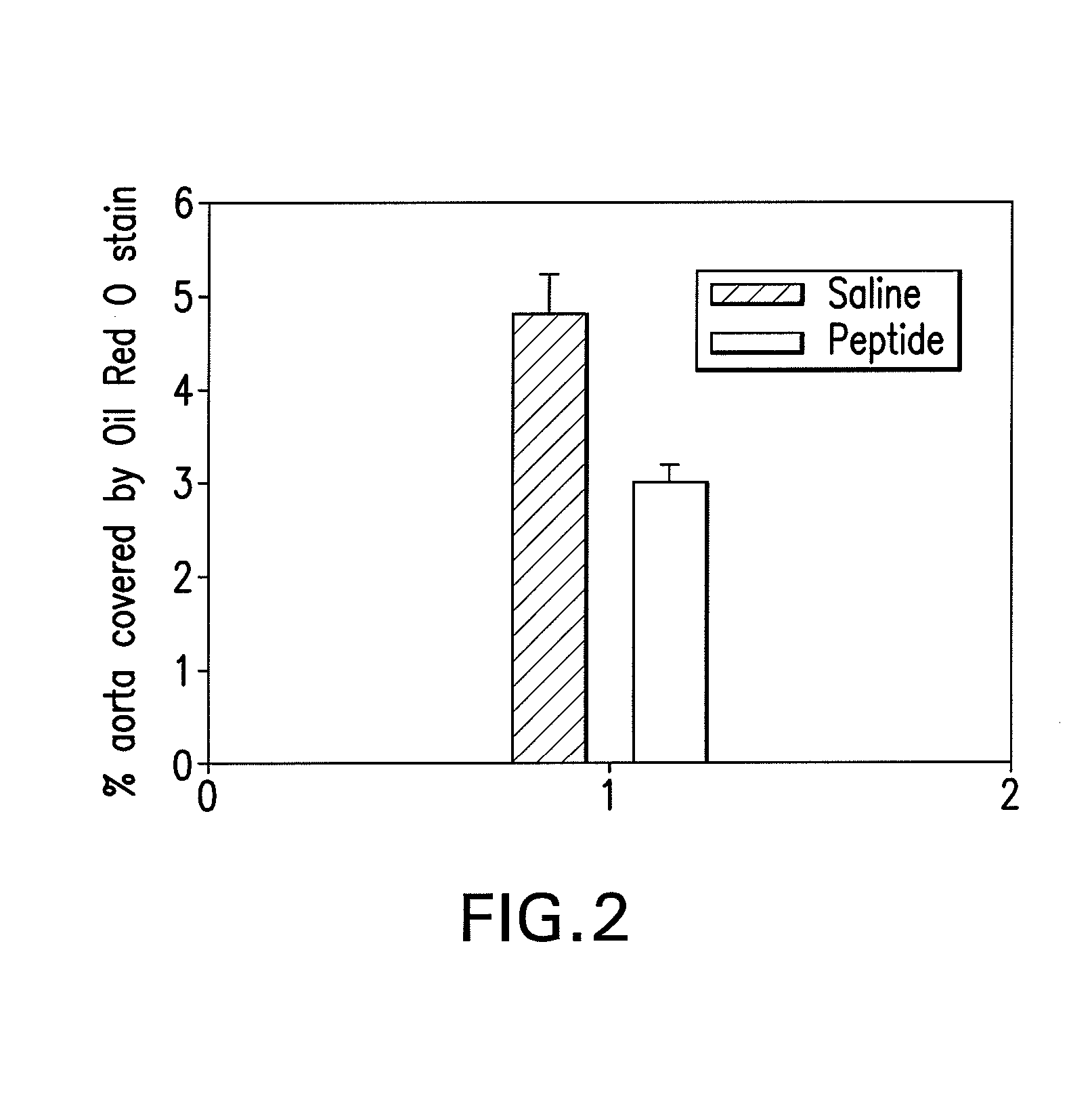
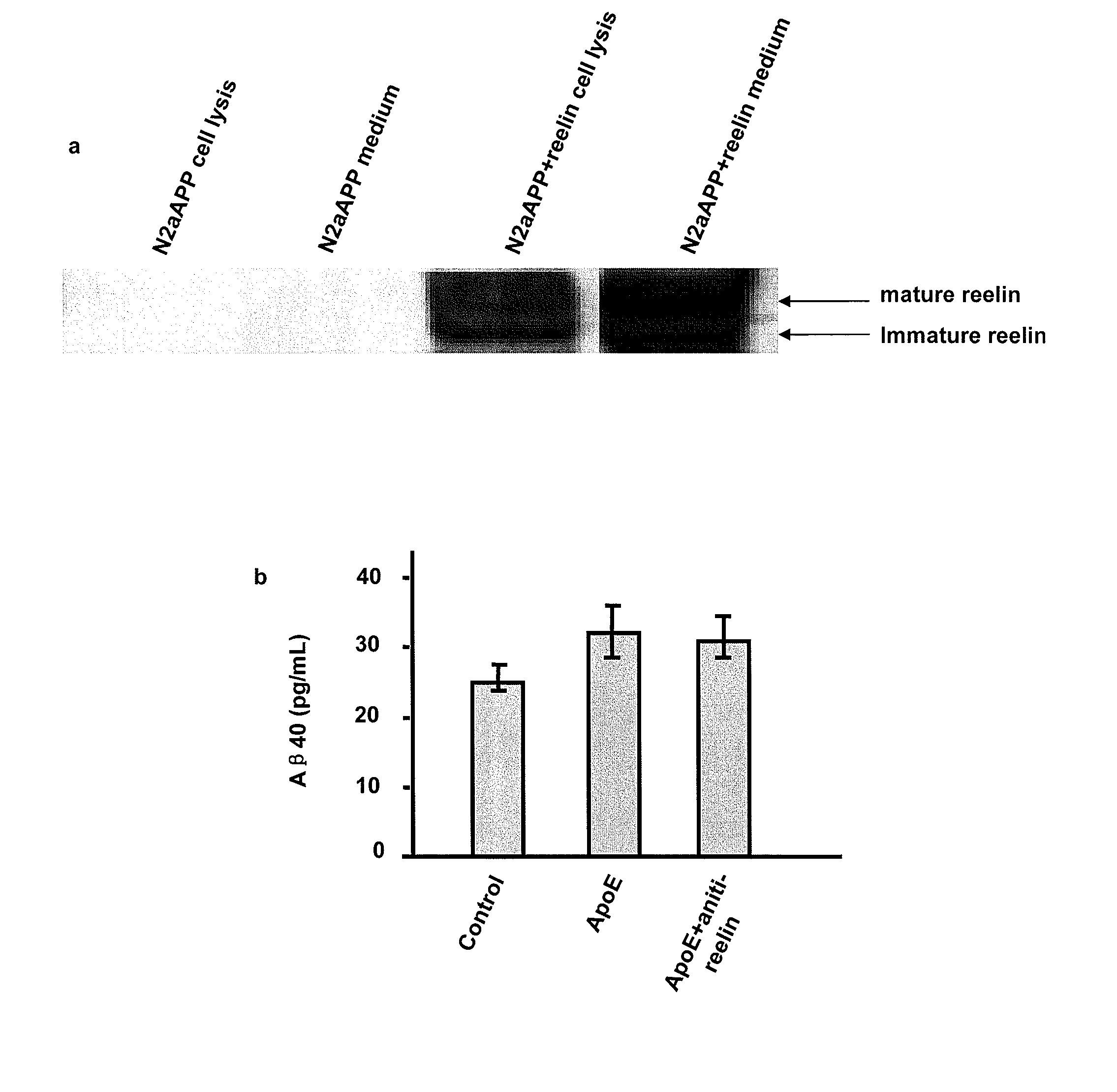
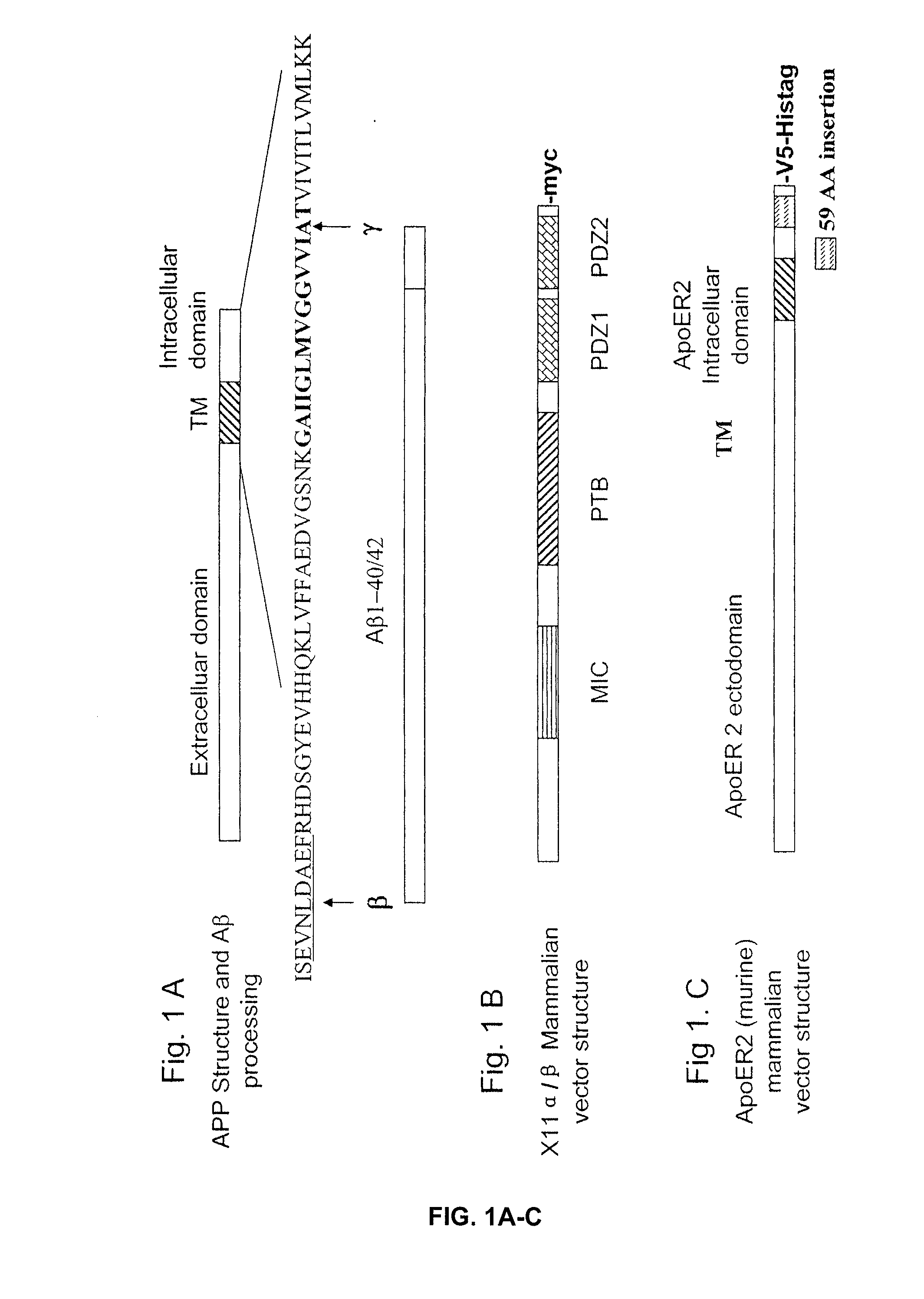
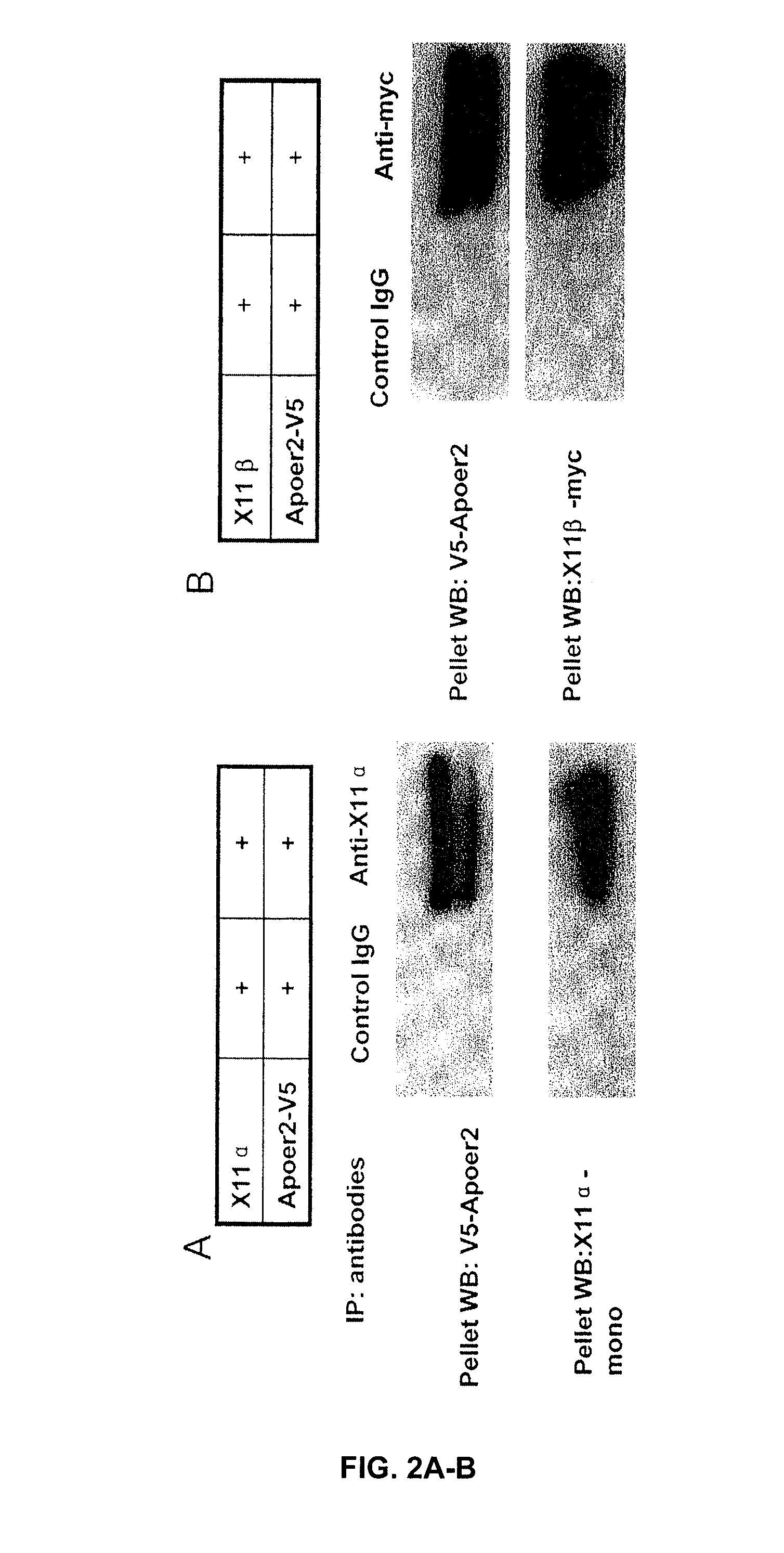
Treatment of alzheimer's disease with inhibitors of apoe binding to apoe receptor
InactiveUS20070248599A1Decrease Aβ productionInhibit bindingOrganic active ingredientsNervous disorderApolipoprotein e4Β protein
Alzheimer's disease (AD) is characterized by the accumulation of amyloid-β peptide (Aβ) in the brain. Aβ is derived from amyloid precursor protein (APP) by β- and γ-secretases. Apolipoprotein E receptor 2 (ApoER2) is a cell-surface receptor for apolipoprotein