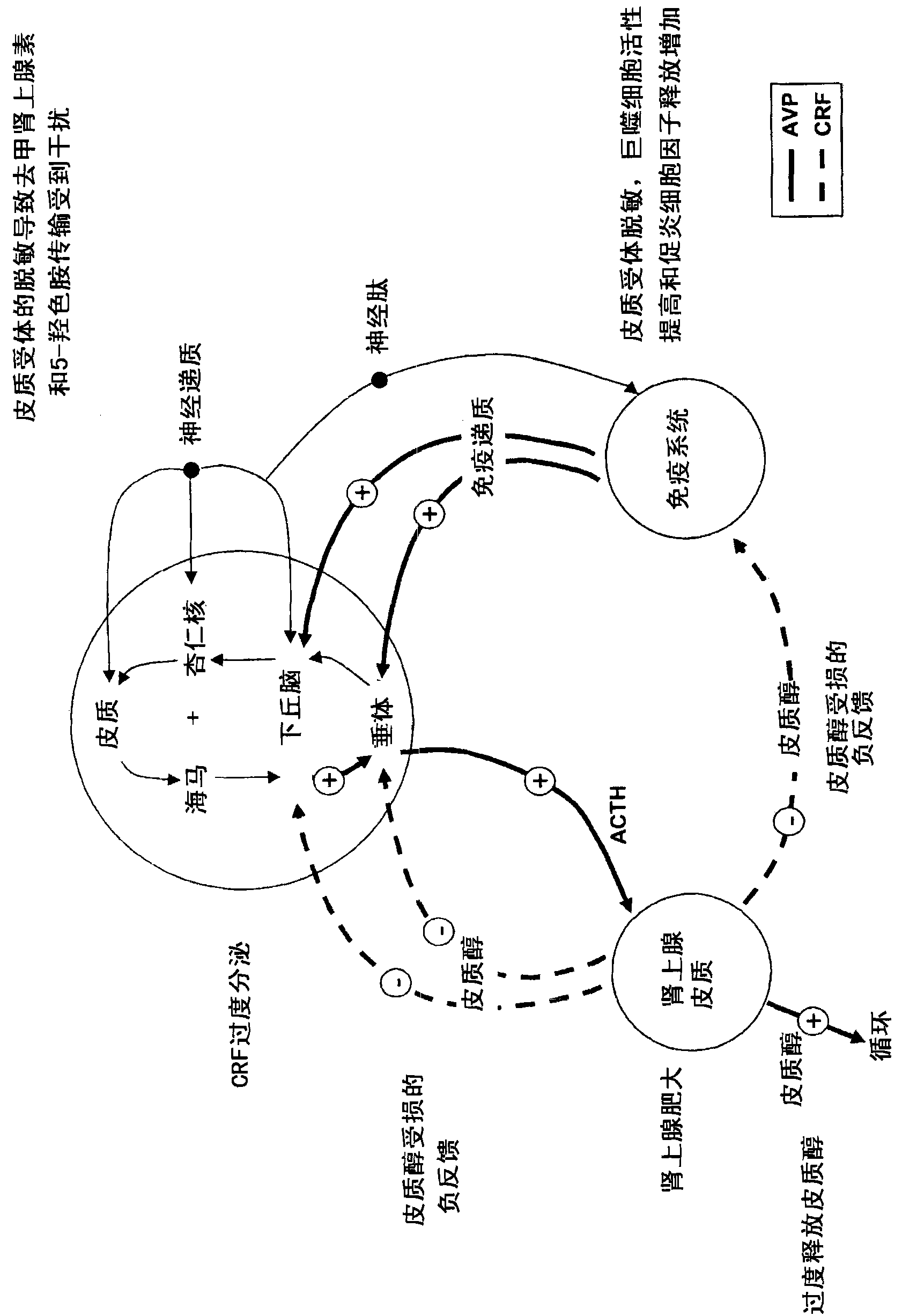
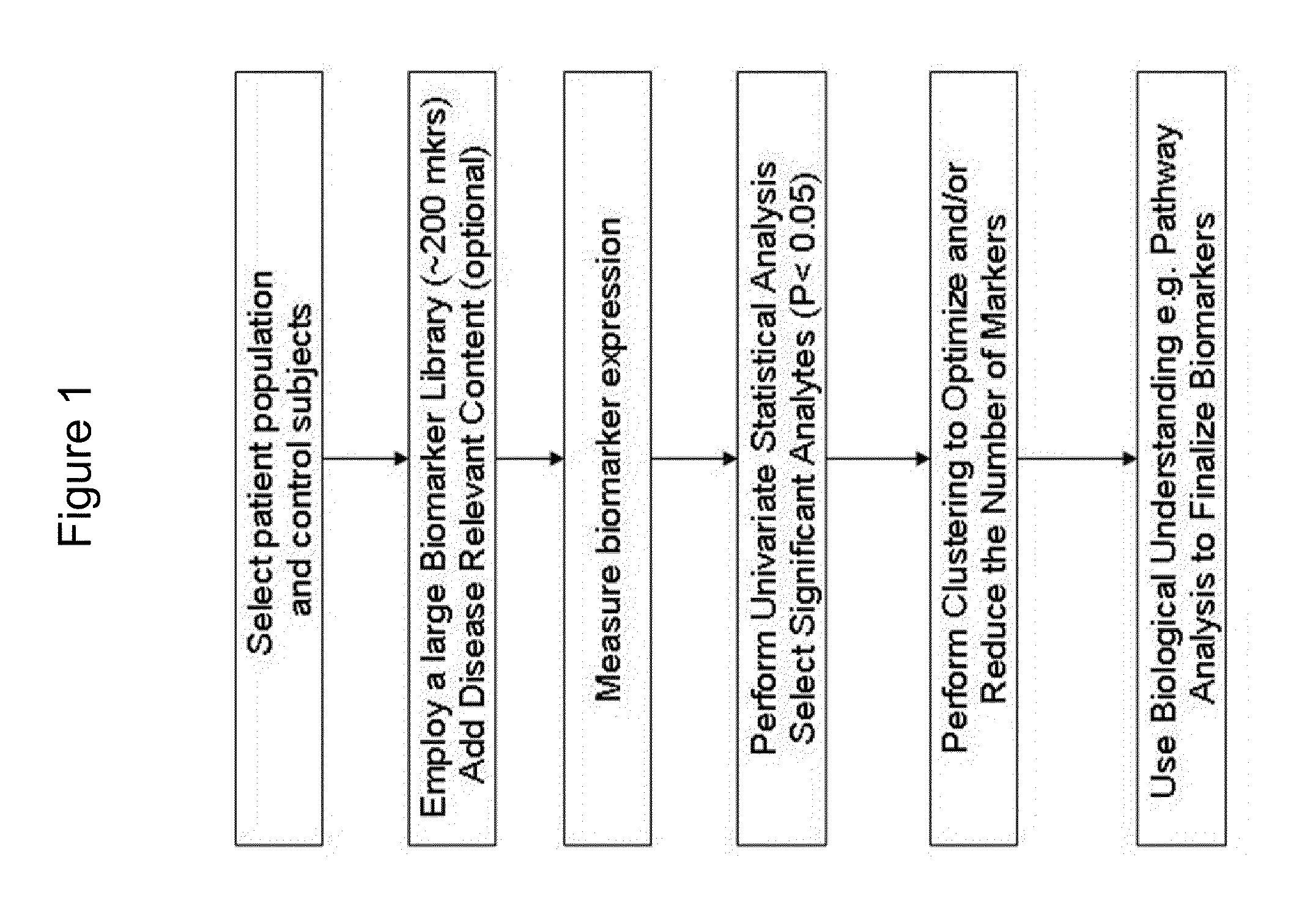
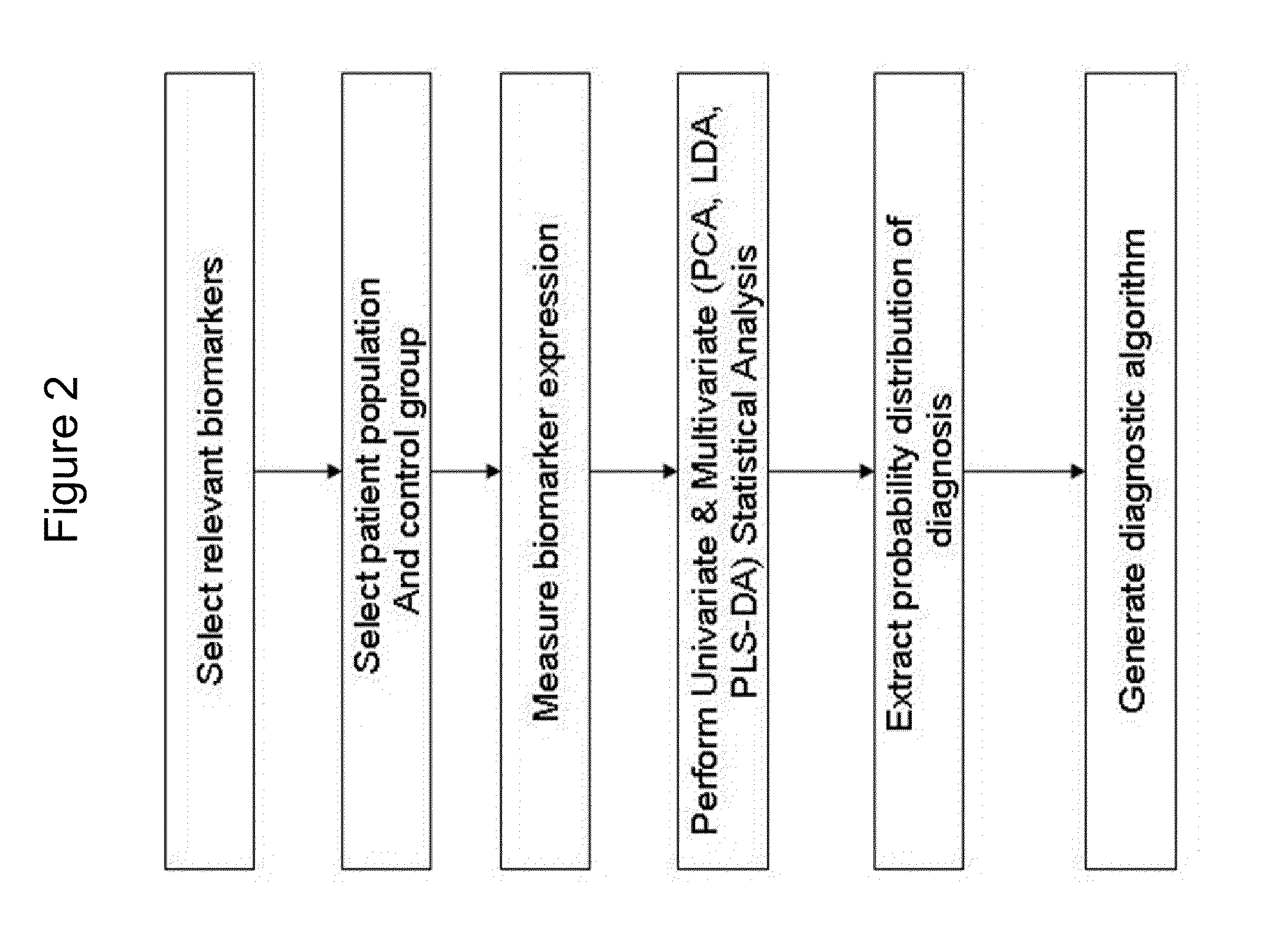
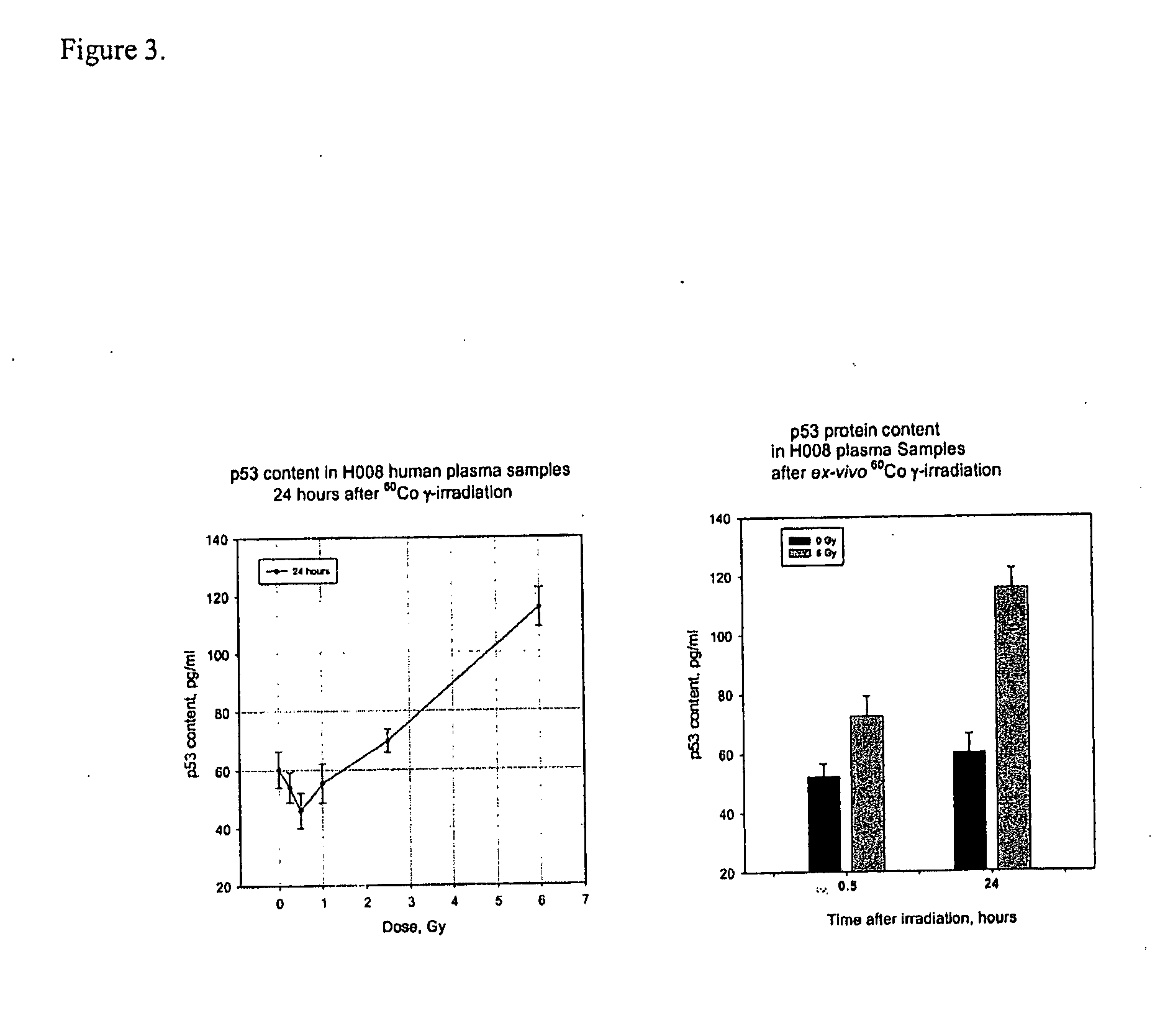
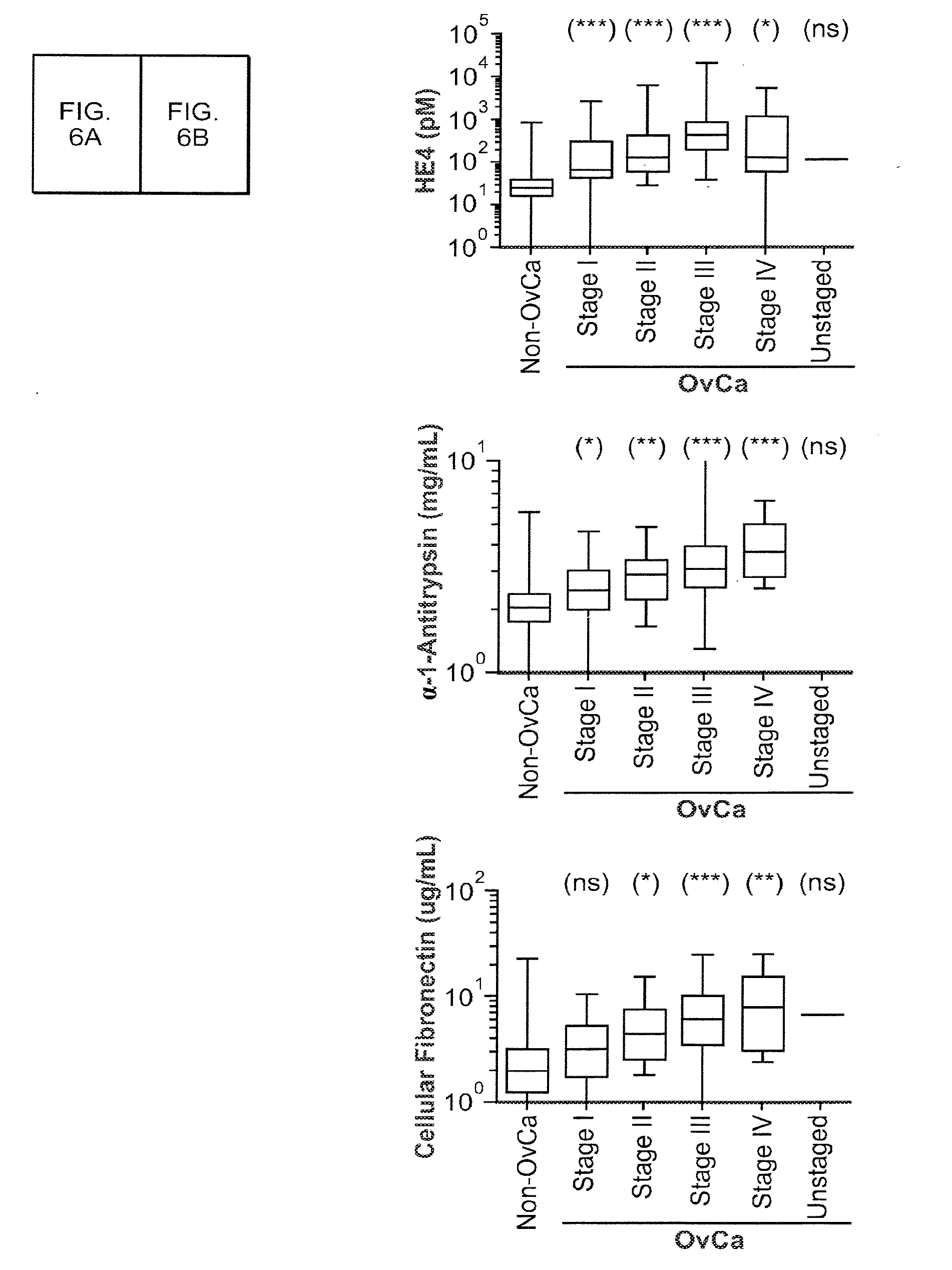
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
107 results about "Biomarker panel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
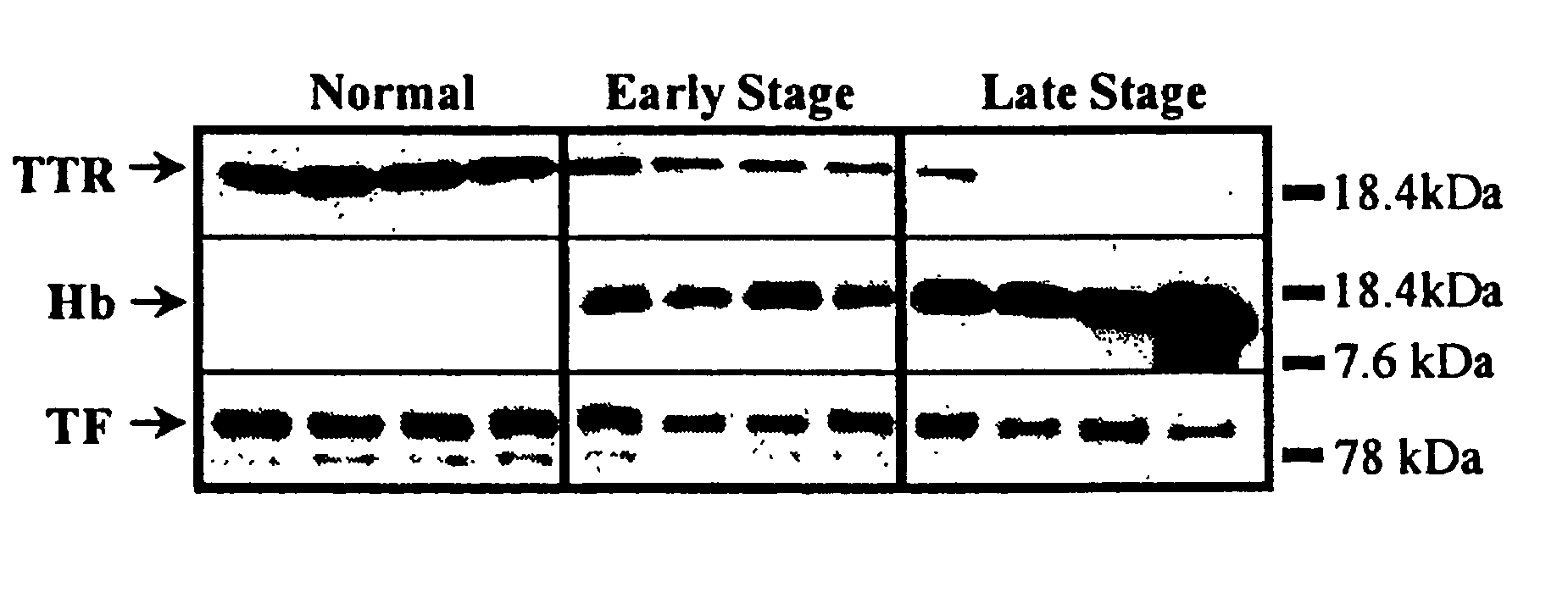
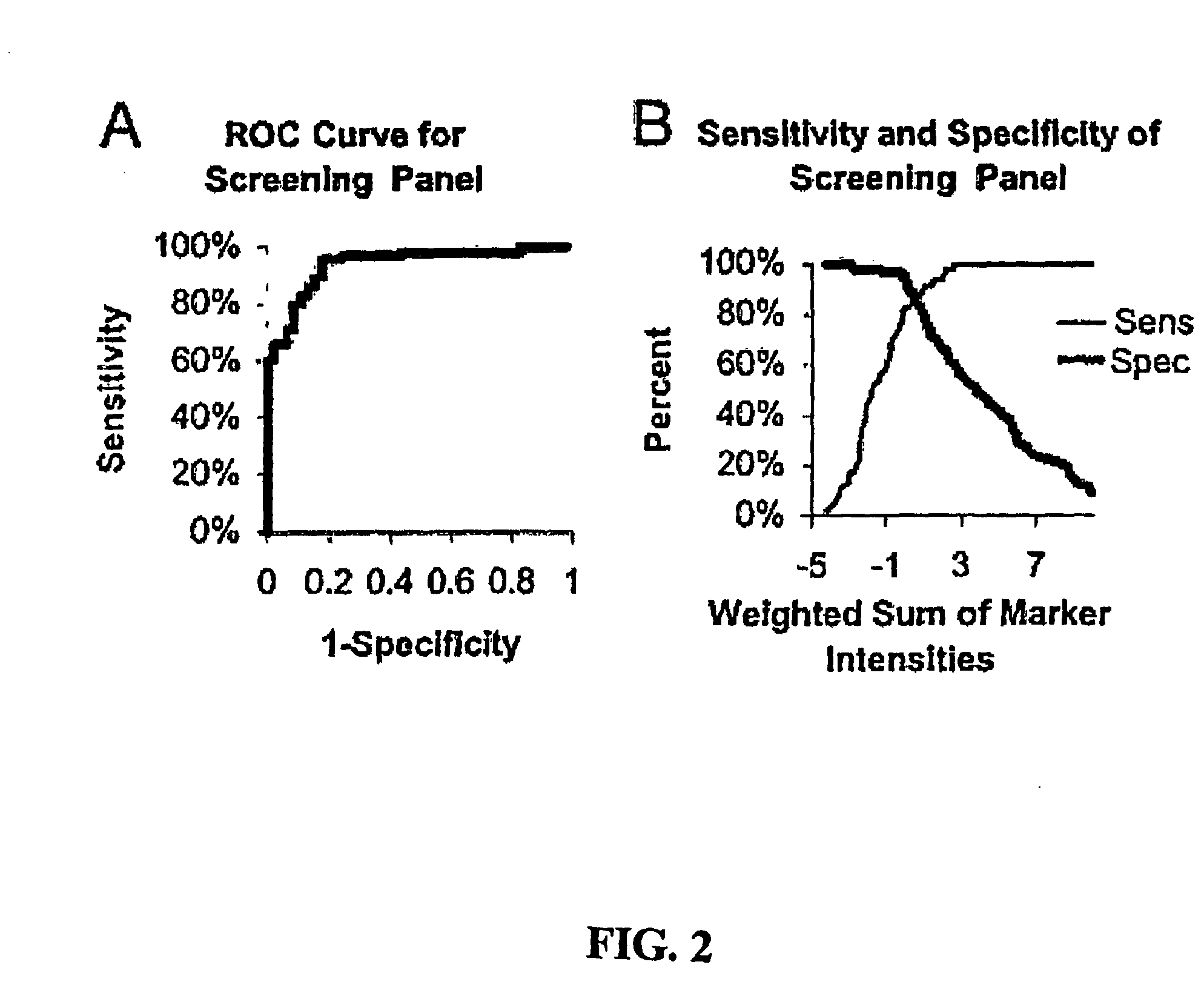
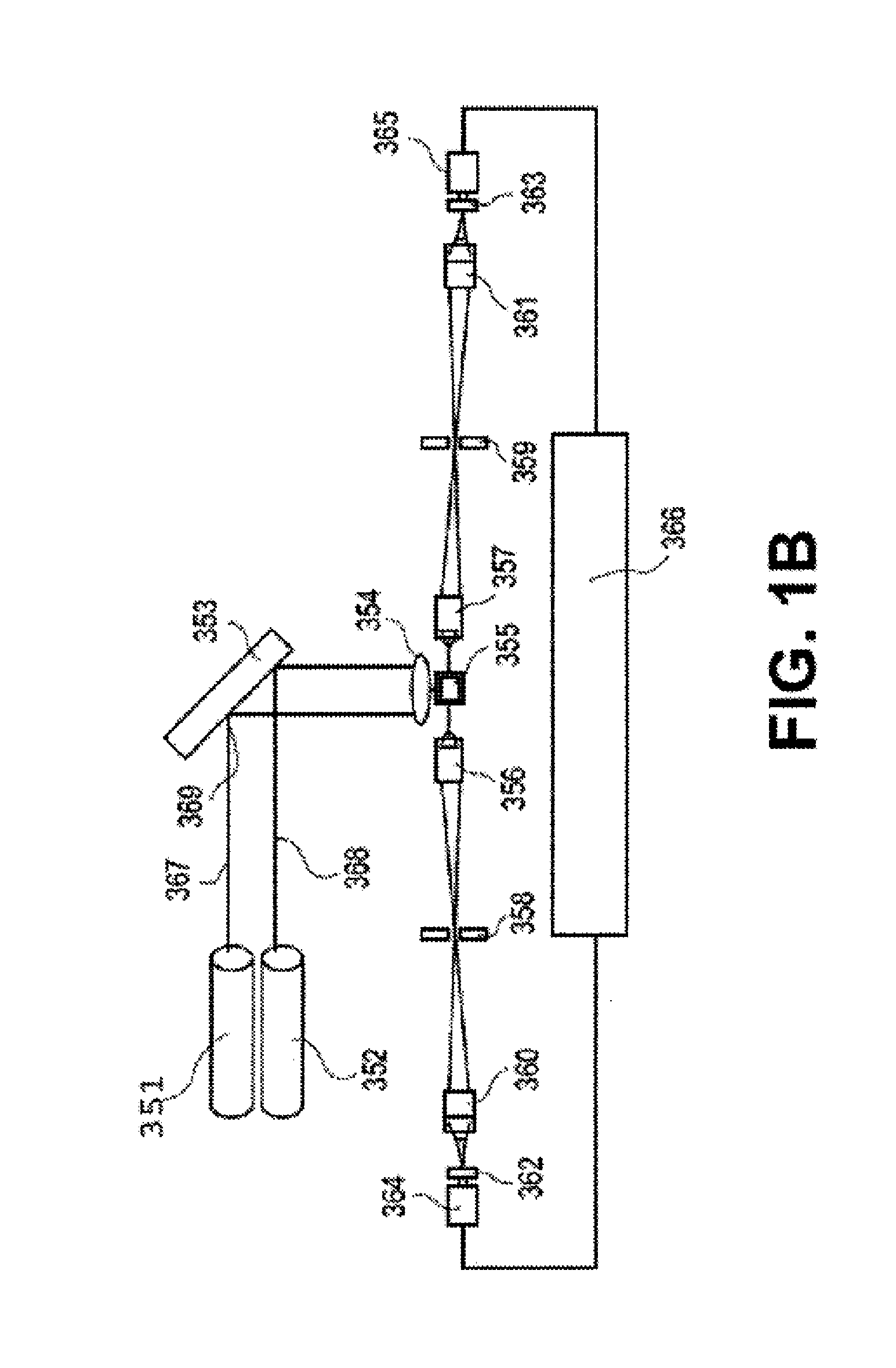
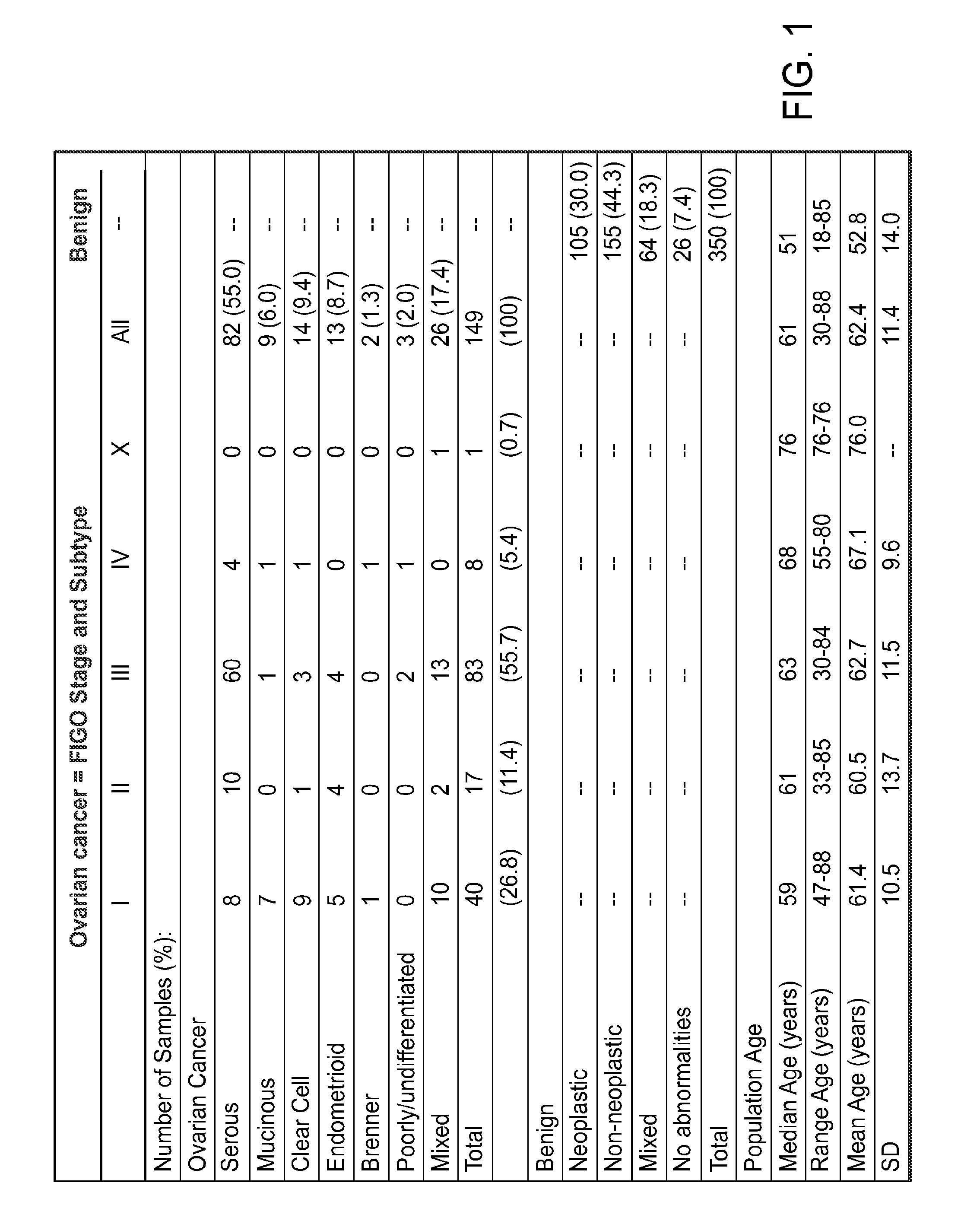
Biomarkers for Early Detection of Ovarian Cancer
Three panels of biomarker proteins that can be used in the diagnosis of early-stage ovarian cancer (OC) are described. The biomarker panels not only permit the distinction of patients with ovarian neoplasia (benign or malignant) from normal subjects, but they also allow the identification of patients with early-stage (stage I / II) ovarian cancer from those patients with benign ovarian tumors or normal individuals. The invention additionally provides methods for detecting and treating various cancers, including cancer of the ovary using OC-related molecules.
Owner:RGT UNIV OF CALIFORNIA
Highly Sensitive Biomarker Panels
InactiveUS20110003707A1Detection moreImprove the level ofLibrary screeningDisease diagnosisVascular inflammationBiomarker panel
Cardiovascular disease, e.g., congestive heart failure, is often first diagnosed after the onset of clinical symptoms, eliminating potential for early intervention. The invention provides a multi-marker immunoassay, including cardiac pathology and vascular inflammation biomarkers, yielding a more sensitive assay for early detection of CHF in plasma. A panel consisting of cardiac pathology (cTnI, BNP) and vascular inflammation (IL-6, TNFα, IL-17a) biomarkers provided a sensitivity of 94% for association with CHF.
Owner:SINGULEX
Modeling of systemic inflammatory response to infection
InactiveUS20070083333A1Microbiological testing/measurementBiostatisticsDisease outcomeBiomarker panel
Models for the systemic inflammatory response to infection, which involve the use of immunocompromised animals, and methods of using the models are described. These models can be used in identifying analytes or biomarker panels that can be used in staging or monitoring sepsis. The models can also be used for predicting an animal's disease outcome or in providing a prognosis for sepsis patients. Further, the invention relates to methods for evaluating potential treatments for sepsis.
