Entecavir high-density lipoprotein enveloping preparation, and preparation method and use thereof
A technology of high-density lipoprotein and entecavir, which is applied in the field of entecavir high-density lipoprotein encapsulation preparation and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
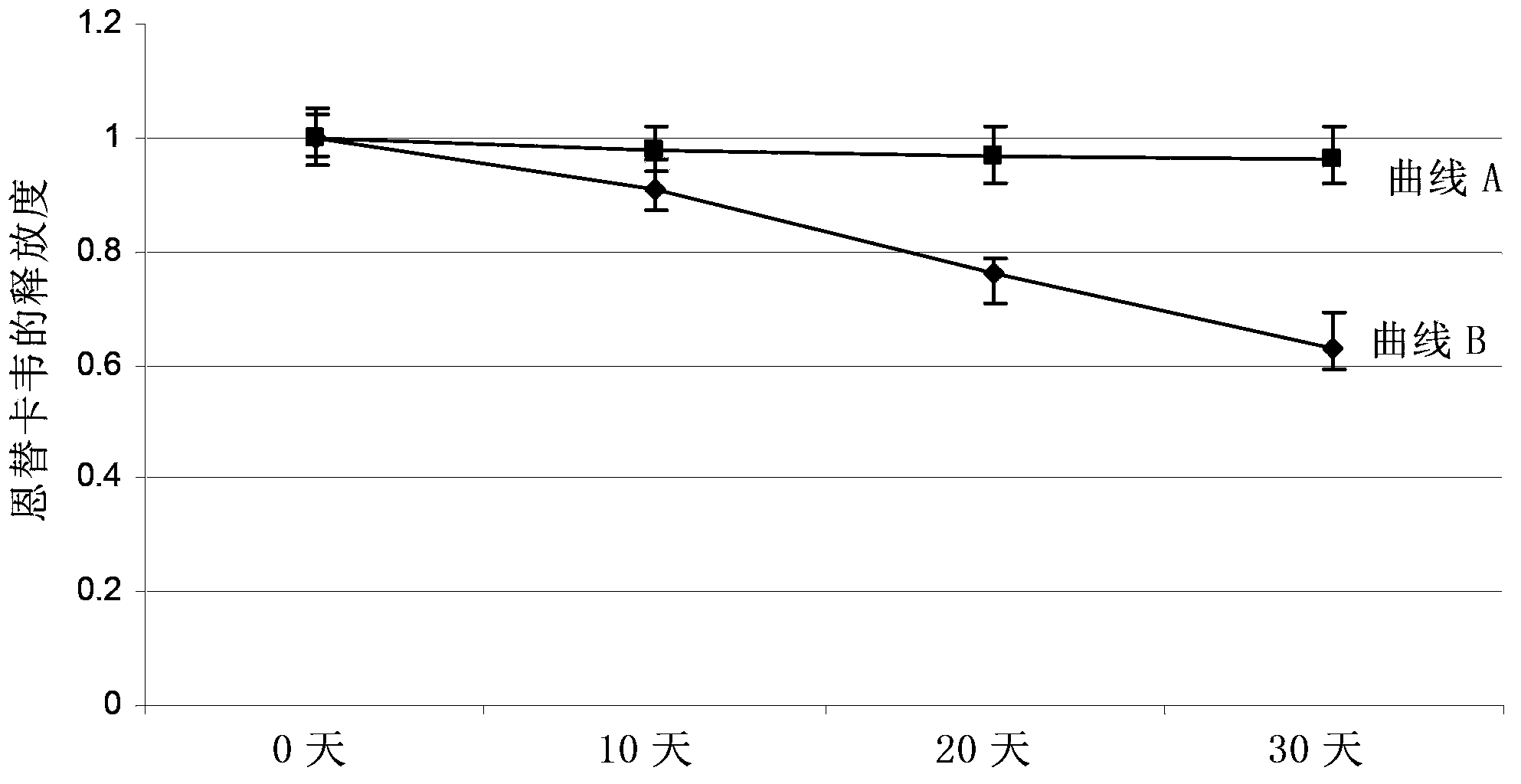
Image
Examples
preparation example Construction
[0051] The invention provides a preparation method of entecavir high-density lipoprotein encapsulation preparation, said method comprising the following steps:
[0052] The first step is to obtain the high-density lipoprotein particle precursor loaded with the internal phase buffer through supercritical fluid technology: mix cholesterol, hydrogenated soybean phospholipid and ammonium salt buffer, and obtain the loaded internal phase buffer through a supercritical fluid reactor. High-density lipoprotein particle precursors;
[0053] The second step is to make the particle size of high-density lipoprotein particle precursor to nanometer level: mix the high-density lipoprotein particle precursor and apolipoprotein A1 loaded with internal phase buffer, and ultrasonically treat the particle size of high-density lipoprotein particle to be 30-50nm, preferably 30-40nm;
[0054] The third step is to replace the external phase buffer with phosphate buffer by molecular sieve, and the pH...
preparation Embodiment 1
[0088] Preparation of Entecavir HDL Encapsulation Preparation 1
[0089] 1. Utilize supercritical fluid technology (putting into the reaction raw material is 50 grams of 0.3M (NH 4 ) 2EDTA (pH=5.0) buffer, 5.5 grams of hydrogenated soybean lecithin HSPC and 2.4 grams of cholesterol, the reaction kettle temperature is 60 ° C, the reaction kettle pressure is 200 bar) preparation loading 0.3M (NH 4 ) 2 High-density lipoprotein particle precursor in EDTA buffer, reacted and equilibrated for 40 minutes;
[0090] 2. Add 9.5 grams of ApoAI polypeptide and ultrasonic treatment (80KHZ, 0.07-0.14W / cm2, 10 minutes) to the prepared high-density lipoprotein particle precursor to reduce the particle size of the high-density lipoprotein particle precursor to about 30-50nm (nano), to obtain high-density lipoprotein particles, the G-50 molecular sieve column replacement high-density lipoprotein particle external phase buffer is phosphate buffer saline (PBS) with pH=8.5, after replacing the ...
preparation Embodiment 2
[0094] Preparation of entecavir encapsulated in liposomes
[0095] The preparation of entecavir encapsulated in liposomes is the same as the process of preparing entecavir encapsulated in high-density lipoprotein, except that ApoA I protein is not added.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com