E. This study shows that ApoER2 interacts with X11α / β proteins and that APP forms association with ApoER2 in the presence of X11s. Significantly, ApoE stimulates the production of Aβ, and ApoE4 produced more Aβ than ApoE2 or ApoE3, correlating with previous studies showing that individuals with the ApoE4 polymorphism were more prone to development of AD. Thus, ApoE binding to ApoER2 on cell surface stimulates the generation of Aβ from APP. Antagonists that interfere with the ApoE-ApoER2 interaction are proposed for the treatment of AD.
Owner:OKLAHOMA MEDICAL RES FOUND
Early prediction marker for gestational diabetes mellitus and detection method thereof
InactiveCN106706928AImprove forecast accuracyEasy to operateBiological testingNeonatal diabetesProteomic Profile
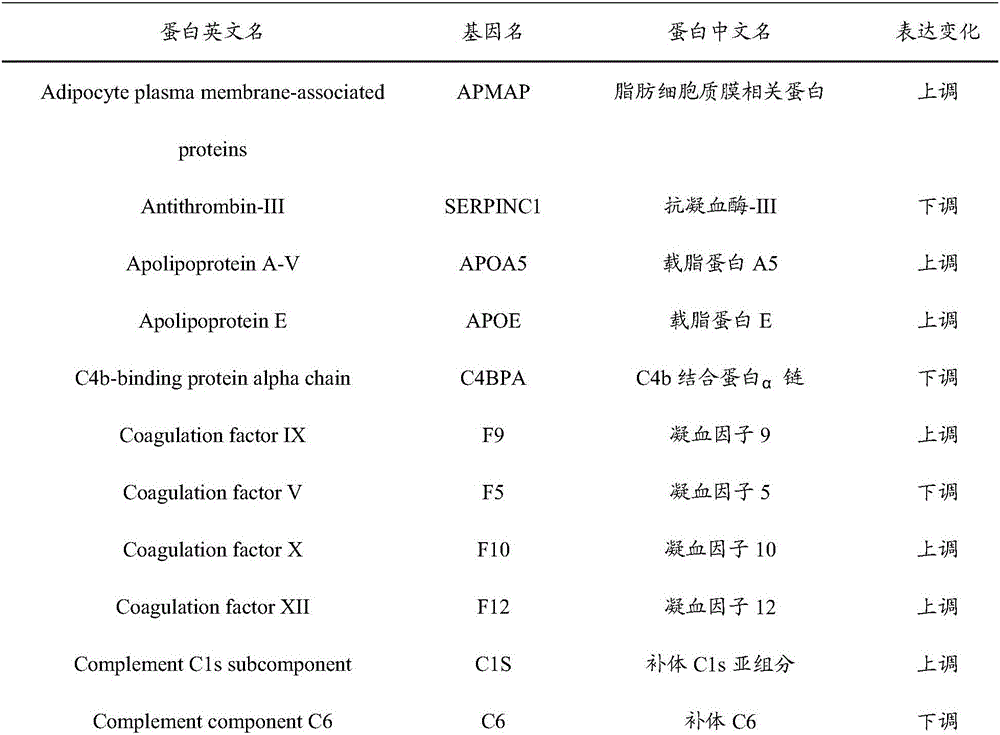
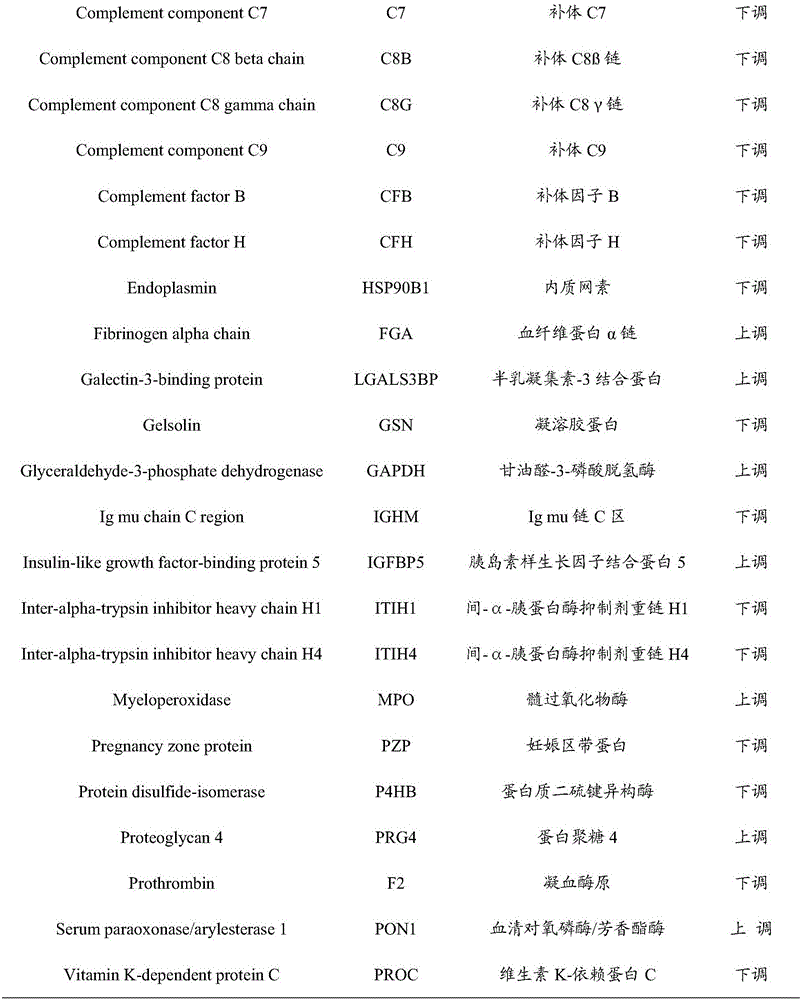
The invention provides an early prediction marker for gestational diabetes mellitus and a detection method thereof. The marker is serum differential protein which comprises 33 proteins including fat plasma membrane related protein, antithrombin-III, apolipoprotein A5, apolipoprotein E and the like; the concentration change of the serum differential protein is detected by one or more of a high-throughput