Owner:JANSSEN PHARMA NV
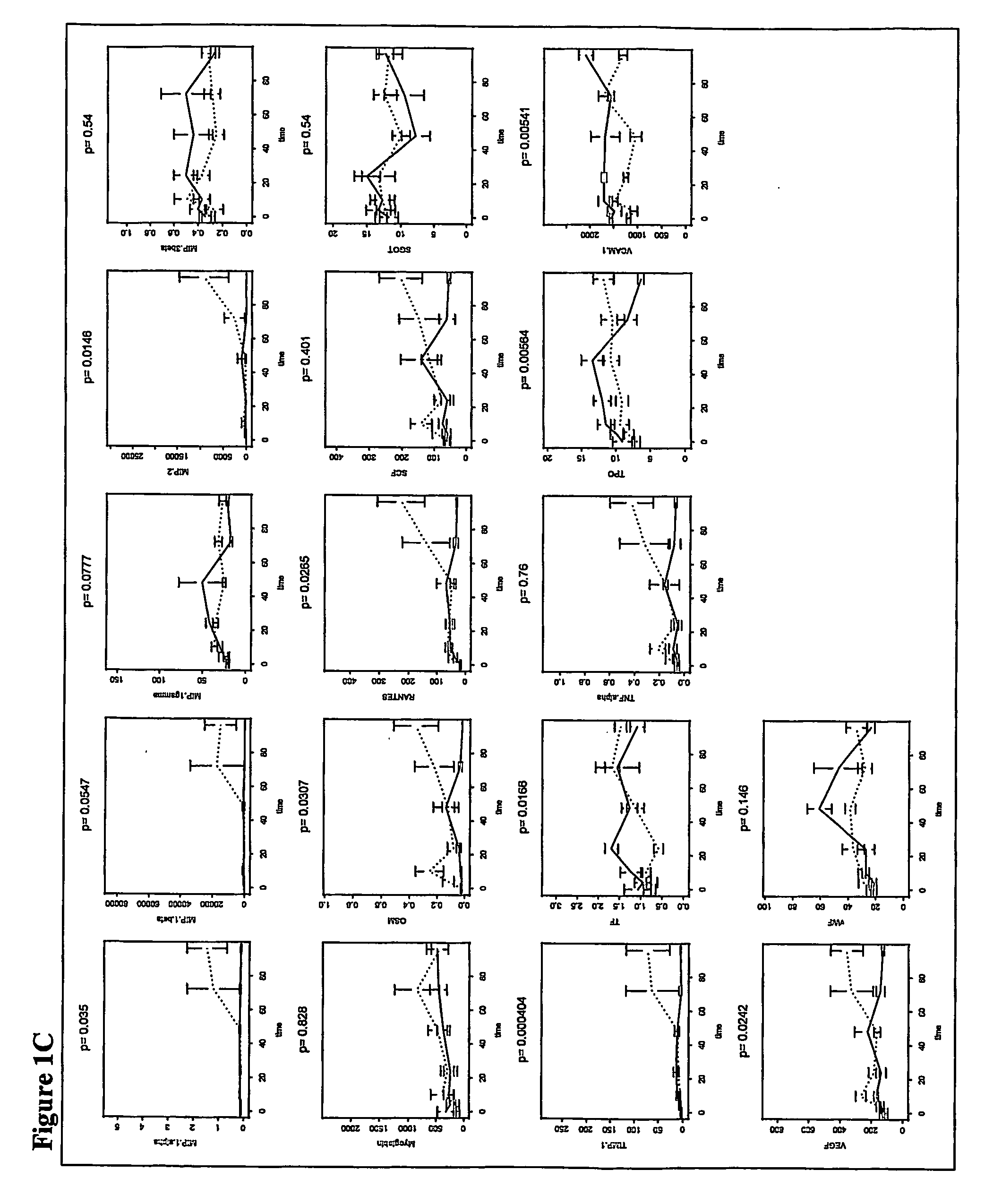
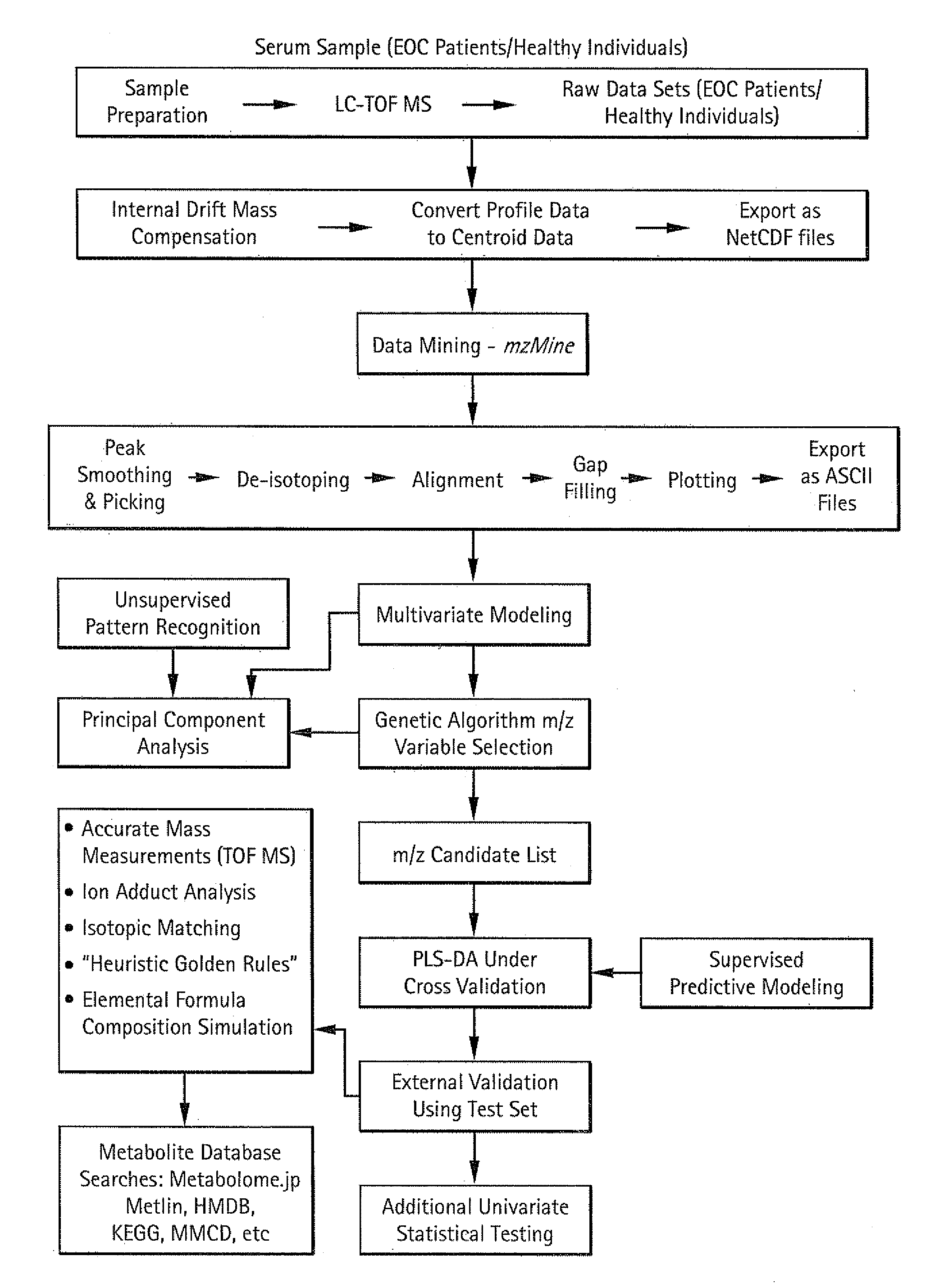
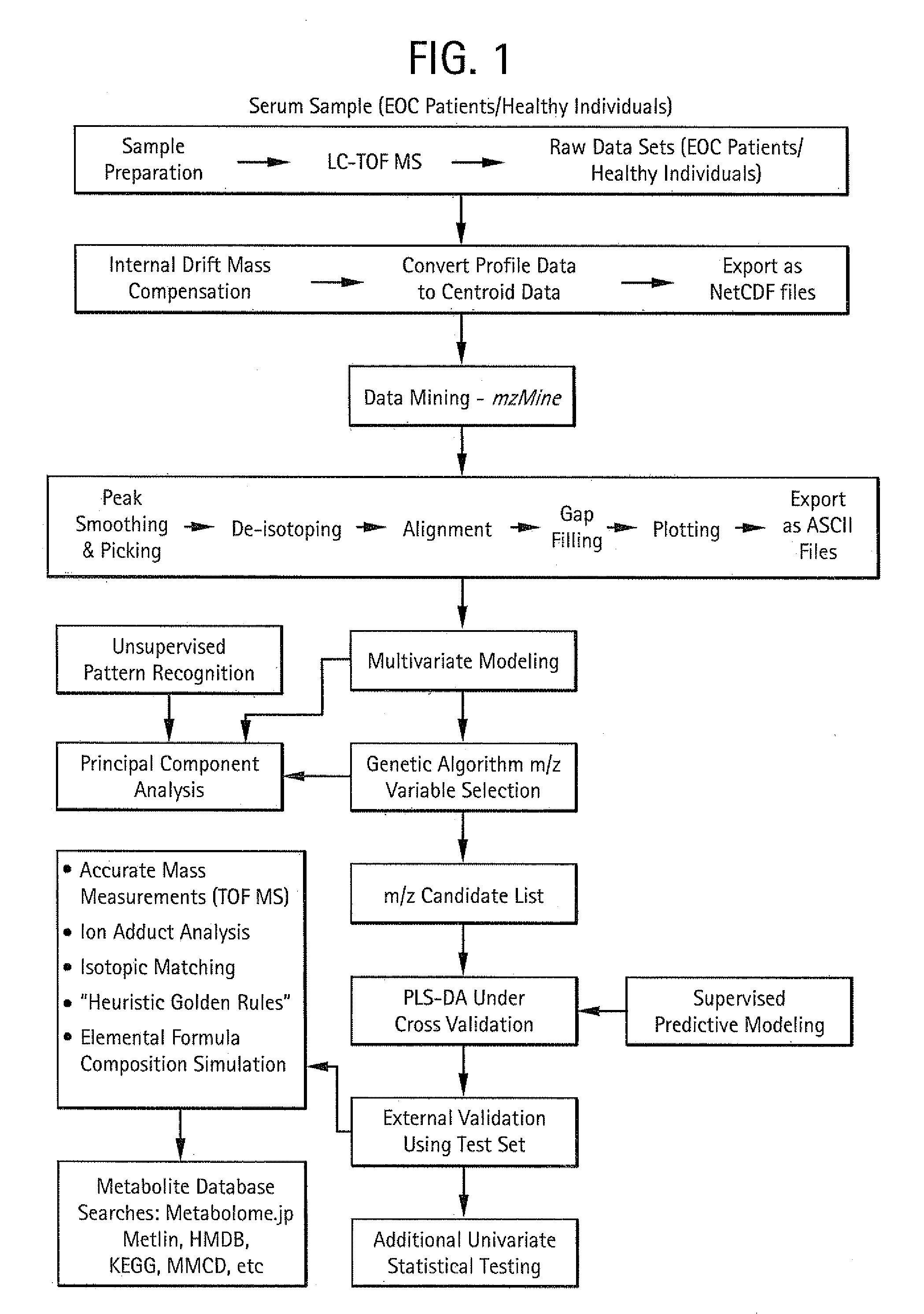
Metabolic biomarkers for ovarian cancer and methods of use thereof
InactiveUS20120004854A1Easy to classifyReduce in quantityBiostatisticsMedical automated diagnosisSerum igeSupport vector machine
Panels of serum metabolic biomarkers and methods of their use in detecting and diagnosing cancer, especially ovarian cancer, are disclosed. The metabolic biomarker panels include 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 50, 75, 100, 150, or more metabolites. Supervised classification methods, such as trained support vector machines (SVMs) are used to determine whether the levels of metabolic biomarkers in a subject are indicative of the presence of cancer. The disclosed biomarkers and methods preferably allow a diagnosis of cancer with an accuracy, a specificity, and / or a sensitivity of at least 80%, 85%, 90%, 95%, 96%, 97%, 98% or 99%.
Owner:GEORGIA TECH RES CORP
Highly sensitive biomarker panels
InactiveUS8450069B2Detection moreImprove the level ofLibrary screeningDisease diagnosisBiomarker panelVascular inflammation
Owner:SINGULEX
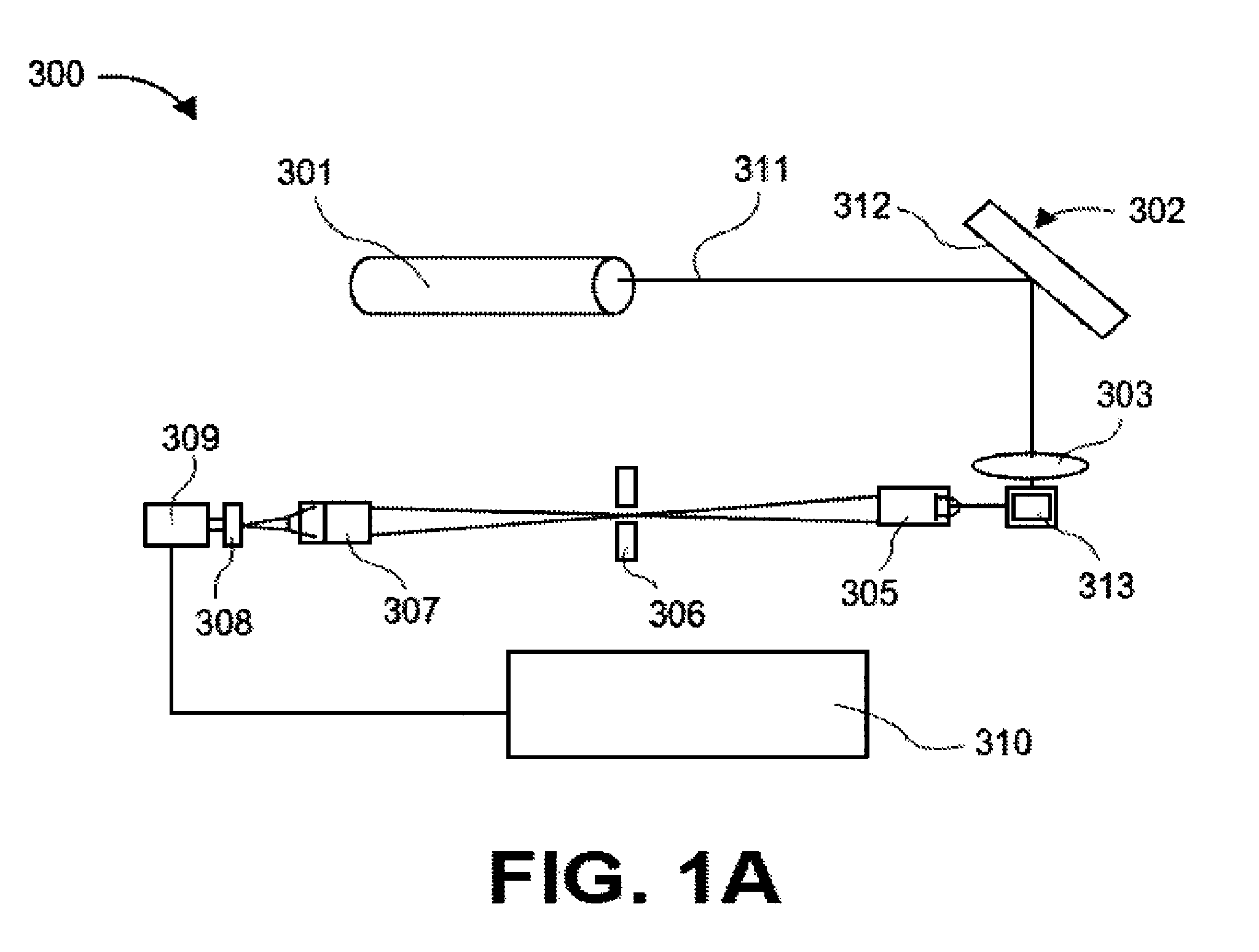
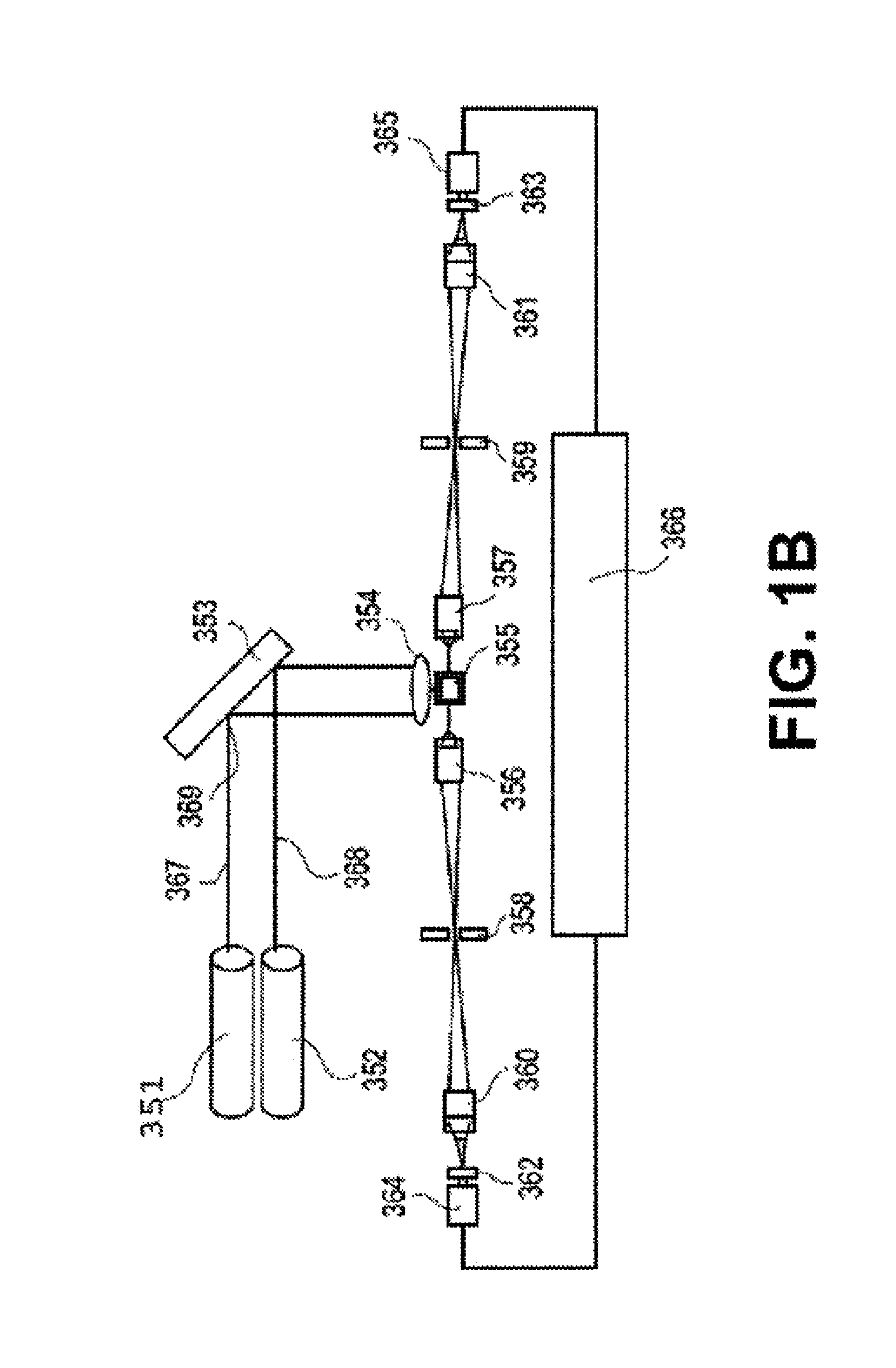
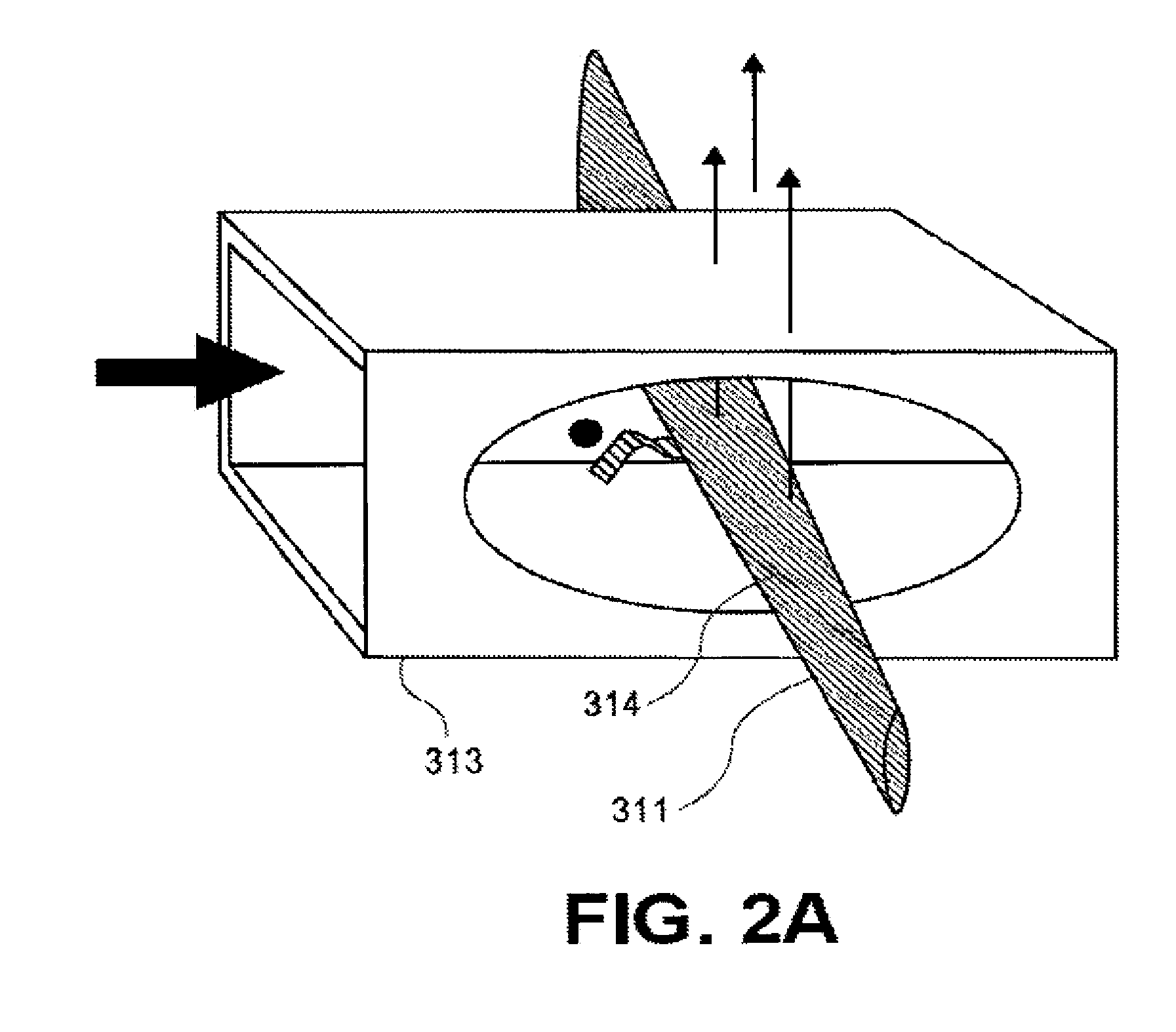
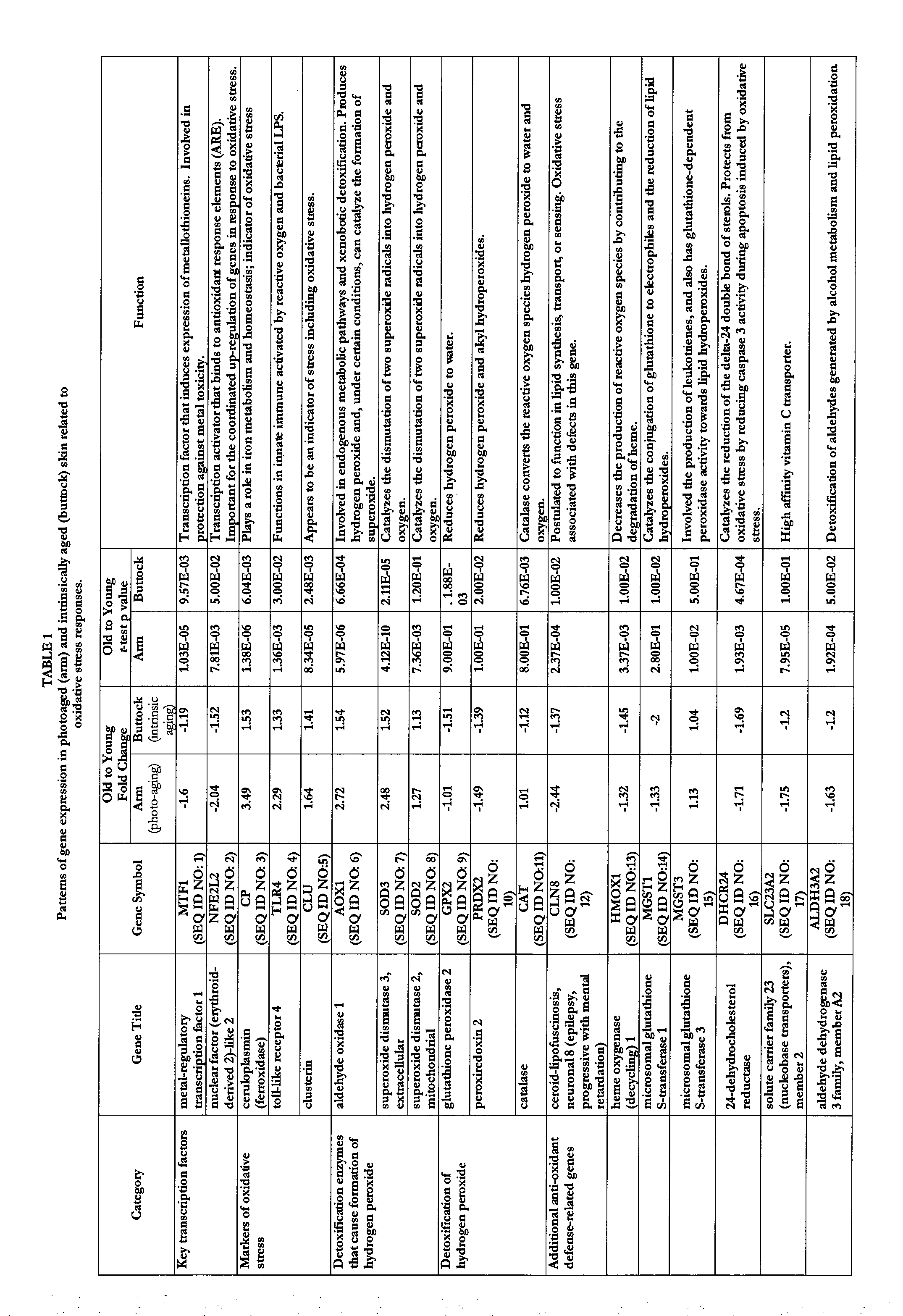
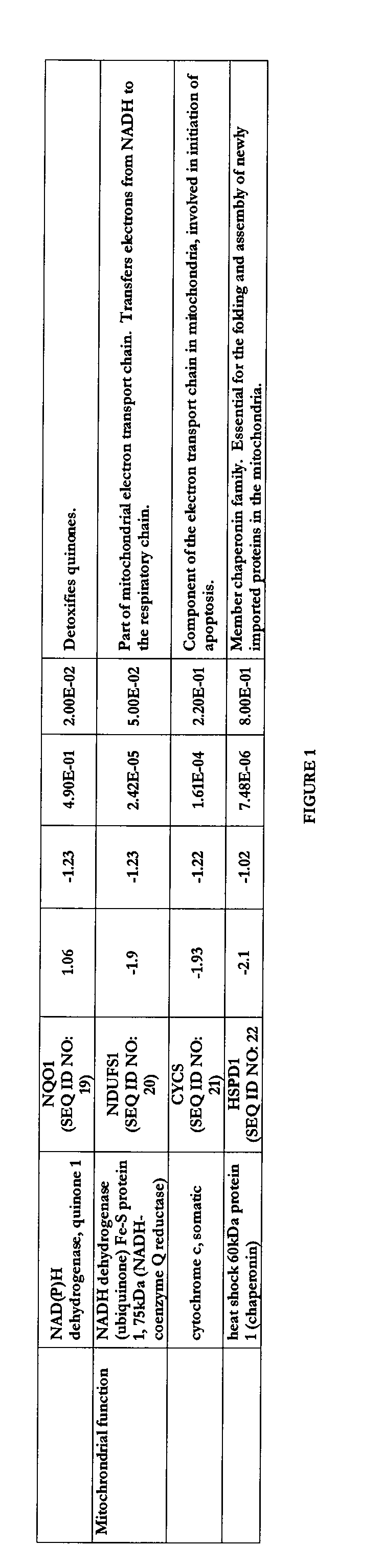
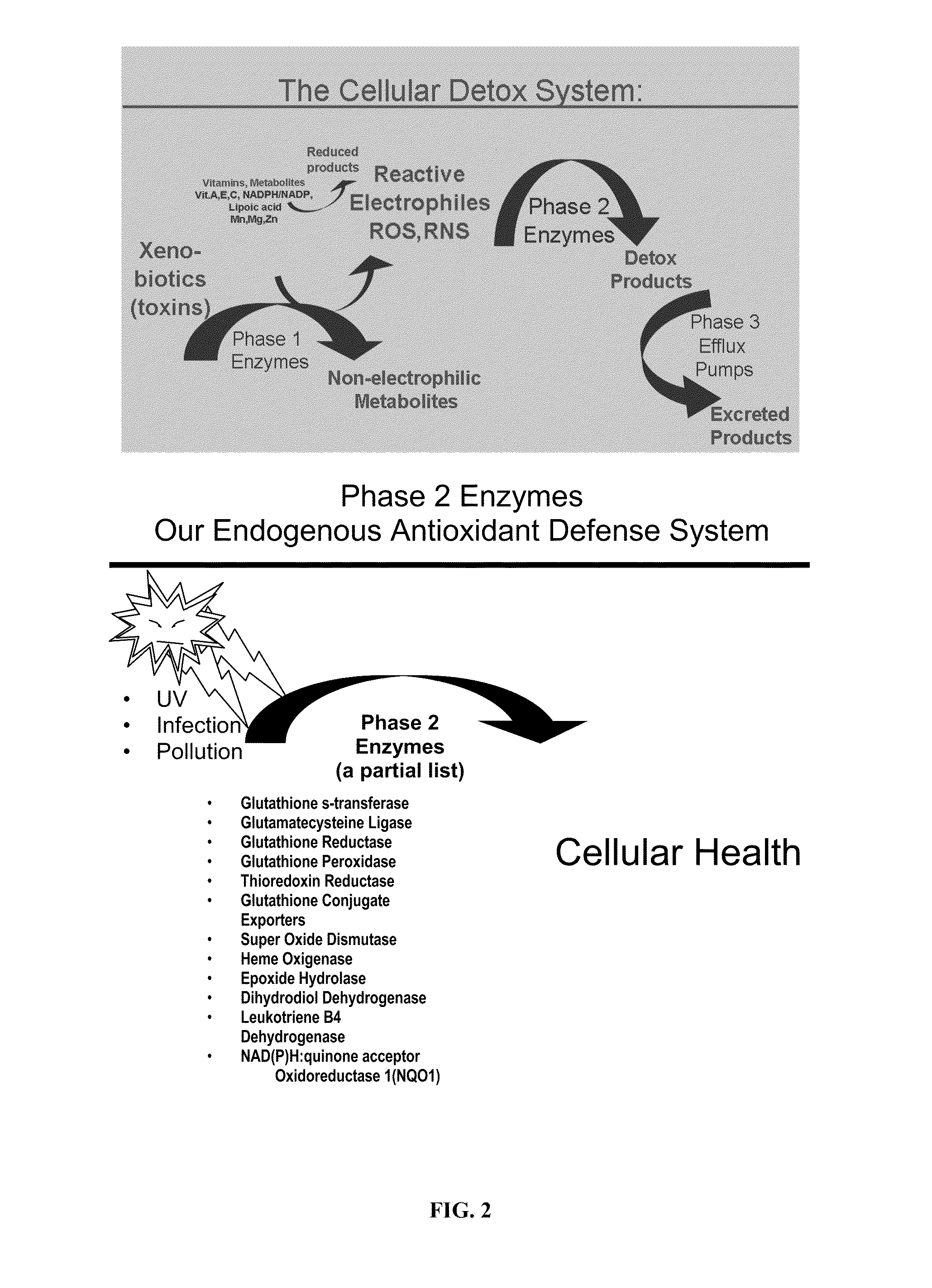
Transcriptional Profiling and Biomarker-Based Methods for Identifying and Evaluating Agents for Antioxidant Efficacy in Cosmetic Skin Care Formulations
InactiveUS20110262570A1Maintain and restore oxidative balanceImprove antioxidant capacityCosmetic preparationsCompound screeningBiomarker panelBiological activation
Gene panels, microarrays and biomarker panels relating to genes and gene products associated with age-related oxidative damage to skin, and transcriptional profiling-based methods for identification and evaluation of cosmetic agents for prevention, reversal, or reduction of oxidative damage to skin. Cosmetic agents and compositions comprising the cosmetic agents, capable of inducing nrf2-mediated activation of the antioxidant response element to increase expression of Phase 2 enzymes, methods for restoring optimal redox status to skin employing the agents, and methods for identifying and evaluating cosmetic agents acting via the nrf2-mediated mechanism.
