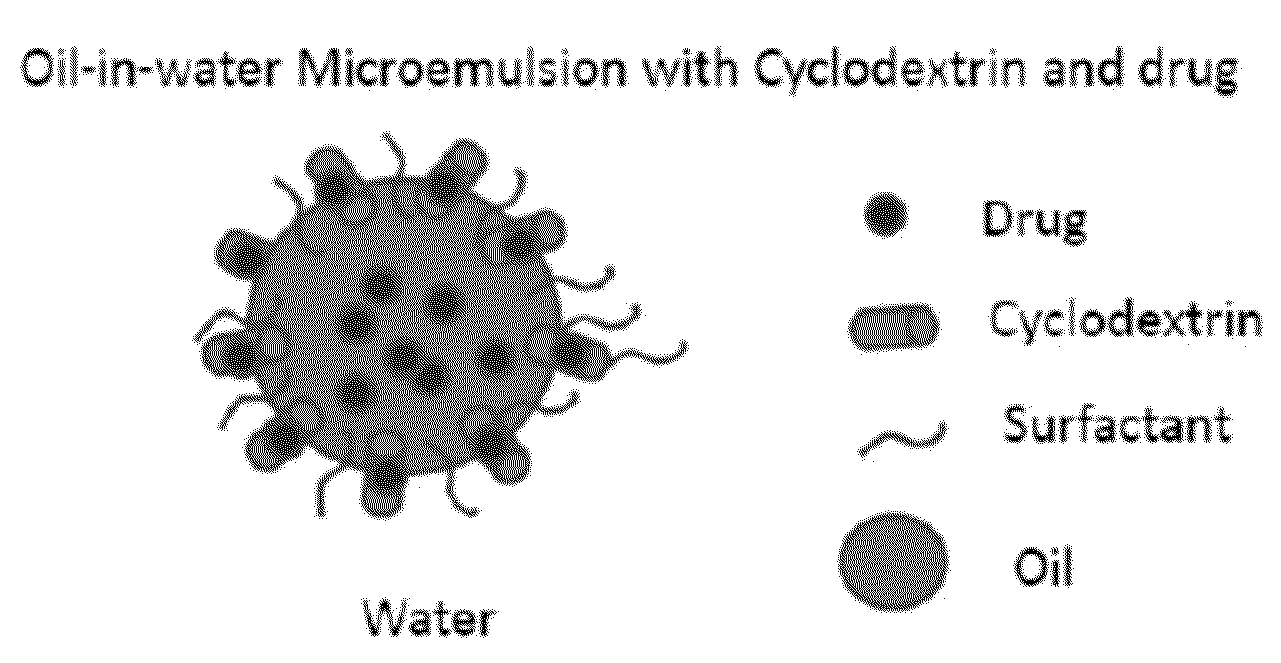
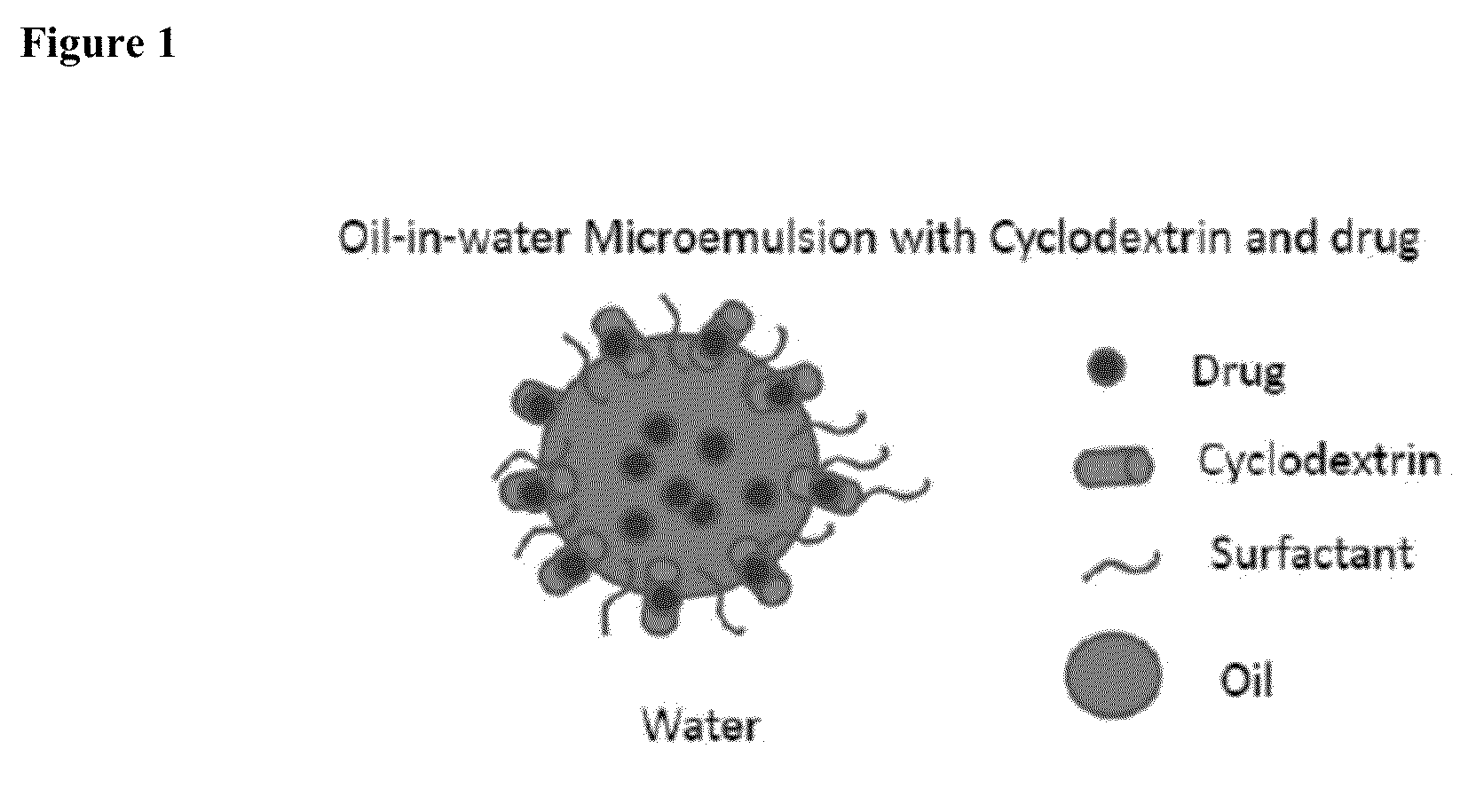
Cyclodextrin-Based Microemulsions, and Dermatological Uses Thereof
a technology of cyclodextrin and microemulsion, which is applied in the direction of biocide, drug composition, aerosol delivery, etc., can solve the problems of skin dryness, volatile lower alcohol composition, extremely flammable volatile lower alcohol microemulsion, etc., and achieve uneven skin tone and increase pore size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Spontaneous Microemulsion Formation by Mixtures of a Caprylocaproyl Polyethylene Glycol-Based Surfactant and Cyclodextrin in Aqueous Media
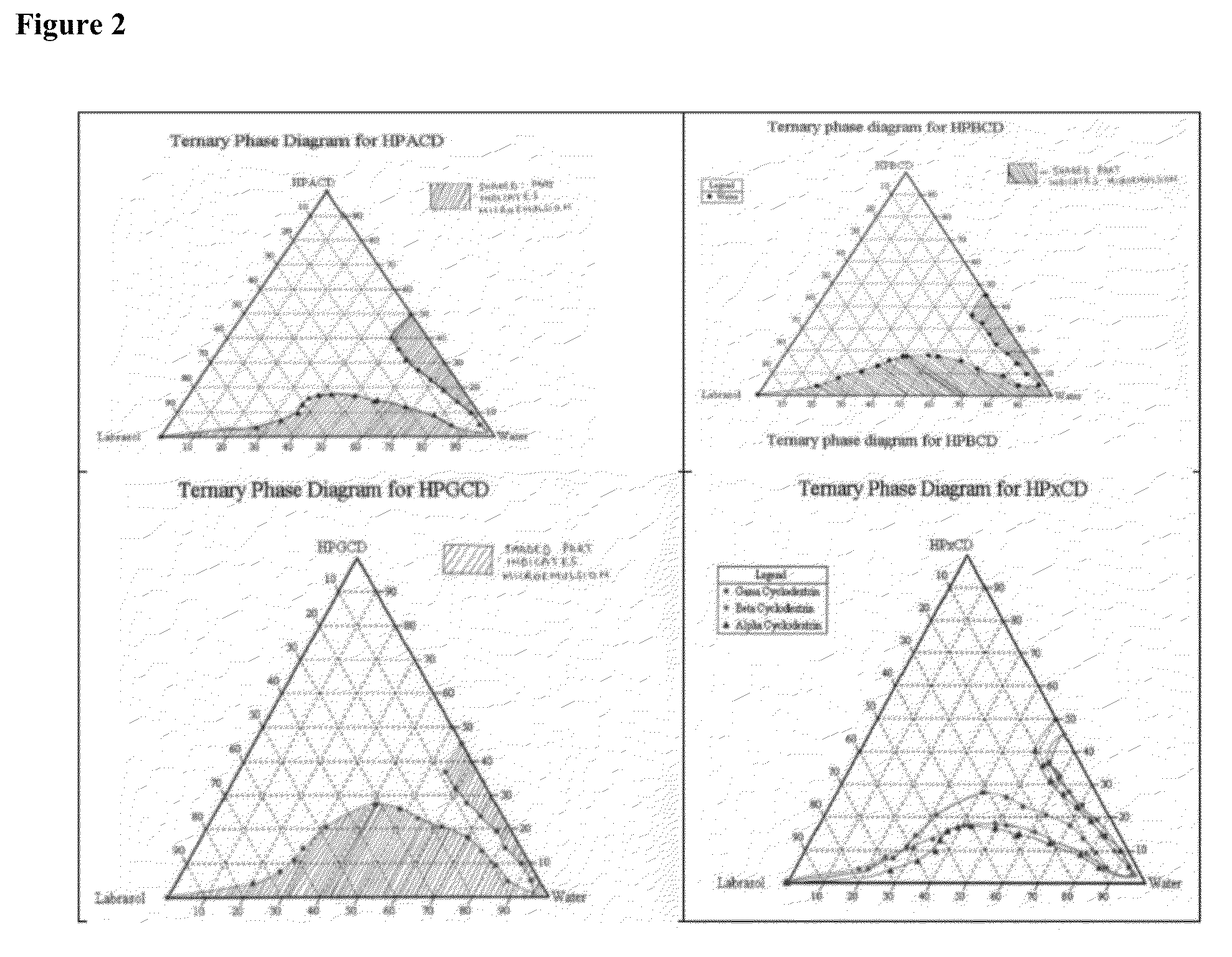
[0936]Microemulsion preparation: Any two components (examples: Labrasol®-water, HPXCD-water, or Labrasol®-HPXCD) were mixed (in different ratios) in different scintillation vials and then the third component was titrated to each vial until a clear-to-turbid or turbid-to-clear transition occurred (in simple situations). Finally wt % percentages data for each component (Labrasol®, water, and cyclodextrin) were plotted to construct the ternary phase diagram. See FIG. 2.