proteomics method, an enzyme linked immunosorbent assay technology and a protein chip technology so as to predict whether a to-be-detected object in the 12th-16th week of pregnancy is to suffer from gestational diabetes mellitus in the 24th-28th weeks of pregnancy; the prediction accuracy of the marker is high; and the detection method is simple to operate and can assist in early diagnosis of potential patients suffering from gestational diabetes mellitus so as to avoid adverse impacts of the disease on the health of patients and fetuses thereof.
Owner:SHENZHEN UNIV
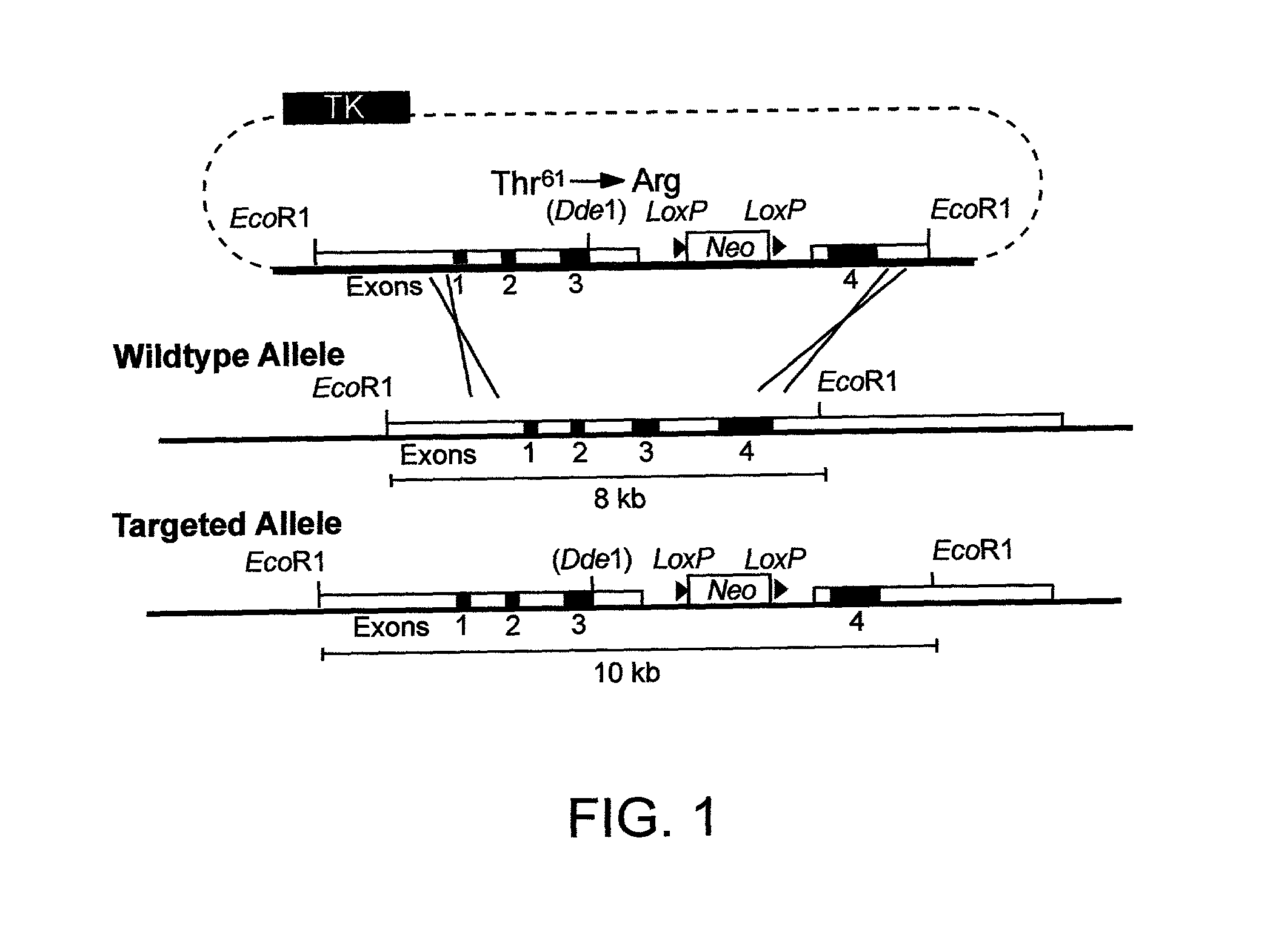