Owner:THE PROCTER & GAMBLE COMPANY
Biomarker panels, diagnostic methods and test kits for ovarian cancer
InactiveCN103582815AHighly accurate testingAssess carcinogenic potentialHealth-index calculationParticle spectrometer methodsBiomarker panelBiomarker (petroleum)
Methods are provided for predicting the presence, subtype and stage of ovarian cancer, as well as for assessing the therapeutic efficacy of a cancer treatment and determining whether a subject potentially is developing cancer. Associated test kits, computer and analytical systems as well as software and diagnostic models are also provided.
Owner:VERMILLION INC
Methods to determine candidate biomarker panels for a phenotypic condition of interest
InactiveUS20150119289A1Reduce exposureAccurate classificationLibrary screeningData visualisationBiomarker panelRelated disorder
A panel of lymphoma related biomarkers are provided. The panel allows the identification of a subject at risk for a lymphoma. Further provided are methods of optimizing therapeutic efficacy associated with treatment of a lymphoma related disorder. Methods of identifying biomarkers affiliated with a condition of interest are provided.
Owner:MEDEOLINX
Diagnosing and monitoring depression disorders based on multiple biomarker panels
InactiveCN102037355AHealth-index calculationMicrobiological testing/measurementUnipolar DepressionsDisease
Owner:RIDGE DIAGNOSTICS
Brain injury biomarker panel
InactiveUS20120322682A1Rapid assessmentPromote resultsPeptide librariesLibrary screeningInjury brainBiomarker panel
Owner:RICE UNIV
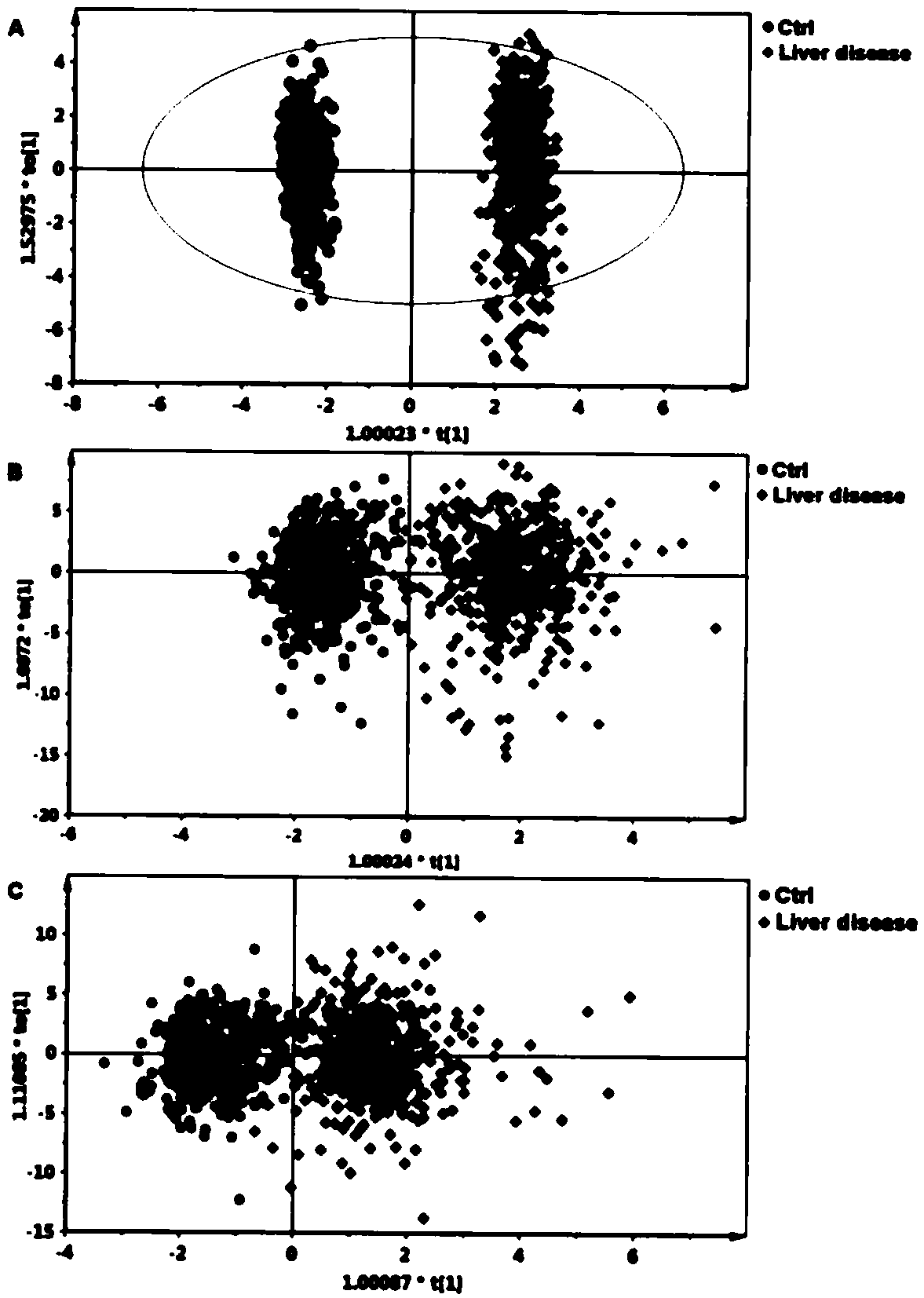
Liver disease-related biomarkers and methods of use thereof
The present invention provides biomarker and biomarker panels useful for diagnostic methods evaluating liver disease status in a subject, monitoring liver disease, distinguishing between liver diseases, treating subjects evaluated by diagnostic methods of the invention, providing diagnostic tests for evaluating liver disease status in a subject, and kits there for.