[0937]In FIG. 3, the “water” phase (above) was replaced with an “aqueous” phase. An example of an aqueous phase is shown in FIG. 5.
example 2
Solubility / Dispersibility of Retinol in the Labrasol®-HPGCD-Water Systems and in Labrasol®, HPGCD, and Water, Individually
[0938]Materials
[0939]Drug used: 1. Solid Retinol (98%) from Sigma, 2. Retistar (5% Retinol, caprylic / capric triglyceride, sodium ascorbate, Tocopherol) from BASF, 3. Retinol 50C (50% retinol, ethoxylated sorbitan monolaurate (Tween 20), butylated hydroxytoluene (BHT), 2-(1,1-dimethylethyl)-4-methoxy-phenol) from BASF.
[0940]Cyclodextrin used: hydroxypropyl-X-cyclodextrin (X=alpha, beta, or gamma).
[0941]Methods
[0942]Vial L from FIG. 4(a) shows the formation of a novel microemulsion (clear transparent system) as well as complete miscibility of Retistar (final retinol concentration: 0.06%) in Labrasol®-HPGCD-water microemulsion system (Note: capric / caprylic triglycerides coming from the Retistar are also contributing to the microemulsion system). The miscibility of retinol may be attributed to the drug incorporation in the cyclodextrin system as well as the micro emu...
example 3
Encapsulation of Pharmaceutical / Cosmetic Ingredients in CD-Stabilized Microemulsions
[0946]Materials
[0947]Retinol used: 1. Solid retinol (98%) from Sigma, 2. Retistar (5% retinol, caprylic / capric triglyceride, sodium ascorbate, tocopherol) from BASF, 3. Retinol 50C (50% retinol, ethoxylated sorbitan monolaurate, BHT, 2-(1,1-dimethylethyl)-4-methoxy-phenol from BASF
[0948]Methods
[0949]Microemulsion Preparation:
[0950]Any two components (examples: Labrasol®-water, cyclodextrin-water, or Labrasol®-HPBCD) were mixed (in different ratios) in different scintillation vials and then the third component was titrated to each vial until a clear-to-turbid or turbid-to-clear transition occurred (in simple situations). Finally wt % percentages data for each component (Labrosol, water, and cyclodextrin) were plotted to construct the ternary phase diagram.
[0951]Incorporation of Drug:
[0952]Drug can be dissolved in the individual components or any of the two-component combination systems, followed by mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com