Gene-targeted animal model of apolipoprotein E4 domain interaction and uses thereof
InactiveUS20020194628A1Lowering of total serum cholesterol levelGood conditionCompound screeningApoptosis detectionGene targetsApolipoprotein e4
The invention provides gene-targeted non-human animals comprising a genetically modified apoE gene encodes a recombinant apoE polypeptide displaying domain interaction. The invention further provides cells isolated from the gene-targeted animals, which cells produce a recombinant apoE polypeptide displaying domain interaction. The invention further provides methods of identifying agents that reduce apoE4 domain interaction, and which are useful to treat apoE4-related neurological and cardiovascular disorders.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Antibody specific for apolipoprotein and methods of use thereof
The present disclosure provides synthetic antibodies specific for an epitope present on an apolipoprotein E polypeptide. The antibodies are useful in various treatment, diagnostic, and monitoring applications, which are also provided.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
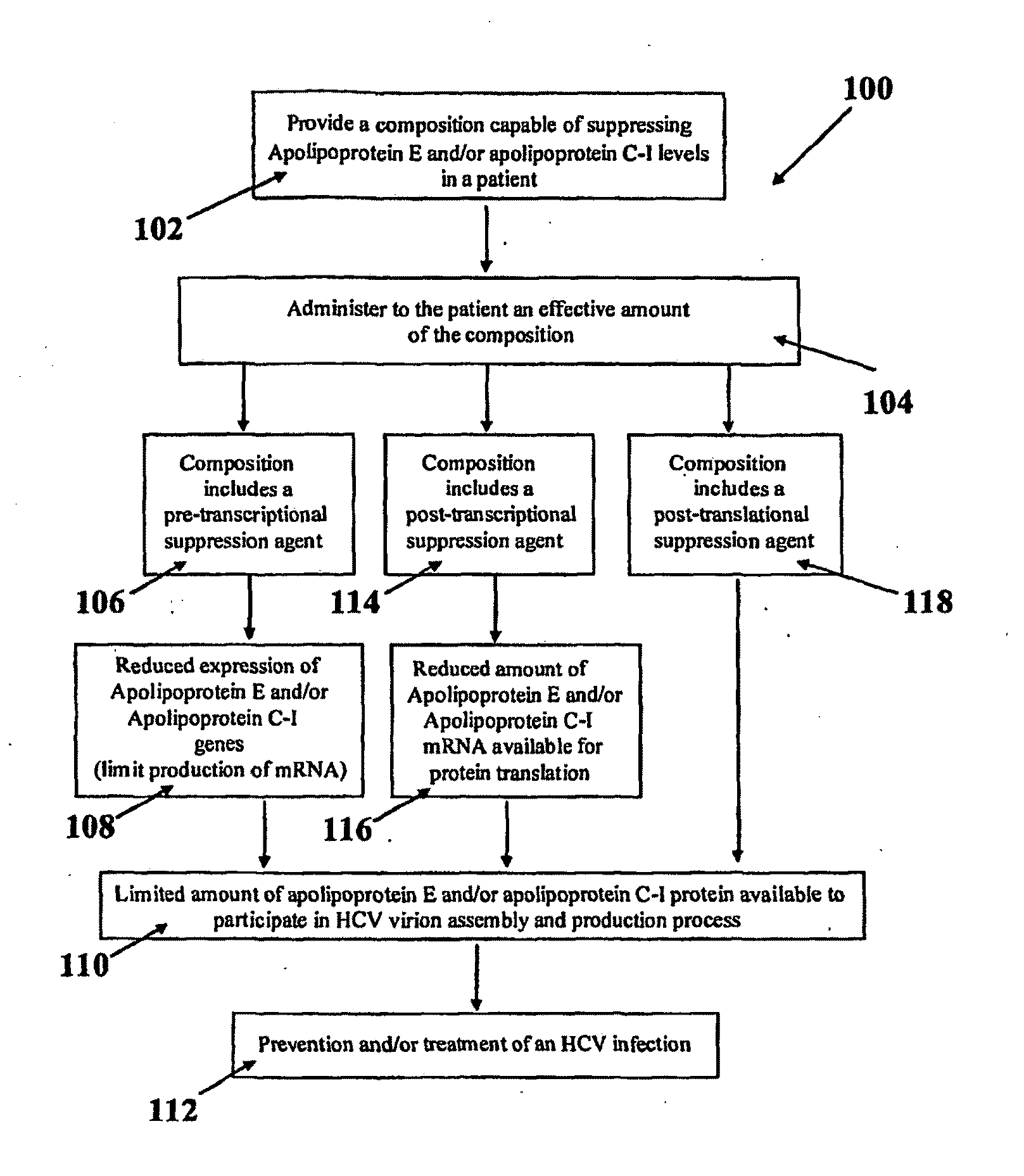
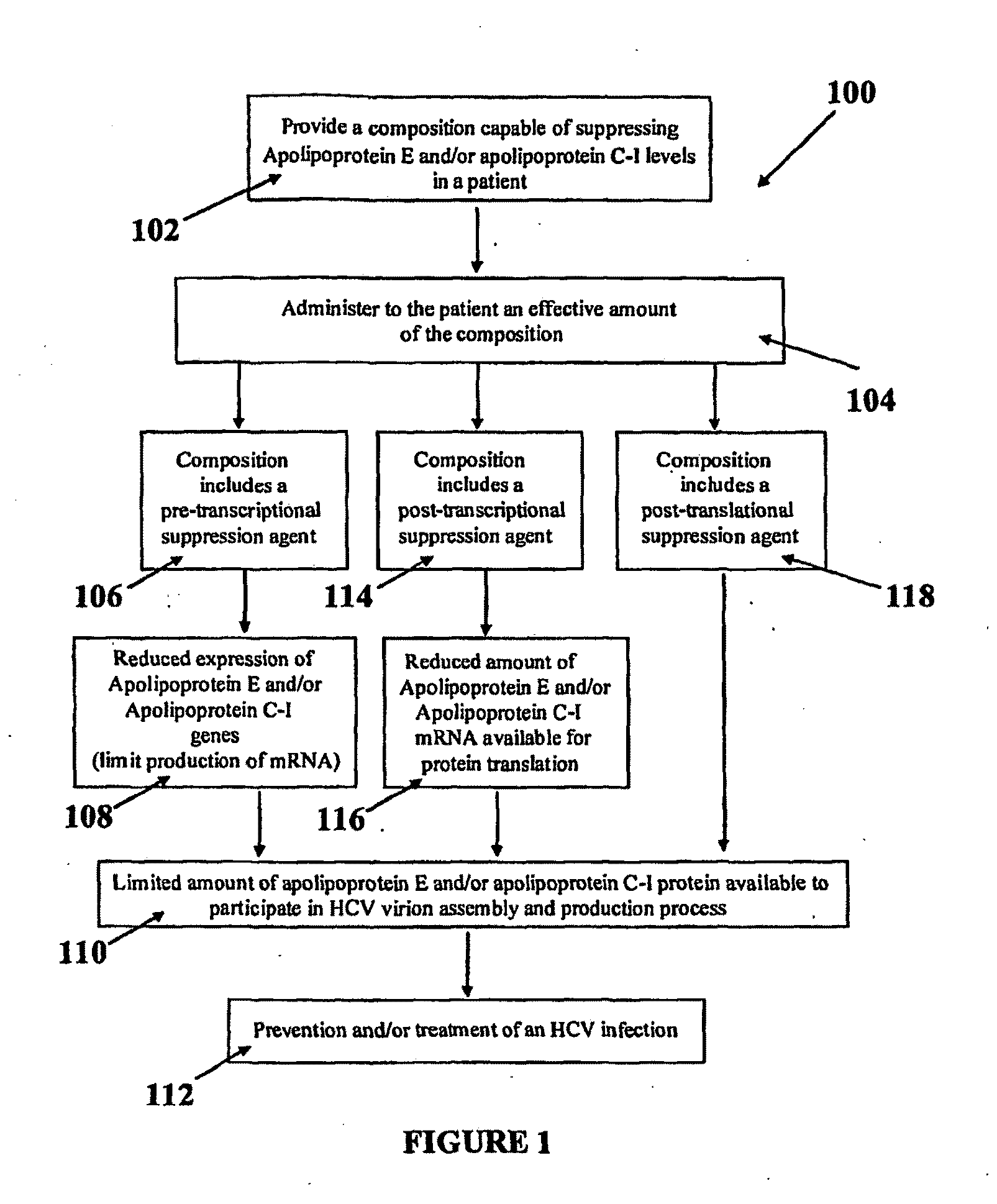
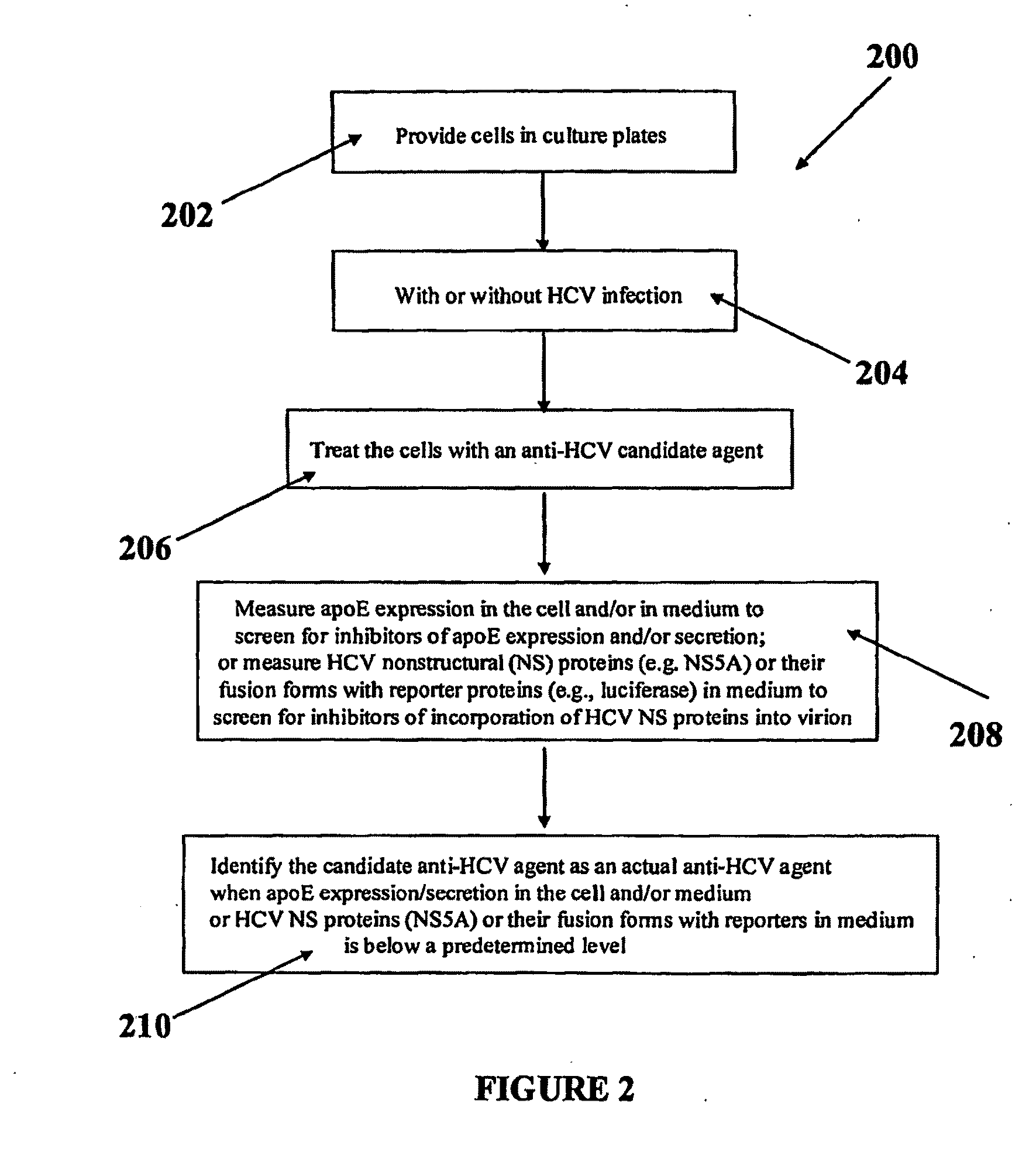
Compositions and Methods for Controlling Hepatitis C Virus Infection
Disclosed herein are methods and compositions for the treatment and prevention of Hepatitis C Virus (HCV) infection and methods of screening for antiviral agents against HCV infection and / or production. A method of using compositions of certain apolipoprotein-specific monoclonal or polyclonal antibodies to inhibit HCV infectivity is disclosed. Further, methods of using small interfering RNAs (siRNAs) specific to apolipoproteins for treating and / or preventing HCV infection are disclosed. Also disclosed are methods of using siRNAs specific and / or small molecule inhibitors to certain lipoprotein biosynthetic genes and of using recombinant apolipoprotein E and / or their forms of lipoproteins to treat and / or prevent HCV infections. Screening methods for anti-HCV agents include assessing the effect of a candidate agent on apolipoprotein E and / or apolipoprotein C-I gene expression, assembly, and / or secretion and assessing the effect of a candidate agent on the blockage of the interaction and / or incorporation of HCV nonstructural proteins and / or their fusion forms with reporter proteins into HCV virions.
Owner:LUO GUANGXIANG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com