Owner:HUMAN METABOLOMICS INST INC
Biomarkers of osteoarthritis
Owner:UNIVERSITY OF MISSOURI
Multiple Biomarker Panels to Stratify Disease Severity and Monitor Treatment of Depression
InactiveUS20110213219A1Accurately stratify disease severityMonitor responseDisease diagnosisDiagnostic recording/measuringBiomarker panelDisease severity
Materials and Methods for stratifying disease severity and for monitoring major depressive disorder are provided.
Owner:RIDGE DIAGNOSTICS
Biomarker panels for assessing radiation injury and exposure
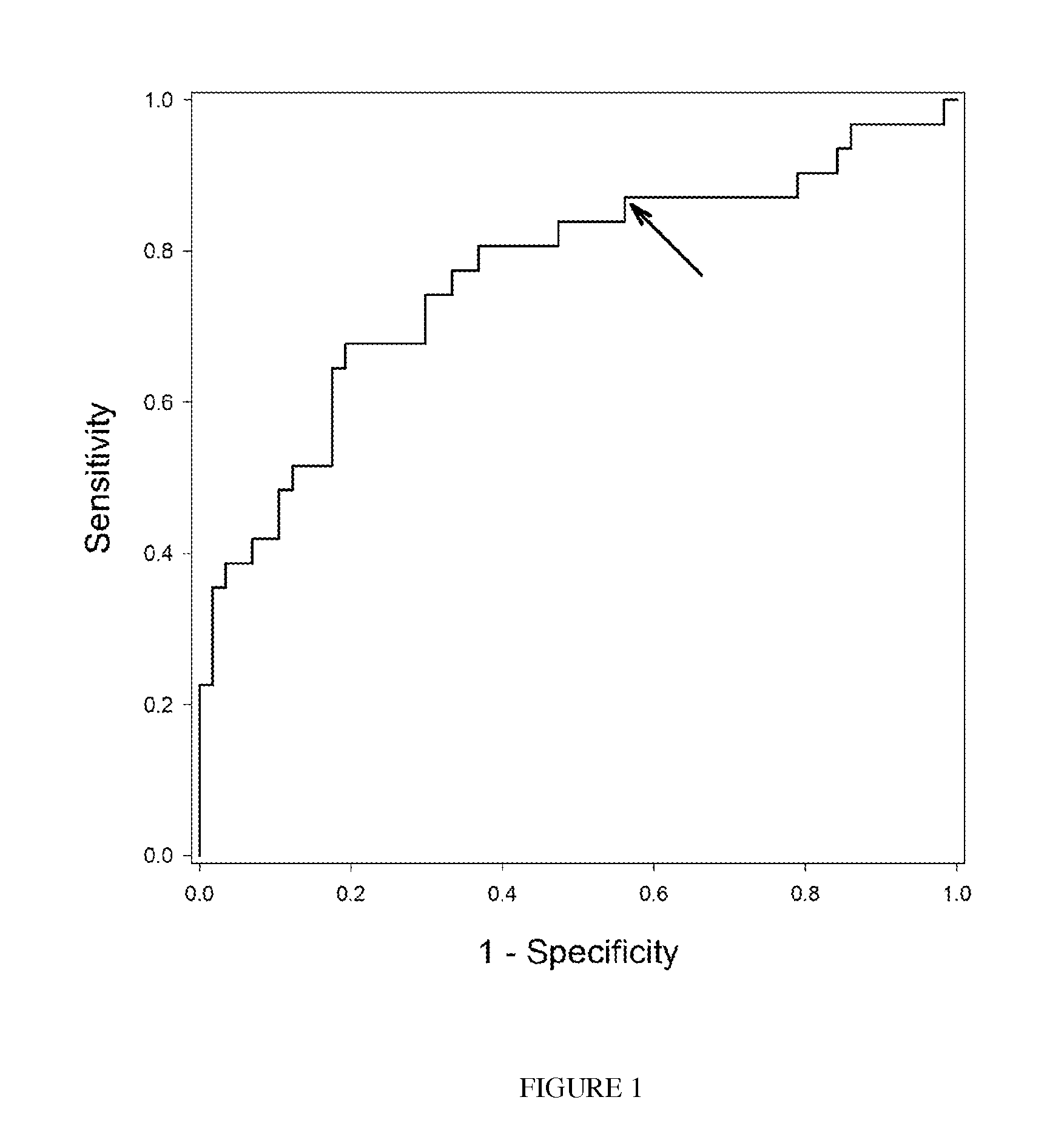
Methods and kits are provided for assessing radiation injury and exposure in a subject. The methods comprise measuring the levels of at least two (2) protein biomarkers from different biological pathways and correlating the levels with an assessment of radiation injury and exposure. Additional use of peripheral blood cell counts and serum enzyme biomarkers, evaluated in the early time frame after a suspected radiation exposure, and use of integrated multiple parameter triage tools to enhance radiation exposure discrimination and assessment are also provided. The information obtained from such methods can be used by a clinician to accurately assess the extent of radiation injury / exposure in the subject, and thus will provide a valuable tool for determining treatment protocols on a subject by subject basis.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Biomarker panels, diagnostic methods and test kits for ovarian cancer
InactiveUS20150031561A1High precision testAccurate measurementPeptide librariesHealth-index calculationBiomarker panelPhases of clinical research
Methods are provided for predicting the presence, subtype and stage of ovarian cancer, as well as for assessing the therapeutic efficacy of a cancer treatment and determining whether a subject potentially is developing cancer. Associated test kits, computer and analytical systems as well as software and diagnostic models are also provided.
Owner:VERMILLION INC
Biomarkers for early detection of ovarian cancer
InactiveUS20100081151A1Microbiological testing/measurementBiological material analysisBiomarker panelOVARIAN NEOPLASIA
Biomarker proteins that can be used in the diagnosis of early-stage ovarian cancer (OC) are described. The biomarker panels not only permit the distinction of patients with ovarian neoplasia (benign or malignant) from normal subjects, but they also allow the identification of patients with early-stage (stage I / II) ovarian cancer from those patients with benign ovarian tumors or normal individuals. The invention additionally provides methods for detecting and treating various cancers, including cancer of the ovary using OC-related molecules.
Owner:RGT UNIV OF CALIFORNIA
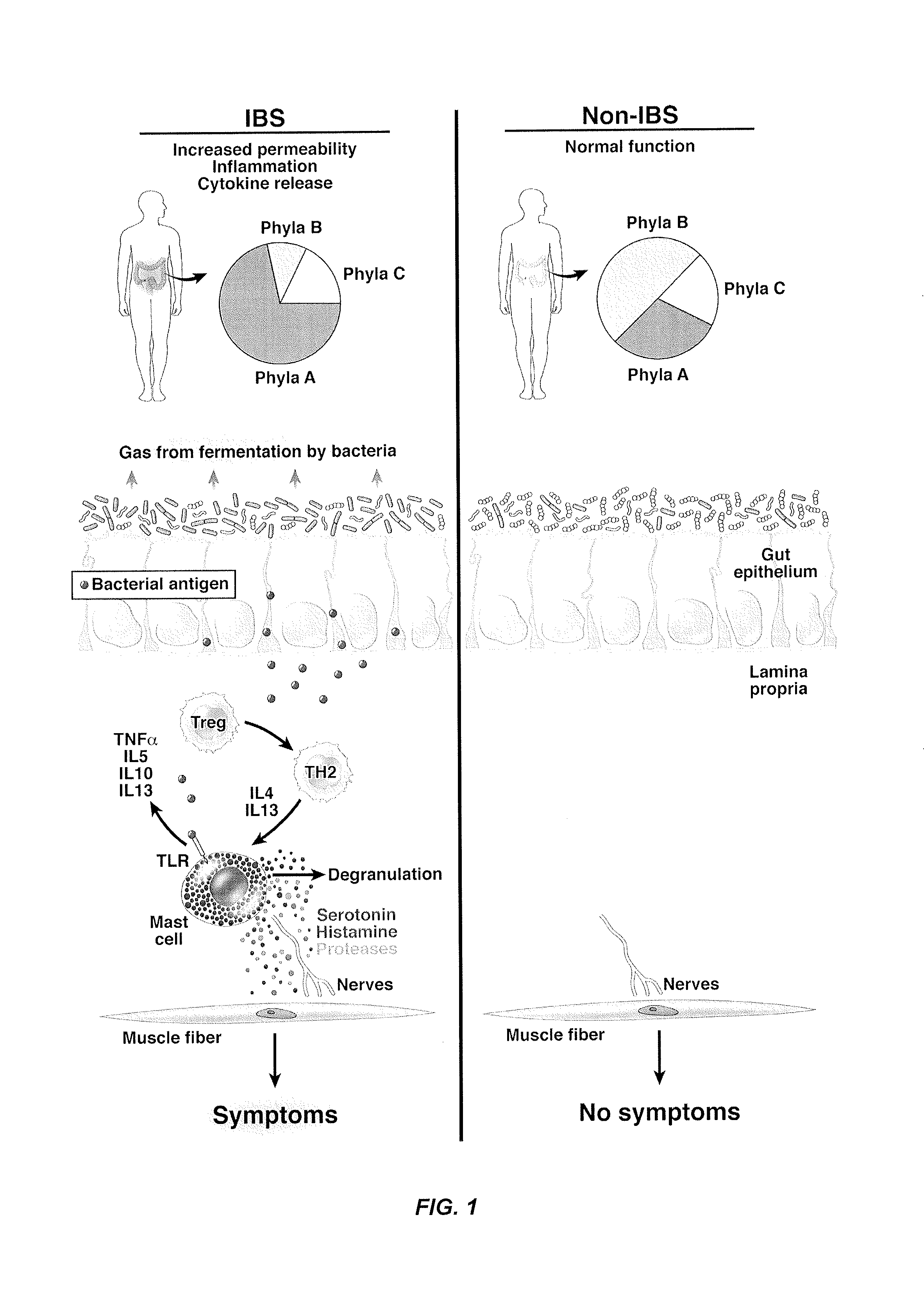
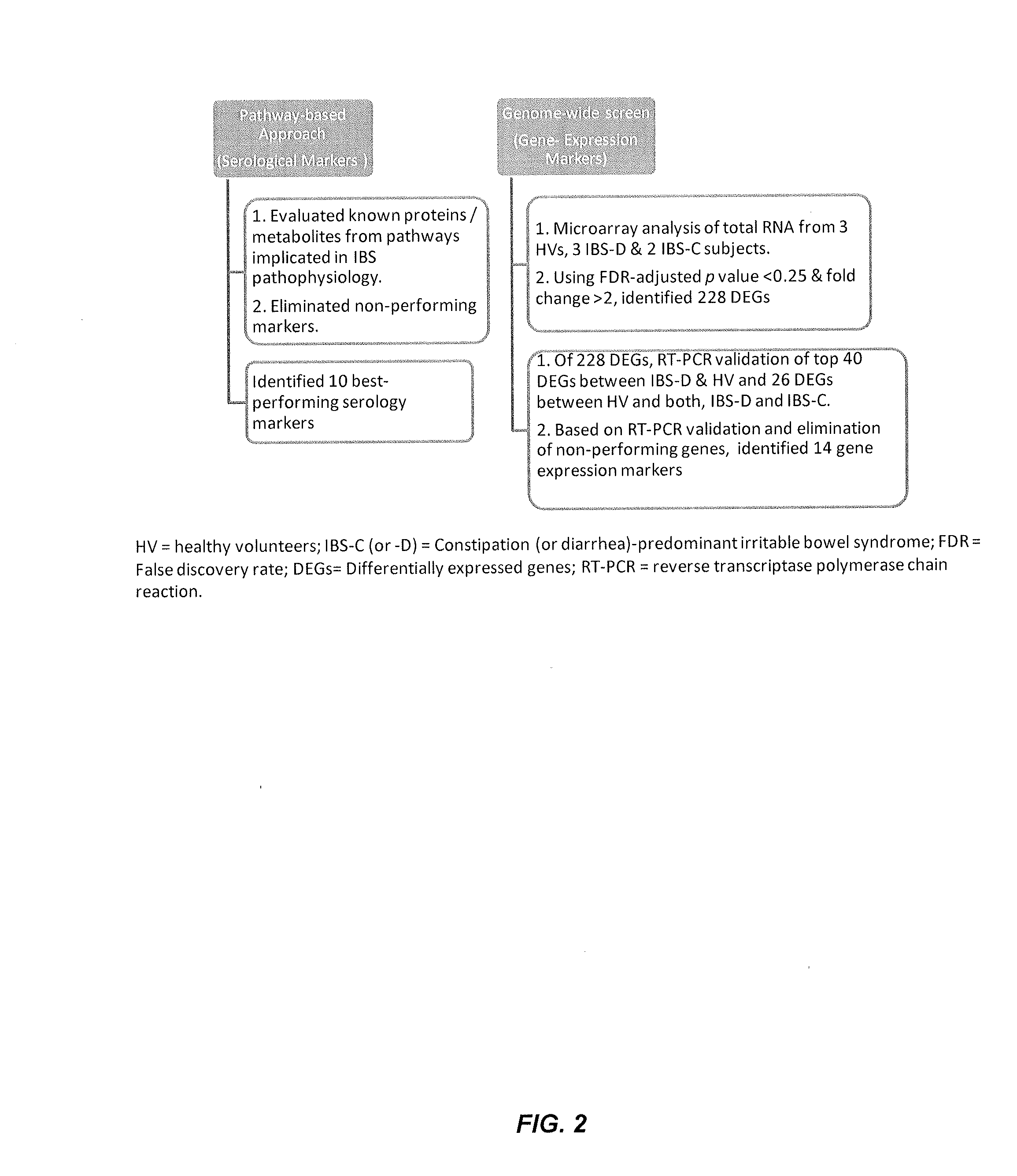
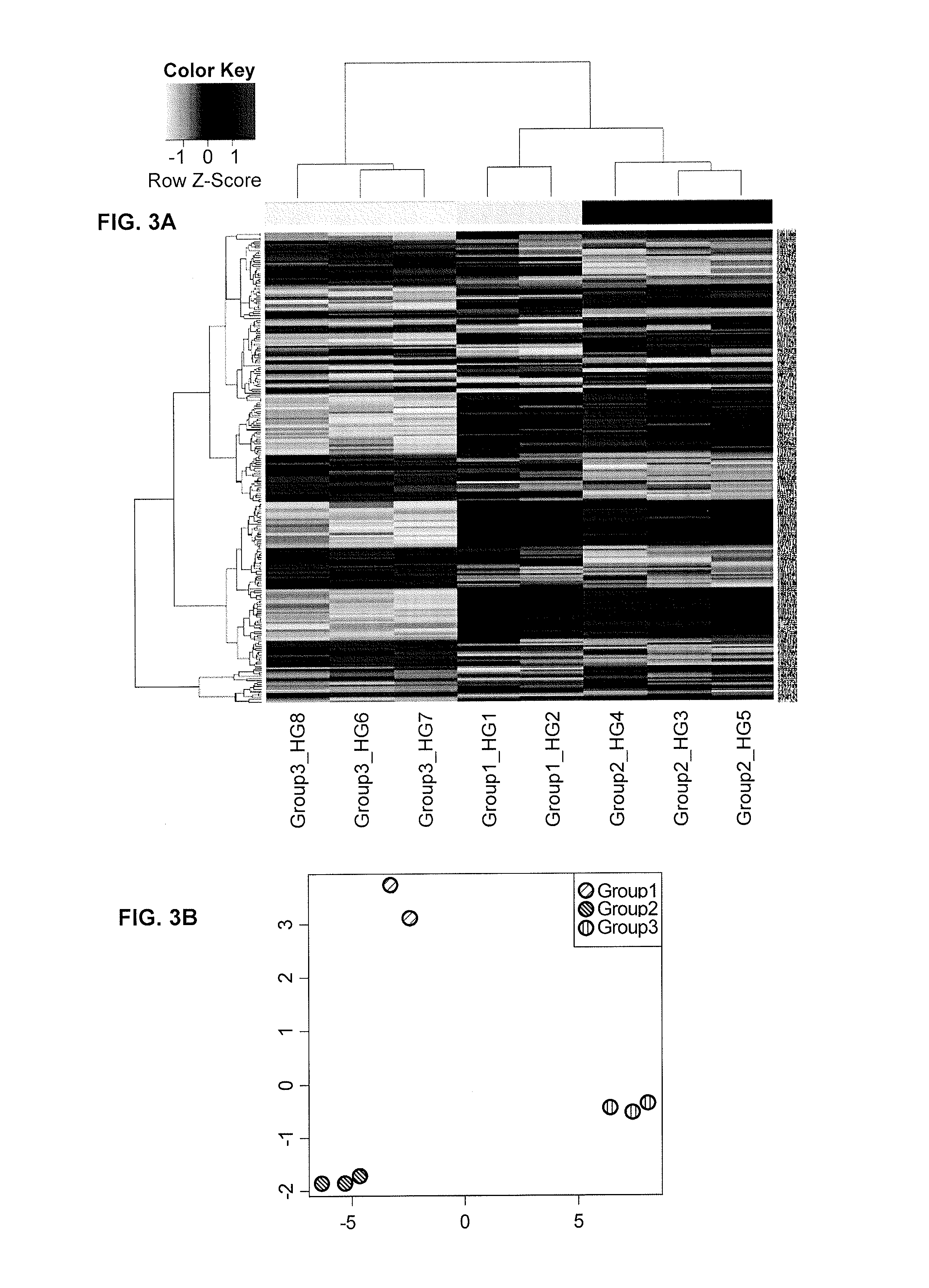
Performance of a biomarker panel for irritable bowel syndrome
InactiveUS20140141990A1Microbiological testing/measurementLibrary screeningBiomarker panelHealthy subjects
The present invention provides a method of using a panel of serological and genetic biomarkers to distinguish IBS subjects from healthy subjects and / or to differentiate IBS subtypes from each other. The present invention also provides a method of using one or more psychological measures of a subject in conjunction with a panel of serological and / or genetic biomarkers to further aid in diagnosing IBS or discriminating IBS subtypes from each other.
Owner:NESTEC SA
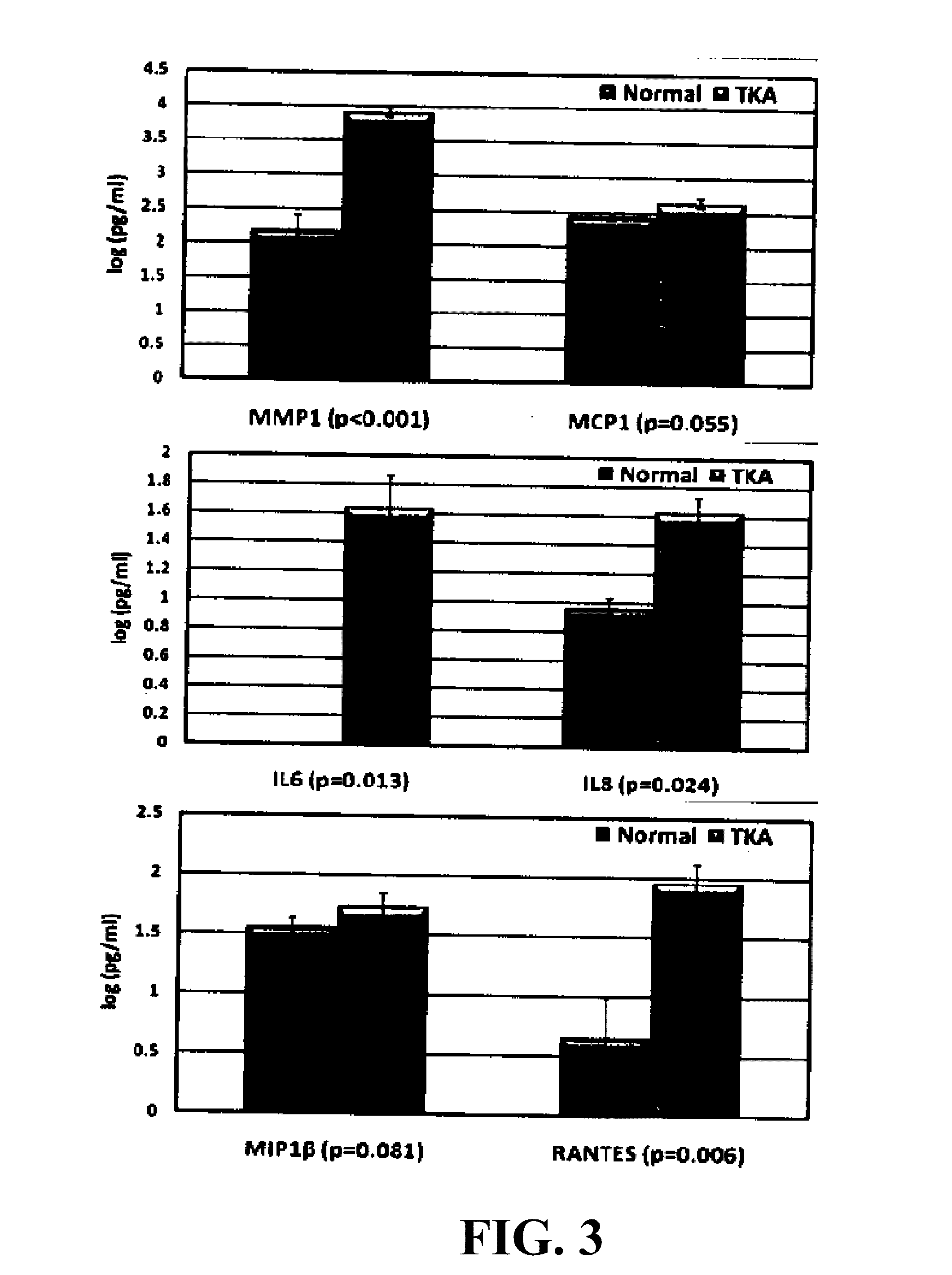
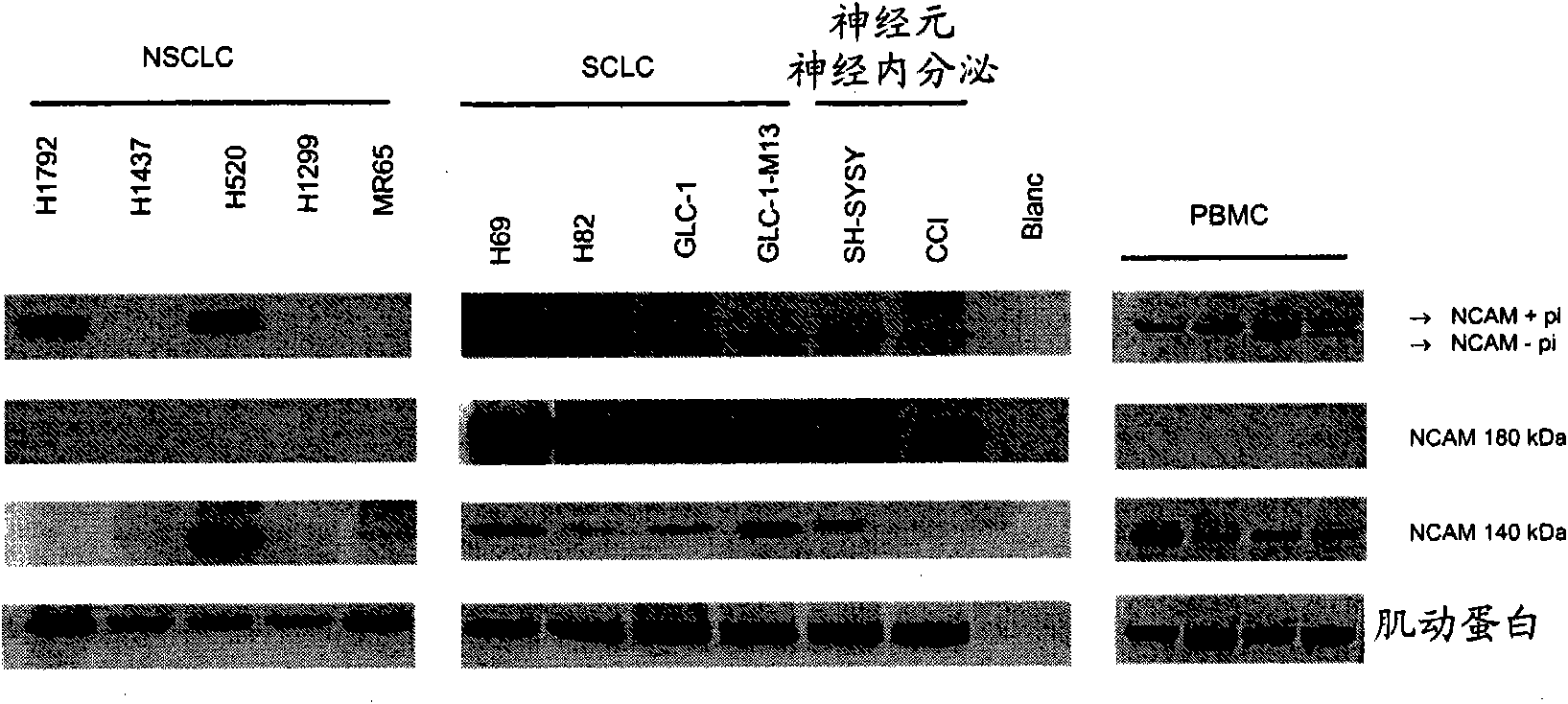
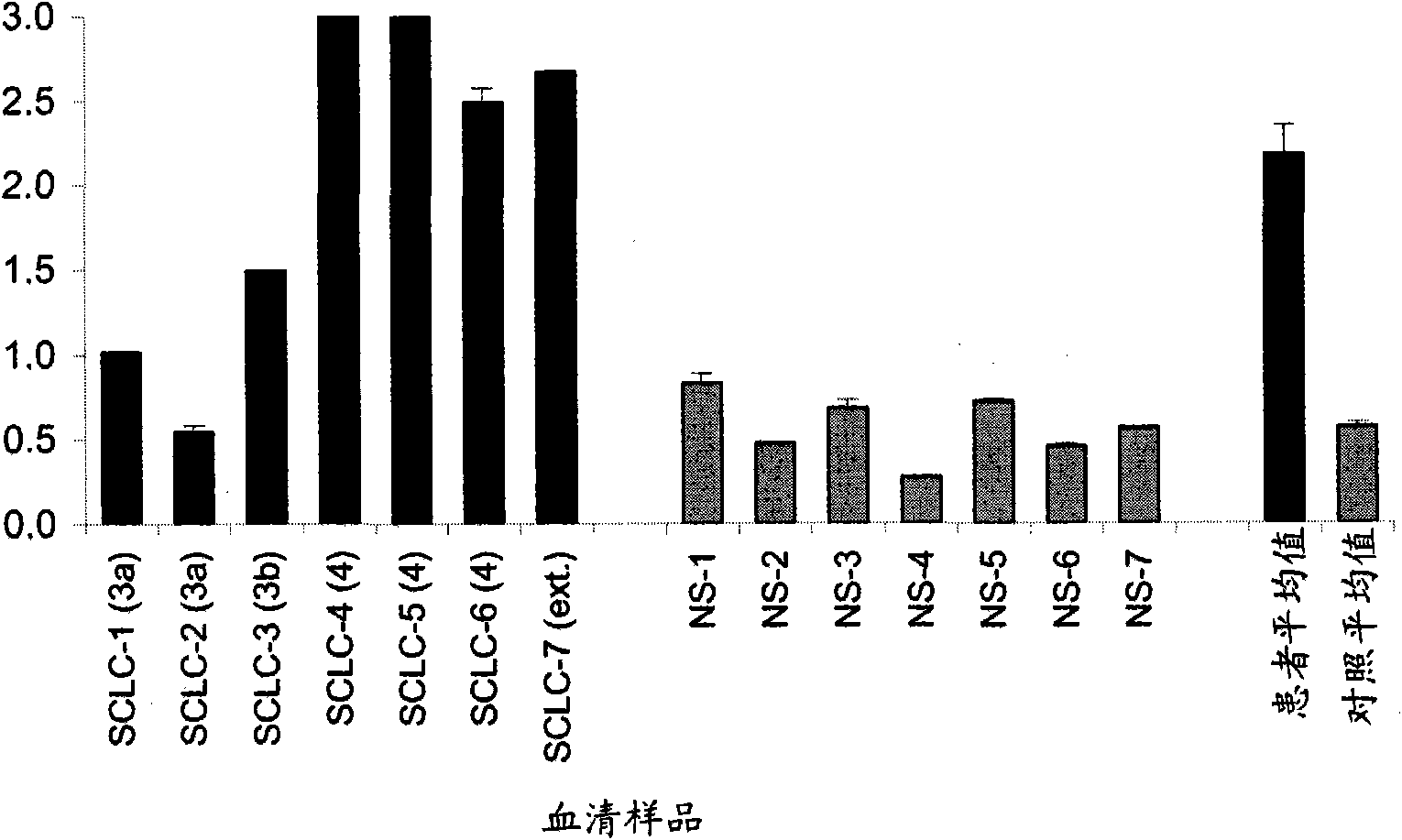
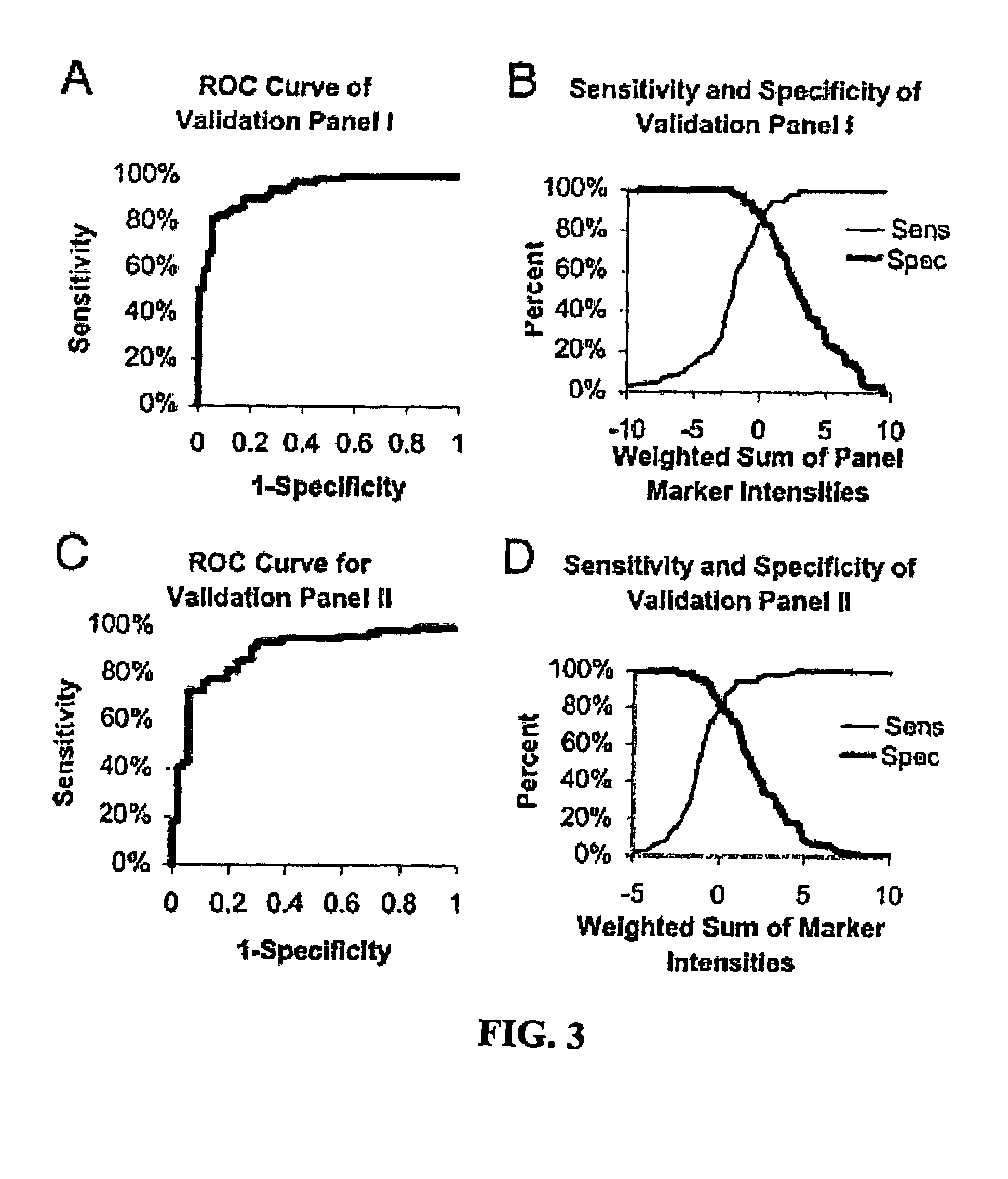
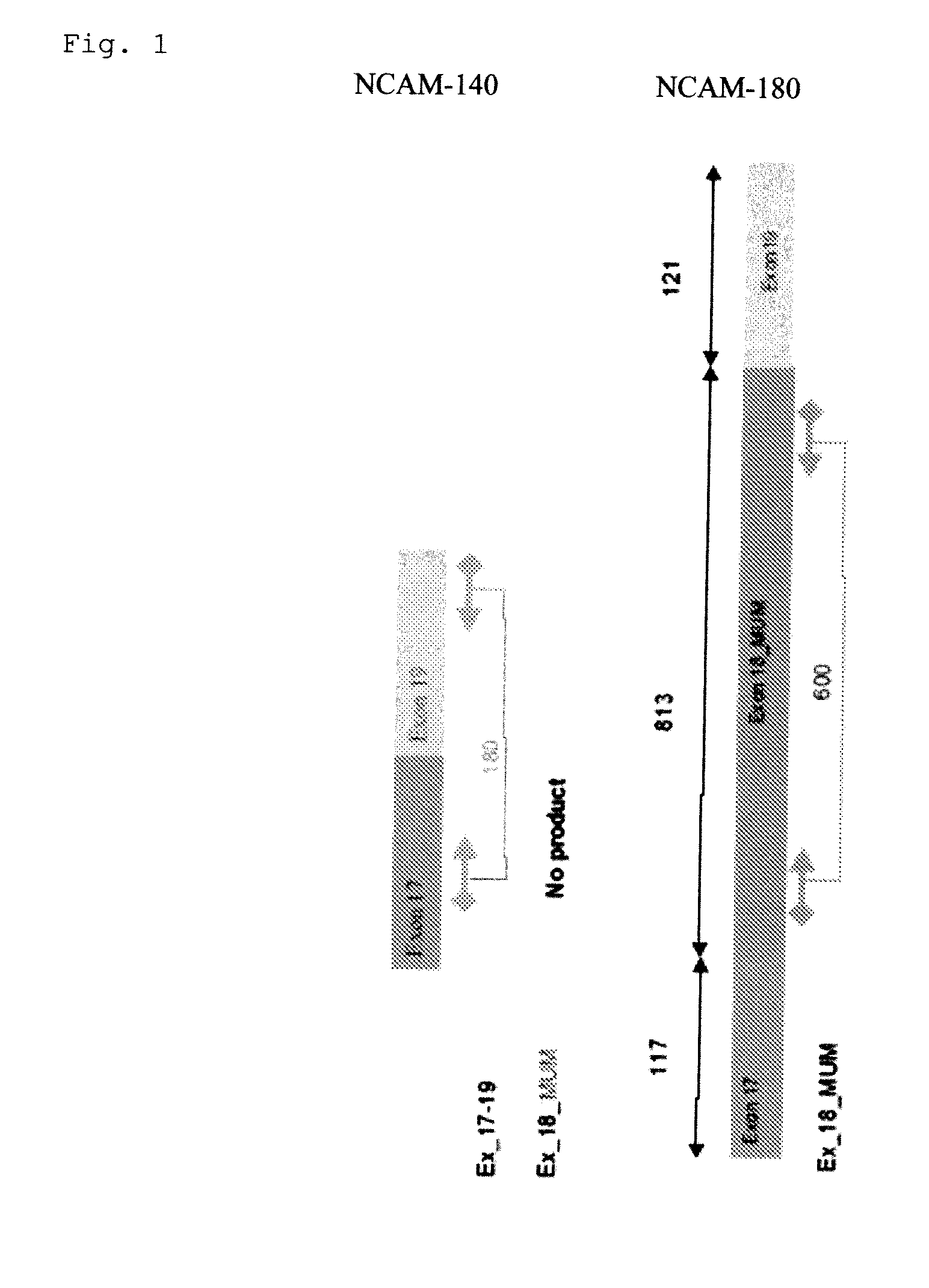
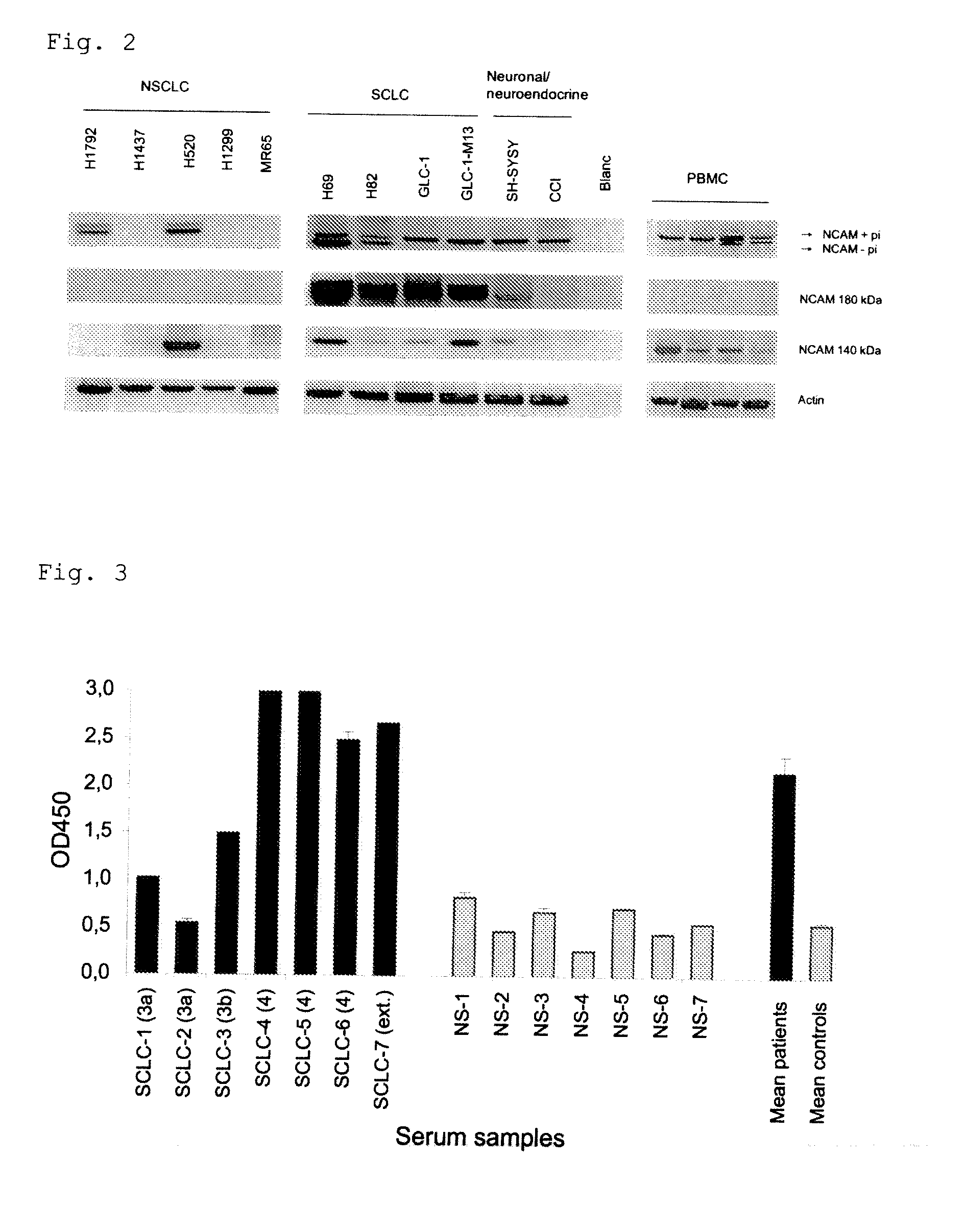
Small cell lung carcinoma biomarker panel
The invention relates generally to the field of cancer detection, diagnosis, subtyping, staging, prognosis, treatment and prevention. More particularly, the present invention relates to methods for the detection, and / or diagnosing and / or subtyping and / or staging of lung cancer in a patient. Based on a particular panel of biomarkers, the present invention provides methods to detect, diagnose at an early stage and / or differentiate small cell lung cancer (SCLC) from non-small cell lung cancer (NSCLC) and within NSCLC to differentiate between squamous cell carcinomas (SCC), adenocarcinomas (AC), within SCC to discriminate G2 and G3 stage and within lung cancer to differentiate for lung cancers with or without neuroendocrine origin. It further provides the use of said panel of biomarkers in monitoring disease progression in a patient, including both in vitro and in vivo imaging techniques. The in vitro imaging techniques typically include an immunoassay detecting protein or antibody of the biomarkers on a sample taken from said patient, e.g. serum or tissue sample. The in vivo imaging techniques typically include chest radiographs (X-rays), Computed Tomography (CT) imaging, spiral CT, Positron Emission Tomography (PET), PET-CT and scintigraphy for molecular imaging and diagnosis and to monitor disease progression and treatment response in patients. It is accordingly a further aspect to provide a kit to perform the aforementioned diagnosing and / or subtyping and / or staging assay and the imaging techniques, comprising reagents to determine the gene expression or protein level of the aforementioned panel of biomarkers for in vitro and in vivo applications.
Owner:MUBIO PRODS BV
Biomarkers for early detection of ovarian cancer
Biomarker proteins that can be used in the diagnosis of early-stage ovarian cancer (OC) are described. The biomarker panels not only permit the distinction of patients with ovarian neoplasia (benign or malignant) from normal subjects, but they also allow the identification of patients with early-stage (stage I / II) ovarian cancer from those patients with benign ovarian tumors or normal individuals. The invention additionally provides methods for detecting and treating various cancers, including cancer of the ovary using OC-related molecules.
Owner:RGT UNIV OF CALIFORNIA
Biomarkers and assay to detect chronic graft versus host disease
ActiveUS20170261518A1Improve expression levelImmunoglobulins against cytokines/lymphokines/interferonsDisease diagnosisBiomarker panelBiomarker (petroleum)
A four-biomarker panel for diagnosis of chronic graft-versus-host disease (cGVHD) and methods of prognosing and / or diagnosing cGVHD using the biomarker panel are disclosed.
Owner:INDIANA UNIV RES & TECH CORP
Small cell lung carcinoma biomarker panel
InactiveUS20110053156A1Peptide/protein ingredientsMicrobiological testing/measurementBiomarker panelCompanion animal
The invention relates generally to the field of cancer detection, diagnosis, subtyping, staging, prognosis, treatment and prevention. More particularly, the present invention relates to methods for the detection, and / or diagnosing and / or subtyping and / or staging of lung cancer in a patient. Based on a particular panel of biomarkers, the present invention provides methods to detect, diagnose at an early stage and / or differentiate small cell lung cancer (SCLC) from non-small cell lung cancer (NSCLC) and within NSCLC to differentiate between squamous cell carcinomas (SCC), adenocarcinomas (AC), within SCC to discriminate G2 and G3 stage and within lung cancer to differentiate for lung cancers with or without neuroendocrine origin. It further provides the use of said panel of biomarkers in monitoring disease progression in a patient, including both in vitro and in vivo imaging techniques. The in vitro imaging techniques typically include an immunoassay detecting protein or antibody of the biomarkers on a sample taken from said patient, e.g. serum or tissue sample. The in vivo imaging techniques typically include chest radiographs (X-rays), Computed Tomography (CT) imaging, spiral CT, Positron Emission Tomography (PET), PET-CT and scintigraphy for molecular imaging and diagnosis and to monitor disease progression and treatment response in patients. It is accordingly a further aspect to provide a kit to perform the aforementioned diagnosing and / or subtyping and / or staging assay and the imaging techniques, comprising reagents to determine the gene expression or protein level of the aforementioned panel of biomarkers for in vitro and in vivo applications.
Owner:MUBIO PRODS BV
Diagnostic assay for urine monitoring of bladder cancer
InactiveUS20190062841A1Increase valueCost effectiveMicrobiological testing/measurementHybridisationBiomarker panelOrganism
An improved diagnostic assay and methods relating to the same that are directed to mutation focused disease diagnosis and surveillance biomarker panels wherein potential genomic regions are selected based on their ability to encompass the genomic diversity of a patient population, maximize the number of unique markers monitored within each patient are maximized while balancing these factors with empirical sequencing performance, geographic clustering of events with a region across diverse patients, and size and cost associated with measuring the respective genomic region. The methods also include quality control steps to reduce noise and maximize the presence of relevant markers.
Owner:CONVERGENT GENOMICS INC
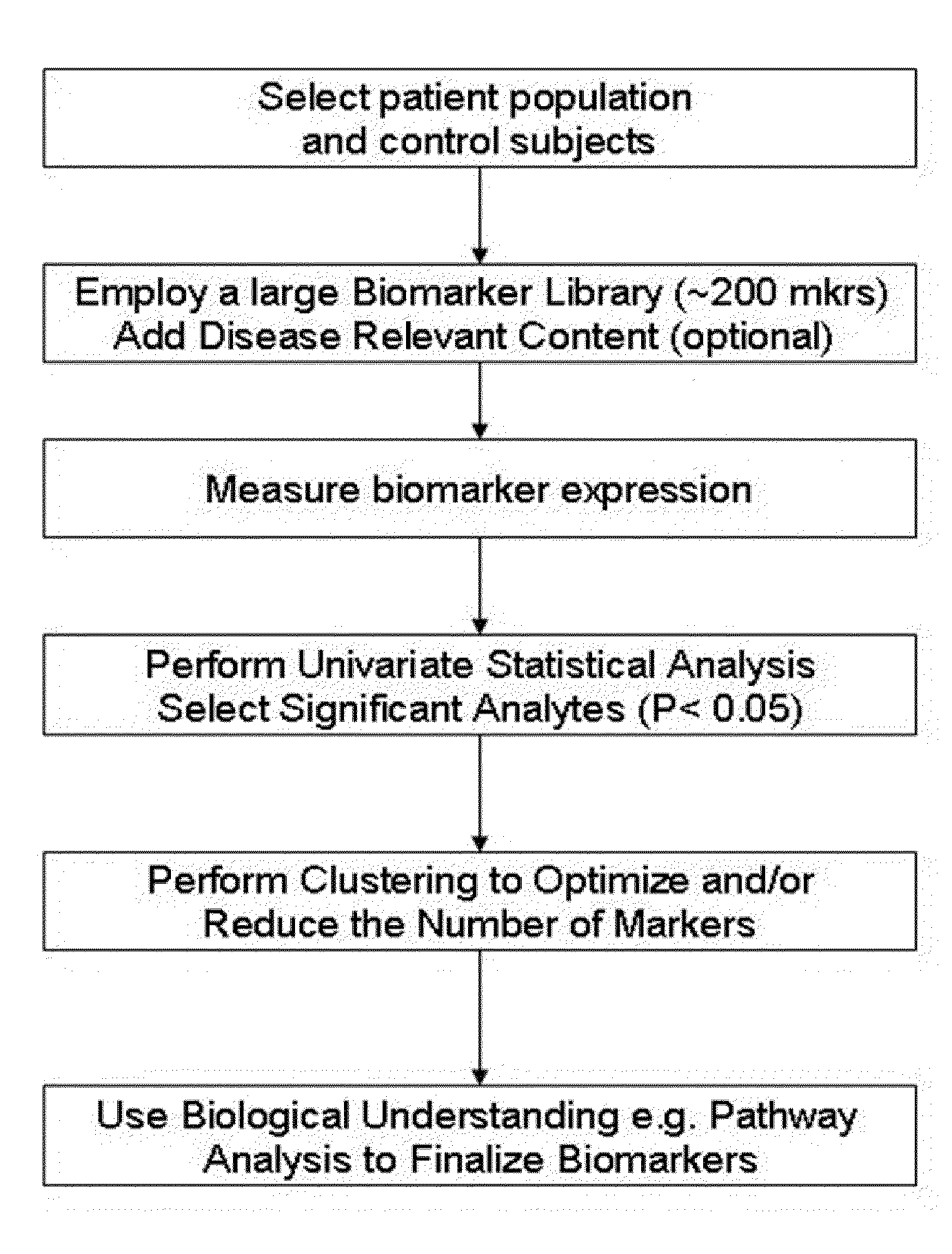
Selection and display of biomarker expressions
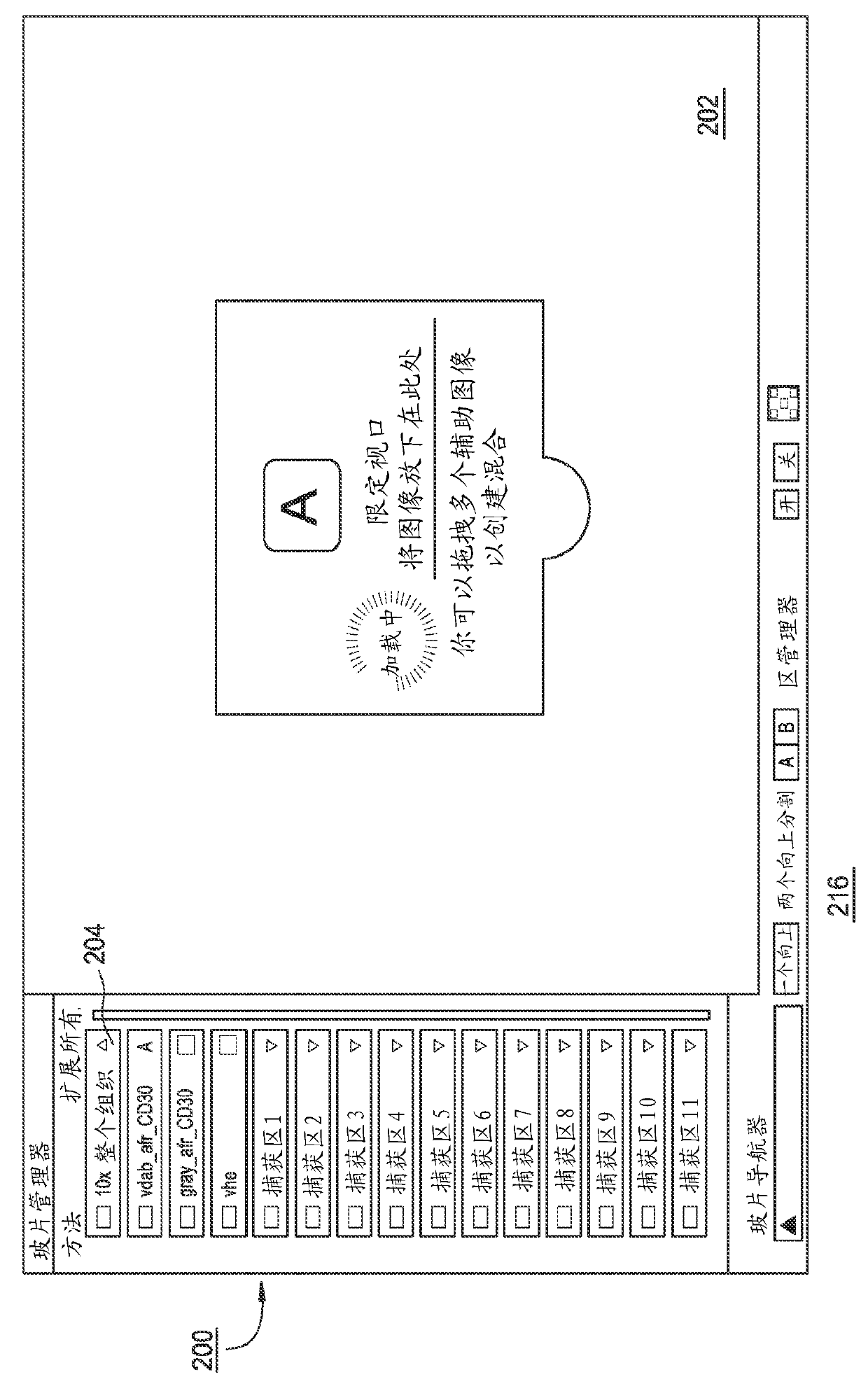
Embodiments provide a user interface including an image panel for displaying a field of view corresponding to an image, or a portion of an image, of a biological specimen, and a biomarker panel for displaying thumbnail images for simultaneously viewing different aspects of a region within the field of view. Upon selection or update of the selected region of the field of view, the thumbnail images may be accordingly updated automatically to display the newly selected region. The image panel may include an interest region selection component for delineating a region within the field of view. The interest region selection component may be overlaid over a portion of the field of view displayed in the image panel, and may be used by a user to select or update the region within the field of view displayed in the biomarker panel. Images of interest may be saved to an electronic record.
Owner:莱卡微系统 CMS +1
Compositions and Methods of Using Micro RNAs
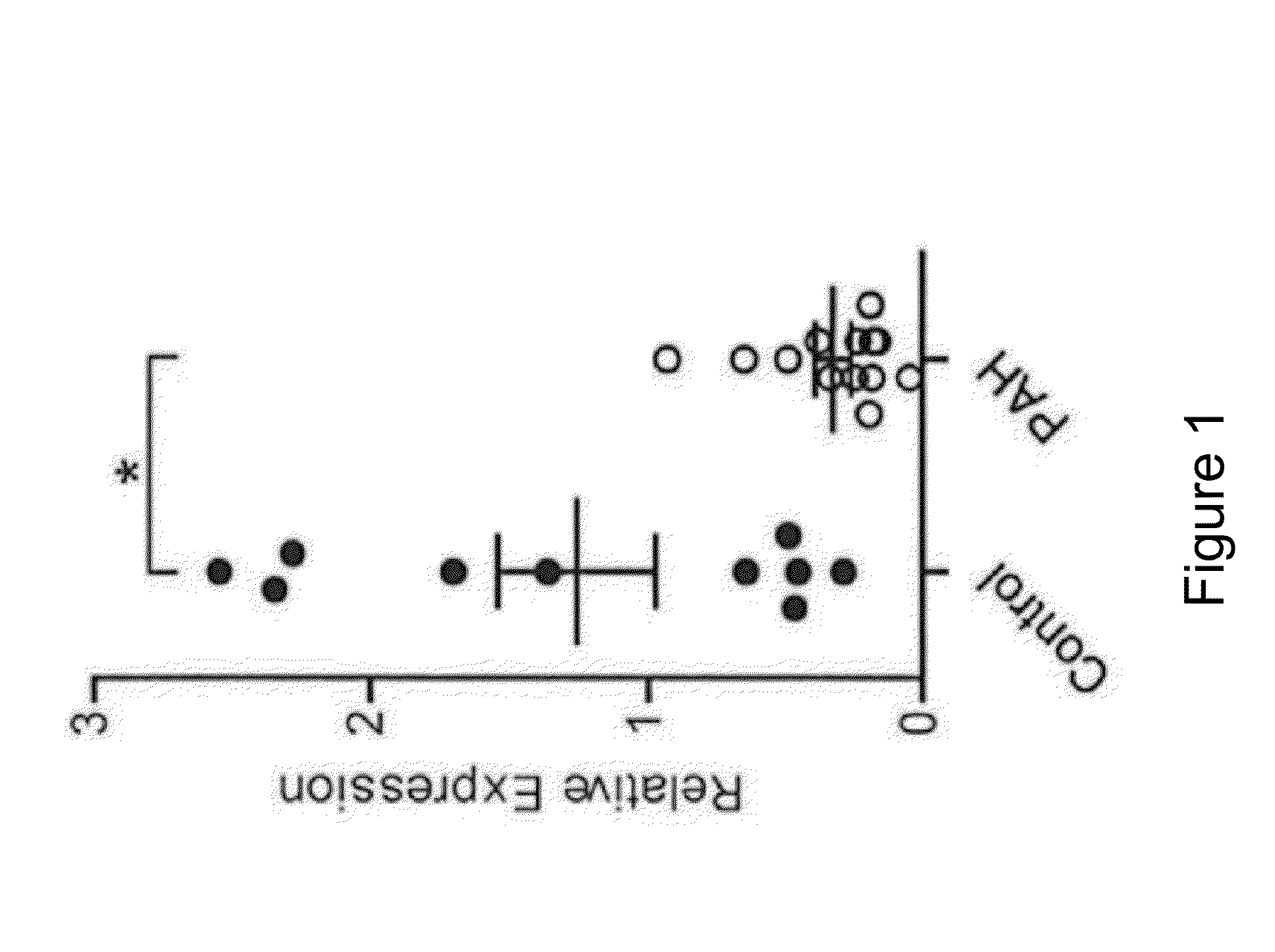
The present invention provides compositions and methods of using microRNAs to treat pulmonary arterial hypertension. In one aspect, methods are disclosed that are useful for identifying a subject in need of therapeutic intervention to reduce or improve a symptom of pulmonary arterial hypertension, reducing proliferation of pulmonary vascular cells in a subject, or treating pulmonary arterial hypertension in a subject. In another aspect, compositions include an inhibitor of fibroblast growth factor 2 (FGF2) expression comprising at least one of: a mature sequence of miR-424 or miR-503; a pri-miRNA of miR-424 or miR-503; a pre-miRNA of miR-424 or miR-503; and the complement thereof. Pharmaceutical compositions for reducing proliferation of pulmonary vascular cells in a subject in need thereof and biomarker panels are also disclosed.
Owner:YALE UNIV
Predictive markers and biomarker panels for ovarian cancer
InactiveUS20120171694A1Accurate measurementMicrobiological testing/measurementDisease diagnosisBiomarker panelBiologic marker
Methods are provided for predicting the presence, subtype and stage of ovarian cancer, as well as for assessing the therapeutic efficacy of a cancer treatment and determining whether a subject potentially is developing cancer. Associated test kits, computer and analytical systems as well as software and diagnostic models are also provided.
Owner:VERMILLION INC
Biomarker composite test for hepatic vein pressure gradient and cirrhosis treatment
Diagnostic biomarker panel, method, kit, and device for diagnosing the severity and / or prognosis of cirrhosis are provided. More specifically, the invention provides a novel biomarker panel correlating to HVPG and esophageal varices. The invention further provides a biomarker panel and non-invasive test methods that predict non-clinically significant portal hypertension HVPG and non-clinically significant esophageal varices when the expression of the biomarker panel correlates with HVPG of less than 12 mmHg. The invention further provides that the patients with the expression of the biomarker panel correlating to non-clinically significant HVPG and esophageal varices can be excluded from undergoing esophagogastroduodenoscopy (EGD) screening and those correlating to HVPG equal to or greater than 12 mmHg are indicated for EGD.
Breast cancer prognostication and screening kits and methods of using same
ActiveUS20160333413A1Improved and more accurate screening and prognostic testsHigh riskMicrobiological testing/measurementSpecial data processing applicationsBiomarker panelRegimen
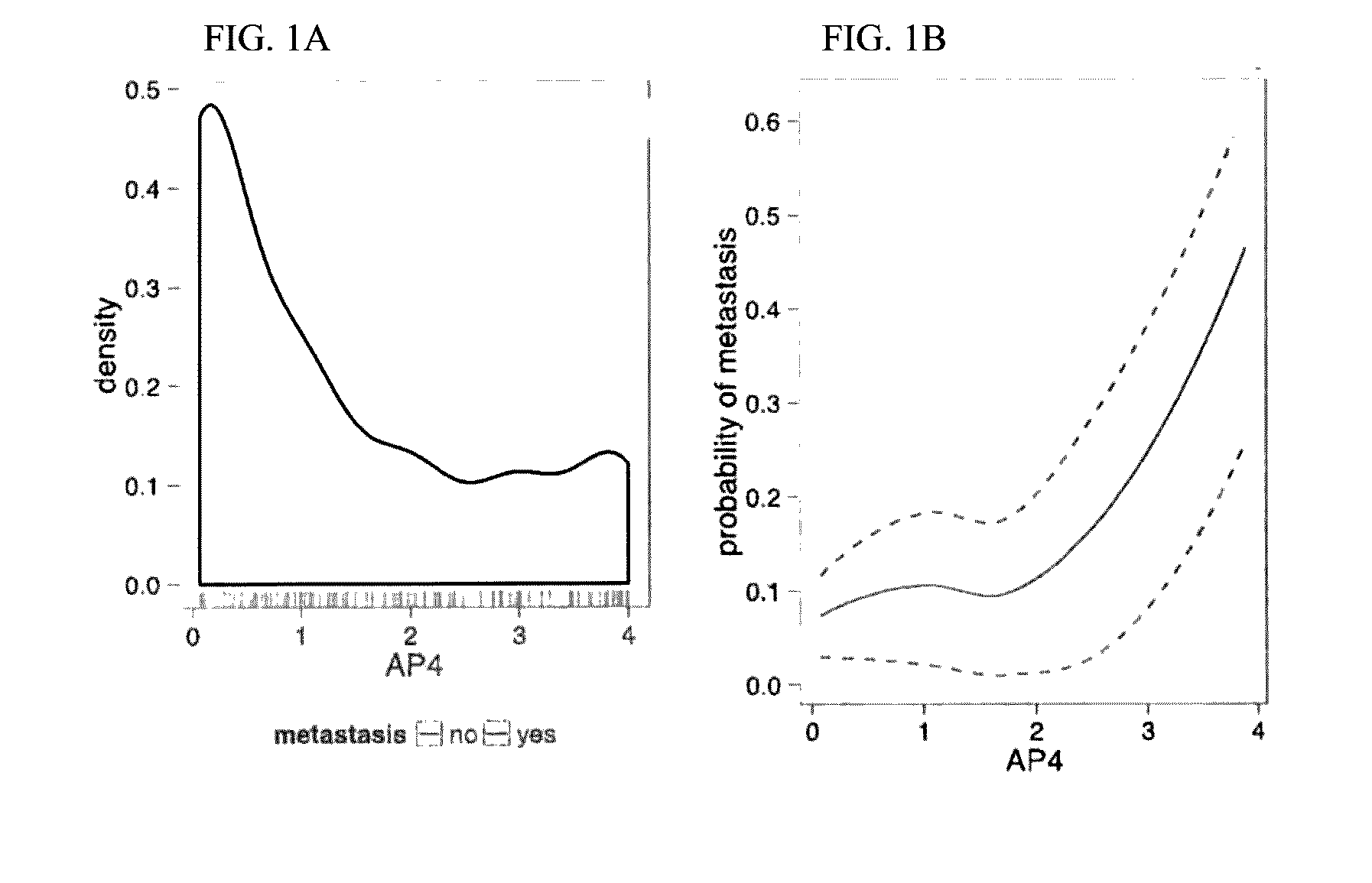
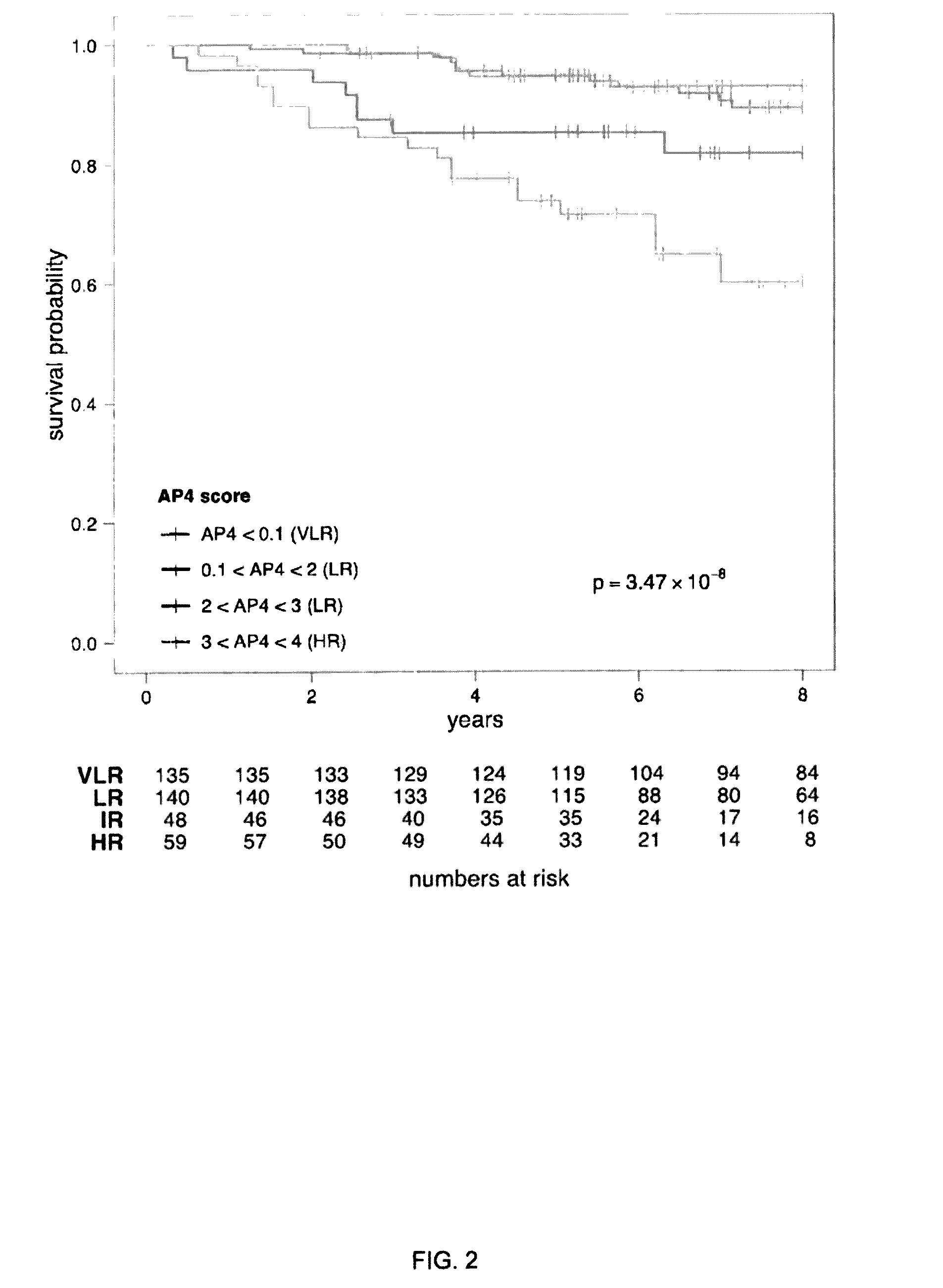
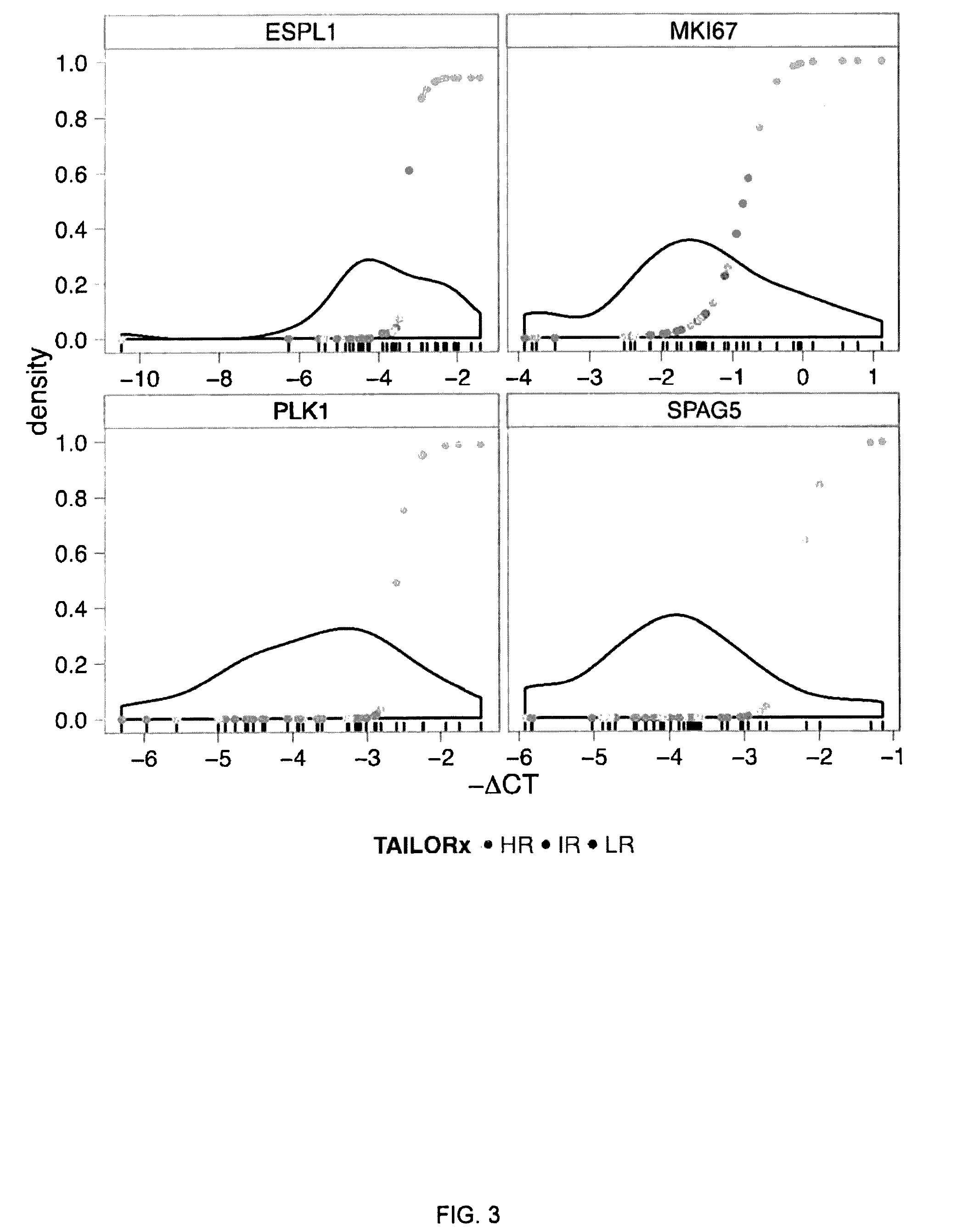
A genetic biomarker panel is provided for prognosing late onset ER+ breast cancer relapse, in a breast cancer survivor patient. Kits are also provided for measuring levels or the presence of an identified panel of genetic biomarkers. Methods are also provided for identifying a breast cancer survivor patient at a relatively high risk of suffering a breast cancer relapse within 8 years of diagnosis, and therefore suitable for treatment with an aggressive chemotherapeutic regimen. The method may also be used for identifying a breast cancer survivor patient not at high risk of suffering a breast cancer relapse within 8 years of diagnosis, and thus not suitable for treatment with an aggressive chemotherapeutic regimen. The genetic biomarker panel includes an oligonucleotide / nucleic acid sequence specific for the following genes: MKI67, SPAG5, ESPL1, PLK1, or a genetic panel for MKI67, SPAG5, ESPL1, PLK1 and PGR.
Owner:INDIANA UNIV RES & TECH CORP +1
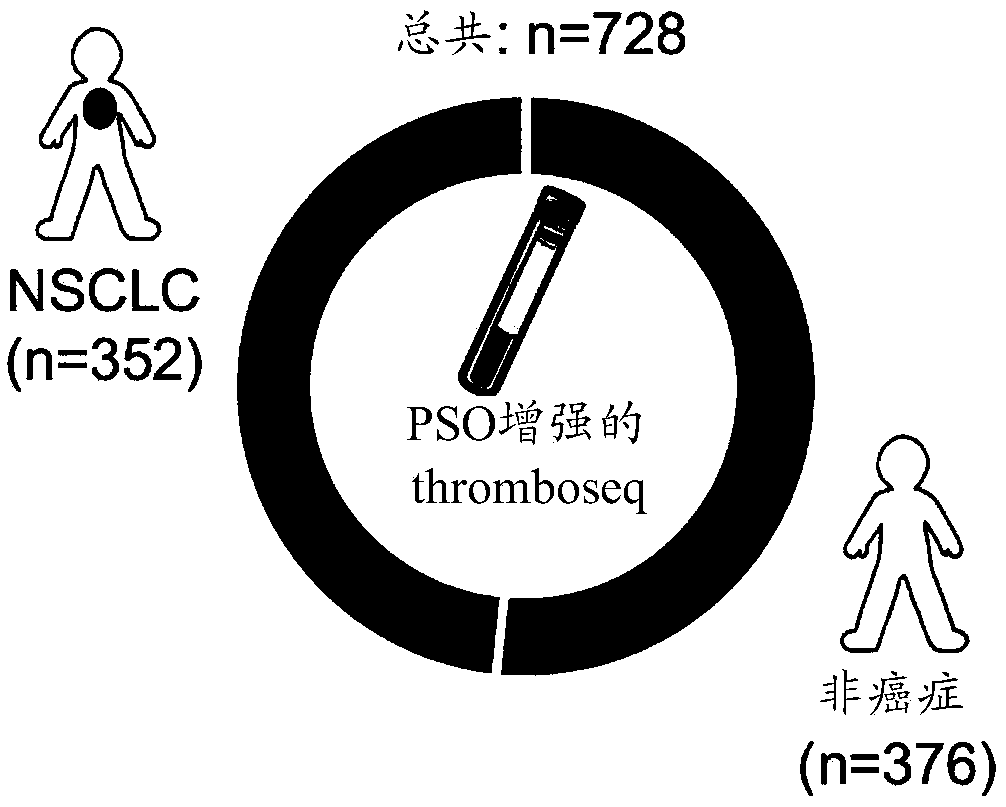
Swarm intelligence-enhanced diagnosis and therapy selection for cancer using tumor- educated platelets
The invention provides methods of administering immunotherapy that modulates an interaction between PD-1 and its ligand, to a cancer patient, based on tumor-educated gene expression profiles obtainedfrom anucleated cells. The invention further provides methods of typing a sample of a subject for the presence or absence of a cancer, based on tumor-educated gene expression profiles obtained from anucleated cells. The invention further provides a method for obtaining a biomarker panel for typing of a sample from a subject using particle swarm optimization-based algorithms.
Owner:スティッチングヴィユーエムシー
Biomarkers for ovarian cancer
Biomarkers and biomarker panels are provided for making ovarian cancer assessments, for example, diagnosing an ovarian cancer, predicting responsiveness of an ovarian cancer to an ovarian cancer therapy, and monitoring an ovarian cancer. A patient may further be treated in accordance with the classification. Also provided are methods, reagents, devices and kits for the use of these biomarkers in making ovarian cancer assessments.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com